Abstract
Allium sativum (garlic), Zingiber officinale (ginger) and Capsicum frutescens (cayenne pepper) are common dietary spices also traditionally used in the treatment of various diseases including diabetes mellitus. The antidiabetic activity of each individual spice is well documented, but their effect when combined is unknown. Polyherbalism is of current interest because polyherbal formulations enhance therapeutic action and reduce the concentrations of single herbs, thereby reducing adverse events. This study evaluated the hypoglycaemic activity of aqueous extract of combined garlic, ginger and cayenne pepper (GGCP) at different doses in alloxan-induced diabetic rats. Diabetic rats were treated with GGCP at 200 mg and 500 mg/kg body weight/day, or glibenclamide (5 mg/kg body weight/day) for 7 days. GGCP extract significantly (p < 0.05) lowered the elevated fasting blood glucose, lipid and haematological indices. The mixture markedly attenuated cellular toxicity, and reduced tubular degeneration and necrosis in the kidney, fatty degeneration and necrosis in the liver and pancreatic hyperplasia in diabetic rats. These effects were more pronounced at 500 mg/kg and equipotent with glibenclamide, suggesting that in addition to its hypoglycaemic activity, GGCP protects the blood, kidney, liver and pancreas against diabetic injury. This is the first pilot study to evaluate a possible role for this spice mixture in the treatment of diabetes.
Introduction
Diabetes mellitus is an endocrine dysfunction that is characterized by defects in insulin secretion or action which result in impaired metabolism of glucose, lipid and protein (Kumar et al. Citation2011). The prevalence of diabetes is on the increase worldwide and may reach 5.4% by 2025 (Moller & Flier Citation1991). According to the World Health Organization, around 173 million adults are diabetic and over 8 million deaths are caused by this disease or its complications annually (Sunmonu & Afolayan Citation2013).
In spite of the appreciable progress that has been made in the management of diabetes using conventional drugs and management strategies, diabetes and its complications continue to be a major medical problem. Most synthetic oral hypoglycaemic agents available for the treatment of the disease have serious side-effects and/or cannot be used during pregnancy, and are also costly (Kumari et al. Citation2013). As a result, large numbers of people are turning to traditional herbal medicines to prevent and treat diabetes throughout the world (Broadhurst et al. Citation2000; Shetti et al. Citation2012). There is therefore an increasing need to search for more effective antidiabetic agents with few or no side-effects.
Spices that show hypoglycaemic, hypolipidaemic and antioxidant activities may have potential roles in the treatment of diabetes. These spices are therefore becoming more popular because of their potential efficacy, minimal or no side-effects and synergistic actions (Panda et al. Citation2013). Some of the spices commonly used in African traditional medicine for the management of diabetes mellitus are Allium sativum (garlic), Zingiber officinale (ginger) and Capsicum frutescens (cayenne pepper). These three spices have been used all over the world as culinary spices since ancient times (Eidi et al. Citation2006). Each of the species is also widely used in the preparation of medicines because of claimed healing properties against many ailments (Amagase Citation2006; Antonious et al. Citation2006).
Garlic (Allium sativum L.) is a bulb-forming herb of the Lilliaceae family. It is a perennial species that originated 5000 years ago in central Asia, where it was used to ward off evil spirits and to improve health, and is now cultivated throughout the world (Barrie et al. Citation1987; Aaron Citation1996). The most common part of this plant used for medicinal purposes is the bulb. Several experimental and clinical investigations have reported many favourable effects of garlic and its preparations. Such effects include: (i) reduction of risk factors for cardiovascular diseases, (ii) reduction of cancer risk, (iii) antioxidant effect, (iv) antimicrobial effect against bacteria, viruses, fungi and parasites, (v) detoxification of foreign compounds and hepatoprotection, and (vi) decrease in platelet aggregation and atherosclerotic plaques (Colín-González et al. Citation2012; Bayan et al. Citation2014).
