Abstract
Three Algerian honey samples (nectar honey, mixed honey and honeydew honey) were analysed for their total phenolic content (TPC) using the Folin–Ciocalteu reagent and total flavonoid content (TFC) by the aluminium chloride method. The antioxidant activity was tested both against reactive oxygen species (ROS) produced by phorbol 12-myristate 13-acetate (PMA)-stimulated equine polymorphonuclear neutrophils (PMNs) and on purified equine myeloperoxidase (MPO) enzyme activity. The ROS production by stimulated PMNs was measured by lucigenin-enhanced chemiluminescence. Specific immunological extraction followed by enzymatic detection (SIEFED) was used to measure specifically the activity of equine MPO. Nectar honey, mixed honey and honeydew honey had TPCs of 59.52 ± 1.31, 68.36 ± 1.20 and 122.76 ± 4.20 mg gallic acid equivalents (GAE)/100 g honey, respectively (mean ± SD). As for the TPC, the highest TFC was observed for honeydew honey [20.55 ± 0.72 mg catechine equivalents (CE)/100 mg honey], followed by mixed honey (7.47 ± 0.07 CE/100 mg honey) and nectar honey (4.80 ± 0.08 CE/100 mg honey). All the tested honeys displayed a dose-dependent inhibitory effect on the oxidant activities of PMNs. By the SIEFED technique, it was shown that most of the honeys interact directly with MPO: the light honeys (nectar honey and mixed honey) were inhibitors of MPO activity, while the dark honey had less inhibiting effect and even behaved as an enhancer of MPO activity at high concentrations.
Introduction
The stimulation of polymorphonuclear neutrophils (PMNs) results, in addition to the release of cytokines, in both intracellular and extracellular generation of reactive oxygen species (ROS). Inside the PMN, the combined action of ROS and proteolytic enzymes kills ingested bacteria and prevents infection. Outside the PMN, the excessive generation of ROS and enzyme release could have detrimental effects on surrounding tissues (Hampton et al. Citation1998). The superoxide radical, produced by the activity of NADPH oxidase of PMNs, is known to be a harmful oxygen species, as it is also the precursor of other ROS such as hydrogen peroxide (Halliwell & Gutteridge Citation2007). PMN stimulation and phagocytosis are generally accompanied by the degranulation and extracellular release of myeloperoxidase (MPO). Although the beneficial role of MPO during the immune response is to kill pathogens, excessive and uncontrolled release can lead to secondary tissue damage (Davies Citation2011). Several publications have shown that high MPO levels are connected to increased levels of free radical production (Eiserich et al. Citation1998; Arnhold & Flemmig Citation2010). However, it has been suggested that the pro-oxidant activity of peroxidases can be modulated by the in vivo environment (Laranjinha et al. Citation1995; Chan et al. Citation2003). It is widely accepted that honey is beneficial for health, but its mode of action has not been fully elucidated and is probably multifactorial (Kwakman et al. Citation2010; Fauzi et al. Citation2011).
The antioxidant properties of honey have been extensively studied, but there are important discrepancies in the published data. Indeed, honey is a very complex mixture, containing a number of ingredients that are involved in oxidant/antioxidant physiological processes, such as hydrogen peroxide, nitrite, nitrate, glucose, glucose oxidase, iron, copper, chlorine, iodine, catalase, tyrosine, tryptophan, arginine, flavonoids and phenolic acids (Ahmed et al. Citation2012). According to Gheldof et al. (Citation2002), the antioxidant activity of honey is due to the combined activities of enzymes including glucose oxidase, catalase and peroxidase. Several studies show that darker honeys possess higher antioxidant activities than lighter honeys (Taormina et al. Citation2001; Socha et al. Citation2009). In honeys from various floral sources, studies have demonstrated a strong correlation between their content of phenolic compounds and their antioxidant and antibacterial activities (Gheldof et al. Citation2002; Escuredo et al. Citation2012; Alvarez-Suarez et al. Citation2013). Various antioxidant activity methods have been used to measure and compare the antioxidant activity of honey. Assay methods differ from one another in terms of reaction mechanisms, oxidant species, reaction conditions and the way in which the final results are expressed (Moniruzzaman et al. Citation2012). So far, there is no standardized method to assess the antioxidant properties of honey (Ahmed et al. Citation2012) and few models have been used to evaluate its anti-inflammatory properties. The aim of this work was to evaluate in vitro the antioxidant and anti-inflammatory activities of three types of raw Algerian honey using PMNs as a cellular model and MPO as an enzymatic model, involving both ROS production and the inflammatory response.
Materials and methods
Reagents
Folin–Ciocalteu reagents, sodium carbonate solution, gallic acid, aluminium chloride and catechin were all purchased from Sigma (represented by the Algerian Chemical Society, Algeria). Analytical grade sodium and potassium salts, calcium chloride (CaCl2), bovine serum albumin (BSA), dimethylsulfoxide (DMSO) and hydrogen peroxide (H2O2, 30% w/v) were from Merck (VWRI, Belgium). Protein A Sepharose was from Amersham Bioscience Benelux (The Netherlands). Trypan Blue was from ICN Biomedicals (Solon, OH, USA). Ethylenediaminetetraacetic acid (EDTA), lucigenin (bis-N-methylacridinium nitrate), Percoll and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma-Aldrich (Bornem, Belgium). Microtitration plates (Cliniplate EB) and White Combiplates were from Fisher Scientific (Aalst, Belgium). Amplex® Red substrate was purchased from Molecular Probes (The Netherlands).
Honey samples
Three samples of Algerian honey: honeydew honey (DH2: darker honey), mixed honey (LH3) and nectar honey (LH1: lighter honey) were obtained directly from beekeepers, belonging to different geographical regions. Raw honeys used in this study were not submitted to thermal treatments or pasteurization, or any other operation able to alter natural composition. Honey samples were stored at 4°C in the dark until further analysis. The botanical origin, colour and accordance with international standards of the three varieties of honey were confirmed by a specialist laboratory (CARI ABSL Laboratory, Louvain La Neuve, Belgium).
Total phenolic content
Total phenolic content (TPC) was determined by the Folin–Ciocateu method (Singleton et al. Citation1999). Thirty microlitres of honey solution (0.1 g/ml) was mixed with 2.37 ml of Milli-Q® water and 150 µl of Folin–Ciocalteu reagent (0.2 N). The solution was thoroughly mixed by vortexing and incubated for 2 min at room temperature. A volume of 450 µl sodium carbonate solution (0.2 g/ml) was added to the reaction mixture and further incubated for 2 h at room temperature. The absorbance was measured at 765 nm. The total phenolic content was determined by comparison with a standard curve prepared using gallic acid (0–200 µg/l). The mean of at least three readings was calculated and expressed as milligrams of gallic acid equivalents (mg GAE)/100 g of honey.
Total flavonoid content
Total flavonoid content (TFC) was determined using the aluminium chloride assay, according to Amaral et al. (Citation2009). A 10 µl volume of a 10% (w/v) honey solution was added to the wells of a 96-well microtitre plate, and then 30 µl of a 2.5% sodium nitrite solution, 20 µl of a 2.5% aluminium chloride solution and 100 µl of a 2% sodium hydroxide solution were sequentially added. The samples were mixed and the absorbance at 450 nm was measured. TFC was expressed as milligrams of catechin equivalents (mg CE)/100 g of honey.
Blood collection and isolation of neutrophils
Blood samples were drawn from healthy horses by jugular venepuncture in 9 ml Vacutainer® tubes with EDTA (1.6 mg/ml blood) as anticoagulant. The horses were clinically healthy: they were fed, bred and housed under identical conditions and were not under medical treatment (Faculty of Veterinary Medicine, University of Liège, Belgium). PMNs were isolated according to the technique previously described by Pycock et al. (Citation1987) on a discontinuous density gradient of Percoll in Hank's balanced salt solution (HBSS) buffer formed by a 85% solution, overlaid by a 70% solution. The anticoagulated whole blood, laid on the top of the gradient, was centrifuged at 400 × g for 20 min at 20°C. The PMNs were collected at the interface between the two gradient layers and washed in two volumes of physiological saline solution. The cell pellets were suspended in 20 mM phosphate-buffered saline (PBS) at pH 7.4 containing 137 mM NaCl and 2.7 mM KCl. The cell preparation was ≥ 96% neutrophils, with a cell viability of 97% as measured by the Trypan Blue exclusion test. Each batch of neutrophils was obtained from 60 ml blood drawn from one horse, the cells were used immediately after isolation, the experiment was completed within 5 h and each assay was performed in triplicate. Each experiment was repeated at least twice with different cell batches.
Chemiluminescence measurement of reactive oxygen species produced by PMA-stimulated neutrophils
Superoxide radical () production by activated neutrophils was followed by chemiluminescence, according to the method described by Benbarek et al. (Citation1996). Neutrophil suspensions (106 cells/200 µl PBS) were distributed in the wells of a 96-well microtitre plate (White Combiplate 8; Fisher Scientific) and incubated for 10 min at 37°C with honey at final concentrations of 2%, 5%, 10% or 20%. After the incubation, 25 µl CaCl2 (1.1 mg/ml) and 2 µl lucigenin (2.5 mg/ml) were added. Just before the chemiluminescence measurement, 10 µl of 6.10–5 M PMA was added to the neutrophil suspension. The chemiluminescence response of stimulated neutrophils was monitored for 30 min using a Multiscan reader (Multiscan Ascent; Fisher Scientific). A control assay, performed with PMA-stimulated PMNs in the presence of PBS without honey, was taken as 100% chemiluminescence response.
Purification of equine myeloperoxidase and polyclonal antibodies against equine myeloperoxidase
The purification of equine MPO and the preparation of the anti-MPO antibodies used in the immunological techniques were previously described in detail (Franck et al. Citation2005). In brief, MPO was extracted from isolated equine neutrophils and purified by two chromatographic steps (ion exchange and gel filtration) to reach a purity of > 98% (as established by electrophoresis with enzymatic detection on electrophoretic bands). Antisera were obtained from rabbits and guinea pigs after their immunization against the purified enzyme. The polyclonal antibodies (immunoglobulin G) were isolated from antisera by affinity chromatography on Protein A Sepharose®.
Measurement of peroxidasic activity of myeloperoxidase by specific immunological extraction followed by enzymatic detection
The specific immunological extraction followed by enzymatic detection (SIEFED) technique was used as previously described by Franck et al. (Citation2006). The first step, immunoextraction, captures MPO from a solution or biological sample using specific immobilized antibodies. The second step consists of washing to eliminate all the compounds or the potential interfering substances of the sample that are not bound to the antibodies. The third step is the in situ detection of MPO enzymatic activity (revelation step) using H2O2, a fluorogenic substrate (Amplex Red) and NaNO2 as the enhancer of the reaction. The peroxidase activity of MPO oxidized Amplex Red into a fluorescent compound. The diluted honey samples at final concentrations of 2%, 5%, 10% and 20% (w/v) were incubated for 10 min with equine MPO (20 ng/ml) before the immunoextraction step. The MPO solution (20 ng/ml) used for SIEFED was prepared with purified equine MPO diluted in PBS buffer at pH 7.4 with 5 g/l BSA and 0.1% Tween 20. The mixture was then loaded on to a microplate coated with rabbit polyclonal antibodies (3 µg/ml) against equine MPO and incubated for 2 h at 37°C to allow the capture of MPO by the antibodies. Afterwards, the samples were eliminated and the wells of the microtitre plate were washed four times with PBS buffer at pH 7.4 containing 0.1% Tween 20. The washing step allows the removal of the excess honey and all the molecules not bound to the antibodies or to MPO, to reveal the enzyme activity. The activity of the enzyme captured by the antibodies was measured by adding H2O2 (10 µM), nitrite anions (NO2–, 10 mM) and Amplex Red (40 µM) as the fluorogenic substrate. The oxidation of Amplex Red into the fluorescent adduct resorufin (λexcitation = 544 nm; λemission = 590 nm) was monitored for 30 min at 37°C with a fluorescent plate reader (Fluoroskan Ascent; Fisher Scientific). A control assay set as 100% MPO activity was performed with purified MPO and with the honey solution replaced by distilled water.
Statistical analysis
TPC and TFC values were determined in triplicate. In terms of the cellular and enzymatic models, within an experiment, each point was repeated three times and each experiment was repeated two to four times with cell batches from different horses, so that the n value of one experimental point ranged from 6 to 12. Data are given as mean ± SD and statistical analysis was performed with GraphPad InStat 3.05 (GraphPad Software, San Diego, CA, USA). A p value < 0.05 was considered significant.
Results
Total polyphenol and flavonoid contents
shows the TPC (mg GAE/100 g of honey) and TFC (mg CE/100 g of honey) values determined using the colorimetric Folin–Ciocalteu method. In this reaction, the oxidant probe is reduced by electron transfer from the antioxidants present in honey samples, resulting in a blue colour complex formed with phosphomolybdic–phosphotungstic acid reagent (Folin–Ciocalteu reagent) with a maximum absorbance between 745 and 765 nm.
Table 1. Total polyphenol and flavonoid contents of the three honey samples.
The colorimetric study indicates that the TPC was 59.52 ± 1.31, 68. 36 ± 1.20 and 122.76 ± 4.20 mg GAE/100 g honey for LH1, LH3 and DH2, respectively () using the standard curve with gallic acid (R2 = 0.9990).
Similarly, according to the standard curve generated by catechin (R2 = 0.9997), the TFC of honey samples was in the same order as observed for polyphenols, with the highest level for DH1 (20.55 ± 0.72 mg CE/100 mg honey) and the lowest level for LH1 (4.80 ± 0.08 mg CE/100 mg honey) ().
Effect of various concentrations of honey on production of reactive oxygen species by PMA-stimulated equine neutrophils
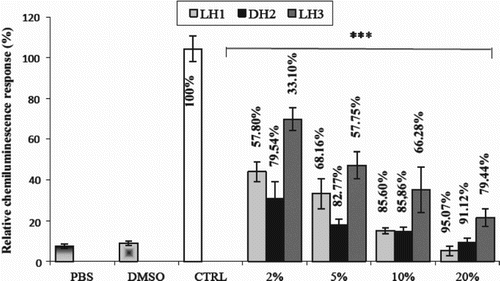
The effect of honey on the ROS production of PMA-activated equine neutrophils was studied by the chemiluminescence technique using lucigenin as a luminescent probe. Before starting the chemiluminescence measurements, neutrophils (106 cells/ml PBS) were incubated with diluted honey samples (2%, 5%, 10% and 20%), and the cell viability was estimated by the Trypan Blue exclusion test to check for possible cytotoxicity of the honey sample. The cell viability was ≥ 96% (data not shown). shows that in the absence of honey samples, the chemiluminescence emission was high (control) compared to the two other controls (non-activated neutrophils with DMSO or PBS). By contrast, when the honey sample was added, at different final concentrations of 2%, 5%, 10% and 20% in the neutrophil suspension, a dose-dependent inhibition of the chemiluminescence was observed (). Comparison of the three honey samples indicated that both DH2 and LH1 samples exerted more pronounced effects than LH3. However, the three samples showed very significant dose-dependent inhibition (), which reached 95.07% for LH1, 91.12% for DH2 and 79.44% LH3 at the highest concentration of 20%. The following order of efficiency was observed: DH2 > LH1 > LH3. (The effect of honey observed by chemiluminescence showed a real inhibitory effect on superoxide production and no toxic effect on equine neutrophils. This remains a subject for discussion.)
Figure 1. Effect of various concentrations of honey on lucigenin-dependent chemiluminescence. The percentage of inhibition of the chemiluminescence of each honey sample was compared to the control (CTRL), taken as 100% (assay performed without honey). Chemiluminescence values were obtained for unstimulated polymorphonuclear neutrophils in phosphate-buffered saline (PBS) or with the addition of dimethylsulfoxide (DMSO; vehicle of phorbol 12-myristate 13-acetate). Data are shown as mean ± SD (n = 9). ***p < 0.0001 for all honey samples compared to control.
Interaction of honey content with the myeloperoxidase active site studied by specific immunological extraction followed by enzymatic detection
The SIEFED technique is a licensed method developed by Franck et al. (Citation2006) for the specific detection of equine MPO by its peroxidasic activity. NO2– is used for the first reduction of the compound I (CpI) to the intermediate oxoferryl form II (CpII) of MPO. Then, CpII is reduced to the ferric form (native enzyme, Por-Fe3+) using Amplex Red as a fluorogenic substrate.
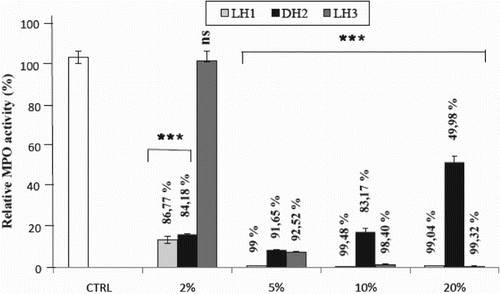
As shown in , the enzyme activity in the absence of any honey sample remained very high, while the incubation of honey samples with the MPO enzyme led to different effects on the activity. Overall, a strong significant inhibition (p < 0.0001) was observed for all types of honey at all dilutions tested (2%, 5%, 10% and 20% in PBS buffer), except for LH3 at 2%, in comparison to the MPO control with PBS, which was set as 100% activity. When honeys were diluted at 20%, the activity of MPO was inhibited by 99.04% and 99.32% with LH1 and LH3, respectively, while the inhibitory effect of DH2 reached a value of 49.98%.
Figure 2. Interaction of honey with myeloperoxidase (MPO) studied by the specific immunological extraction followed by enzymatic detection (SIEFED) technique. Control (CTRL): activity of the enzyme in the absence of honey, taken as 100%. Data are shown as mean ± SD (n = 12). ***p < 0.0001 for all samples and at all concentrations versus CTRL, except for LH3 at 2% concentration; ns = not significant vs control.
Discussion
From the literature, it is known that the antioxidant activity of honeys can be associated with their combined activities with enzymes, oxidases, etc. (Gheldof et al. Citation2002). Also, it is established that honeys from various sources exhibit a strong correlation between their content of phenolic compounds and their antioxidant and antibacterial activities (Gheldof et al. Citation2002; Alvarez-Suarez et al. Citation2013). As summarized in , honeydew honey (DH2) has the highest levels of polyphenols and flavonoids while nectar honey (LH1) has the lowest levels of polyphenols and flavonoids. The phenolic content of honey depends on the geographical and botanical origin. Darker honeys have a higher content of polyphenols than light-coloured honeys (Blasa et al. Citation2006; Bertonceli et al. Citation2007). The results herein are in accordance with those of Khallil et al. (Citation2012), who found a high level of polyphenols and flavonoids in the studied Algerian honeys, which further indicates their high antioxidant potential. Proline was also detected in high concentrations (1692–2712 mg/kg) in all of the Algerian honey samples tested. The authors concluded that the total proline content may be a critical factor responsible for the antioxidant activity of Algerian honey.
Numerous tests have been developed for measuring the antioxidant capacity of food and biological samples. However, there is no universal method that can measure the antioxidant capacity of all samples accurately and quantitatively. The antioxidant capacity depends on the assay method and the radical source used (Prior et al. Citation2005).
In the present study, in vitro cellular and enzymatic models were used to represent biological conditions. A lucigenin-enhanced chemiluminescence assay was used because lucigenin is a good luminescent probe to measure the extracellular by stimulated neutrophils (Vladimirov & Proskurnina Citation2009). All the honeys tested induced dose-dependent inhibition of the
production by stimulated neutrophils. These results are in agreement with those obtained by previous studies. Leong et al. (Citation2011) reported that honey exhibited potent and dose-dependent reduction of human neutrophil superoxide anion production in vitro. Other groups also described a scavenger activity of honey on
using the xanthine–xanthine oxidase system (Henriques et al. Citation2006; Van den Berg et al. Citation2008). Honey was also found to decrease the luminol-enhanced chemiluminescence in opsonized zymosan-stimulated whole blood and isolated leucocytes (Mesaik et al. Citation2008). In the present study, the dark honey (DH2) had the greatest effect on the oxidant response of neutrophils, and this can be explained by its higher content of polyphenol and flavonoids. It is generally found that darker honeys possess stronger antioxidant activities than lighter ones (Frankel et al. Citation1998; Taormina et al. Citation2001; Socha et al. Citation2009). The darker the honey, the higher its phenolic content and its antioxidative power (Gheldof et al. Citation2002; Blasa et al Citation2006; Bertonceli et al. Citation2007; Vela et al. Citation2007; Marghitas et al. 2009). It has also been shown that honeydew honeys possess higher antioxidant capacities than nectar ones (Vela et al. Citation2007; Lachman et al. Citation2010). On the other hand, during the activation of neutrophils, there is a subsequent release of MPO which, in combination with ROS production, can make MPO functional. Indeed, in the presence of superoxide dismutase,
yields H2O2, which is the substrate of MPO. As honey samples were efficient towards ROS production, it appeared interesting to evaluate their putative effect on the activity of the MPO enzyme.
Very few studies have investigated the effects of honey on the activity of MPO. Medhi et al. (Citation2008) reported that manuka honey has the ability to reduce the MPO level in rats induced with ulcerative colitis. According to Papineni and Orton (Citation2012), intraperitoneal administration of honey in a rabbit model significantly enhanced the MPO activity, resulting in neutrophil stimulation. In these studies, MPO was investigated as a marker of neutrophil infiltration or stimulation. This work focused on the effect of honey content on the specific activity of MPO through its active site. The catalytic activity of MPO is partitioned between halogenation and peroxidation via competition between peroxidase substrates and (pseudo)halides for compound I. Such competition is involved in the modulation of the enzyme activity (Davies Citation2011). In addition, variations in the pH value may change the priority of the enzyme with respect to a substrate. At acidic pH values, MPO will catalyse preferably the formation of HOCl via its chlorination cycle, whereas at neutral pH values the oxidation of phenolic substrates can be observed via the peroxidase cycle of MPO (Vlasova et al. Citation2006).
The SIEFED technique used in this study allows the peroxidase cycle of the enzyme to be examined. No chloride anions are added to the revelation solution, so that HOCl cannot be generated by MPO activity and thus cannot react directly with honey. Moreover, by the immunocapture of MPO, SIEFED allows the removal of all interfering substances not bound by the antibodies before measurement of the enzyme activity. Thus, the free honey solutions, after incubation with MPO, were removed before the detection of MPO activity. If inhibition of the enzyme is observed after the elimination of honey, it means that some molecules from honey are bound to the enzyme and inhibit its peroxidase activity.
All the honeys inhibited the MPO activity at all dilutions tested (2–20% in PBS buffer), except for LH3 at the lowest dilution (2%). Some molecules found in the honeys can interact with or bind to MPO, and either modify the enzyme structure or hinder the access of the substrate into the active site, thus acting as competitive substrates. The active site of human MPO is located in a hydrophobic cavity with a narrow, oval-shaped opening (Fiedler et al. Citation2000). It can be expected that polyphenols or other honey compounds could hinder or block the entry of this cavity. Jiao et al. (Citation2006) showed that curcuminoids could act as iron chelators, and it cannot be excluded that such a chelating activity could also be possible with some honey components. Further studies are needed to confirm this hypothesis. The potent iron chelator deferoxamine neither reacts with nor removes the iron of MPO (Klebanoff & Waltersdorph Citation1988).
Surprisingly, the honey DH2, at low concentration, inhibited strongly the activity of MPO but the activity increased progressively when the concentration increased. Such an effect could depend on the concentration ratio between antioxidants and the target (Magalhães et al. Citation2008). This notion is consistent with in vitro observations showing that phenolic compounds can induce pro-oxidant or antioxidant activity depending on the chemical environment (Laranjinha et al. Citation1995; Chan et al. Citation2003). The phenol/peroxidase reaction has been generally regarded as a mechanism by which the pro-oxidant activity of natural polyphenols is mediated in animal cells (Galati et al. Citation2002). However, it has been suggested that MPO may be involved in the antioxidant activity of phenolic compounds in endothelial cells under oxidative stress (Lee et al. Citation2009). Therefore, one must be aware that honey is a very complex mixture containing numerous phenolic components that could modulate the activity of MPO. Further studies are needed to determine whether these honey compounds could have a synergistic or a competitive effect in their binding to MPO.
Acknowledgements
The authors would like to thank Mrs Ariane Niesten and Jennifer Romainville for their technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Centre for Oxygen, Research and Development (CORD), University of Liege, funded by the National Fund for Scientific Research (NFSR) Belgium.
References
- Ahmed M, Aissat S, Djebli N. 2012. How honey acts as an antioxidant? Medicinal Aromatic Plants. 1:1–2. doi: 10.4172/2167-0412.1000e132
- Alvarez-Suarez JM, Giampieri F, Battino M. 2013. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr Med Chem. 20:621–638. doi: 10.2174/092986713804999358
- Amaral S, Mira L, Nogueira JM, Da Silva AP, Florêncio HM. 2009. Plant extracts with anti-inflammatory properties –A new approach for characterization of their bioactive compounds and establishment of structure–antioxidant activity relationships. Bioorg Med Chem. 17:1876–1883. doi: 10.1016/j.bmc.2009.01.045
- Arnhold J, Flemmig J. 2010. Human myeloperoxidase in innate and acquired immunity. Arch Biochem Biophys. 500:92–106. doi: 10.1016/j.abb.2010.04.008
- Benbarek H, Deby-Dupont G, Deby C, Caudron I, Mathy-Hartert M, Lamy M, Serteyn D. 1996. Experimental model for the study by chemiluminescence of the activation of isolated equine leucocytes. Res Vet Sci. 61:59–64. doi: 10.1016/S0034-5288(96)90112-5
- Bertonceli J, Dobersek U, Jamnik M, Golob T. 2007. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 105:822–828. doi: 10.1016/j.foodchem.2007.01.060
- Blasa M, Candiracci M, Accorsi A, Piacentini MP, Albertini MC, Piatti E. 2006. Raw Millefiori honey is packed full of antioxidants. Food Chem. 97:217–222. doi: 10.1016/j.foodchem.2005.03.039
- Chan TS, Galati G, Pannala AS, Rice-Evans C, O'Brien PJ. 2003. Simultaneous detection of the antioxidant and pro-oxidant activity of dietary polyphenolics in a peroxidise system. Free Radic Res. 37:87–794. doi: 10.1080/1071576031000094899
- Davies MJ. 2011. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr. 48:8–19. doi: 10.3164/jcbn.11-006FR
- Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. 1998. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 391:393–397. doi: 10.1038/34923
- Escuredo O, Silva LR, Valentão P, Seijo MC, Andrade PB. 2012. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 130:671–678. doi: 10.1016/j.foodchem.2011.07.107
- Fauzi AN, Norazmi MN, Yaacob NS. 2011. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem Toxicol. 49:871–878. doi: 10.1016/j.fct.2010.12.010
- Fiedler TJ, Davey CA, Fenna RE. 2000. X-ray crystal structure and characterization of halide-binding sites of human myeloperoxidase at 1.8Å resolution. J Biol Chem. 275:11964–11971. doi: 10.1074/jbc.275.16.11964
- Franck T, Grulke S, Deby-Dupont G, Deby C, Duvivier H, Peters F, Serteyn D. 2005. Development of an enzyme-linked immunosorbent assay for specific equine neutrophil myeloperoxidase measurement in blood. J Vet Diagn Invest. 17:412–419. doi: 10.1177/104063870501700502
- Franck T, Kohnen S, Deby-Dupont G, Grulke S, Deby C, Serteyn D. 2006. A specific method for measurement of equine active myeloperoxidase in biological samples and in in vitro tests. J Vet Diagn Invest. 18:326–334. doi: 10.1177/104063870601800402
- Frankel S, Robinson GE, Berenbaum MR. 1998. Antioxidant capacity and correlated characteristics of fourteen unifloral honey. J Apic Res. 37:27–31.
- Galati G, Sabzevari O, Wilson JX, O'Brien PJ. 2002. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology. 177:91–104. doi: 10.1016/S0300-483X(02)00198-1
- Gheldof N, Wang XH, Engeseth NJ. 2002. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 50:5870–5877. doi: 10.1021/jf0256135
- Halliwell B, Gutteridge JMC. 2007. Free radicals in biology and medicine. 4th ed. Oxford, UK: Oxford University Press.
- Hampton MB, Kettle AJ, Winterbourn CC. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 92:3007–3017.
- Henriques A, Jackson S, Cooper R, Burton N. 2006. Free radical production and quenching in honeys with wound healing potential. J Antimicrob Chemother. 58:773–777. doi: 10.1093/jac/dkl336
- Jiao Y, Wilkinson JIV, Pietsch EC, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. 2006. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003
- Khallil MI, Moniruzzaman M, Boukraâ L, Benhanifia M, Islam A, Islam N, Sulaiman SA, Gan SH. 2012. Physicochemical and antioxidant properties of algerian honey. Molecules. 17:11199–11215. doi: 10.3390/molecules170911199
- Klebanoff SJ, Waltersdorph AM. 1988. Inhibition of peroxidase-catalyzed reactions by deferoxamine. Arch Biochem Biophys. 264:600–606. doi: 10.1016/0003-9861(88)90326-8
- Kwakman PH, te Velde AA, de Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA. 2010 Jul. How honey kills bacteria. FASEB J. 24(7):2576–82. doi: 10.1096/fj.09-150789
- Lachman J, Orsăk M, Alena H, Eva K. 2010. Evaluation of antioxidant activity and total phenolics of selected Czech honey. LWT-Food Sci Technol. 43:52–58. doi: 10.1016/j.lwt.2009.06.008
- Laranjinha J, Vieira O, Madeira V, Almeida L. 1995. Two related phenolic antioxidants with opposite effects on vitamin E content in low density lipoproteins oxidized by ferrylmyoglobin: consumption vs regeneration. Arch Biochem Biophys. 323:373–381. doi: 10.1006/abbi.1995.0057
- Lee SJ, Mun GI, An SM, Boo YC. 2009. Antioxidant effect of p-coumaric acid in endothelial cells exposed to high glucose plus arachidonic acid. BMB reports 561.
- Leong AG, Herst PM, Harper JL. 2011. Indigenous New Zealand honeys exhibit multiple antiinflammatory activities. Innate Immun. 18:459–466. doi: 10.1177/1753425911422263
- Magalhães LM, Segundo MA, Reis S, Lima JL. 2008. Methodological aspects about in vitro evaluation of antioxidant properties. Analyt Chim Acta. 613:1–19. doi: 10.1016/j.aca.2008.02.047
- Medhi B, Prakash A, Avti PK, Saikia UN, Pandhi P, Khanduja KL. 2008. Effect of manuka honey and sulfasalazine in combination to promote antioxidant defense system in experimentally induced ulcerative colitis model in rat. Indian J Exp Biol. 46:583–590.
- Mesaik MA, Azim MK, Mohiuddin S. 2008. Honey modulates oxidative burst of professional phagocytes. Phytother Res. 22:1404–1408. doi: 10.1002/ptr.2509
- Moniruzzaman M, Khalil MI, Sulaiman SA, Gan SH. 2012. Advances in the analytical methods for determining the antioxidant. J Tradit Complement Altern Med. 9(36):42.
- Papineni RVL, Orton S. 2012. Intraperitoneal Administration of Honey Elicit Robust Luminescence Signals from Myelo, peroxidase Activation. Presentation at World Molecular Imaging Congress Dublin, Ireland September 5–8. 2012. http://fr.scribd.com/doc/100813301/Honey-Medicinal-http://fr.scribd.com/doc/100813301/Honey-Medicinal-Value-and-Mechanism-Dr-Rao-Papineni.
- Prior RL, Wu XL, Schaich K. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 53:4290–4302. doi: 10.1021/jf0502698
- Pycock JF, Allen WE, Morris TH. 1987. Rapid, single-step isolation of equine neutrophils on a discontinuous Percoll density gradient. Res Vet Sci. 42:411–412.
- Singleton VL, Orthofer R, Lamuela-Raventós RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 299:152–178. doi: 10.1016/S0076-6879(99)99017-1
- Socha R, Juszczak L, Pietrzyk S, Fortuna T. 2009. Antioxidant activity and phenolic composition of herb honeys. Food Chem. 113:568–574. doi: 10.1016/j.foodchem.2008.08.029
- Taormina PJ, Niemira BA, Beuchat LR. 2001. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int J Food Microbiol. 69:217–225. doi: 10.1016/S0168-1605(01)00505-0
- Van den Berg AJ, van den Worm E, van Ufford HC, Halkes SB, Hoekstra MJ, Beukelman CJ. 2008. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J Wound Care. 17: 172–4– 176–8. doi: 10.12968/jowc.2008.17.4.28839
- Vela L, De Lorenzo C, Pérez RA. 2007. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J Sci Food Agric. 87:1069–1075. doi: 10.1002/jsfa.2813
- Vladimirov YuA, Proskurnina EV. 2009. Free radicals and cell chemiluminescence. Biochemistry (Mosc). 74:1545–1566. doi: 10.1134/S0006297909130082
- Vlasova II, Arnhold J, Osipov AN, Panasenko OM. 2006. pH-Dependent regulation of activity of myeloperoxidase. Biochemistry (Mosc). 71:667–677. doi: 10.1134/S0006297906060113
- Zuckerman ZH, Bryan N. 1996. Inhibition of LDL oxidation and myeloperoxidase dependent tyrosyl radical formation by the selective estrogen receptor modulator raloxifene (LY139481 HCL). Atherosclerosis. 126:65–75. doi: 10.1016/0021-9150(96)05894-7