Ginger (Zingiber officinale Roscoe) is a spice that is indigenous to South-East Asia. It is also cultivated in the USA, India, China, the West Indies and tropical regions as a spice and condiment to add flavour to food. Besides this, the rhizome of ginger has also been used in traditional herbal medicine. The rhizome of ginger contains many bioactive components, such as [6]-gingerol (1-[4′-hydroxy-3′-methoxyphenyl]-5-hydroxy-3-decanone), which is the primary pungent ingredient that is believed to exert a variety of remarkable pharmacological and physiological activities (Bode & Dong Citation2011). Ginger has been used for years in Asian, Ayurvedic and traditional medicine as an anti-inflammatory and antipyretic agent to treat indigestion, nausea, stomach ache, toothache, insomnia, respiratory and urinary tract infections, rheumatism, diabetes and infertility, and as a memory enhancer (Grzanna et al. Citation2005; Morakinyo et al. Citation2011).
Cayenne pepper (Capsicum frutescens L.) is a temperate plant of the Solanaceae (nightshade) family. The genus Capsicum comprises five cultivated species with worldwide distribution and about 20 wild species (Walter Citation1986). Traditionally, cayenne pepper has a variety of uses both in the diet (as an ingredient in hot sauces) and medicinally. The active ingredient in cayenne is capsaicin, although carotenoids, flavonoids and vitamins A and C have also been reported to play active roles in its use as a medicine (Ahuja et al. Citation2006). Cayenne pepper has reportedly been used in ethnomedicinal practices of postnatal care, as nutrition therapy, in pain management, to treat erectile dysfunction, as a circulatory tonic, as a remedy for rheumatic and arthritic pains, in the reduction of blood sugar levels, for the excretion of cholesterol, as well as for its analgesic, antimicrobial, anti-inflammatory and immunological effects (Renault et al. Citation2003; Ahuja et al. Citation2006; Nwose Citation2009; Jolayemi & Ojewole Citation2013).
Previously, Otunola et al. (Citation2012, Citation2014) have reported the hypolipidaemic and antioxidative stress potential of garlic, ginger and cayenne pepper singly and combined in rat models. Although each of the spices (garlic, ginger and pepper) has been reported to exhibit antidiabetic activities such as increasing insulin secretion, and lowering of blood glucose and urine sugar (Tolan et al. Citation2001; Liu et al. Citation2007; Gall & Shenkute Citation2009; Rackova et al. Citation2013), there is no report on the antidiabetic action of a combination of the three spices. In view of the above effectiveness of the spices for diabetics, it is proposed to formulate a combination of the three and evaluate the efficacy of the combined spices compared with a reference drug, glibenclamide.
Materials and methods
Drugs and chemicals
Alloxan monohydrate was procured from Sigma-Aldrich Chemical Co. (St Louis, MO, USA), while glibenclamide was a product of Taj Pharmaceuticals (Mumbai, India). The assay kits used for biochemical analysis were products of Randox Laboratories (Ardmore, Co. Antrim, UK). All other reagents were of analytical grade.
Preparation of the spice mixture
The spice mixture was prepared as described previously. Garlic, ginger and pepper were purchased from the local vegetable shop in Alice (Eastern Cape, South Africa). The spices were cleaned separately, thinly sliced, dried in an oven at 60°C for 72 h and then homogenized. Equal weights of each species were blended to give a homogeneous spice material. Fifty grams of the spice was extracted in 1000 ml of distilled water at 100°C for 60 min, allowed to cool and filtered. The filtrate was freeze-dried and kept in an airtight bottle at 4°C. This was then reconstituted in distilled water to give the required calculated doses equivalent to 200 mg/kg and 500 mg/kg body weight of the rats for the experiment.
Experimental animals
Male albino rats of Wistar strain (300–350 g) were obtained from the Animal Unit of the Central Analytical Laboratory of the University of Fort Hare (Alice, South Africa). The animals were housed in polypropylene rat cages and maintained under controlled room temperature (22 ± 2°C) and humidity (45 ± 5%) with a 12 h diurnal cycle. All the rats had free access to a normal rat pellet diet and drinking water ad libitum. The study was approved by the institutional board after obtaining approval for the protocol from the Animal Use Ethics Committee of the university. Ethical clearance number AFO011 was assigned to the project. The animals were allowed to acclimatize to the experimental environment for 1 week before the study.
Induction of diabetes
Diabetes was induced in rats fasted for 16 h, by a single intraperitoneal injection of a freshly prepared solution of alloxan monohydrate (150 mg/kg body weight) in normal saline (Kulkarni et al. Citation2002). The animals were allowed to drink 1% glucose solution immediately after the injection to prevent transient hypoglycaemia. Control rats were injected with normal saline alone. At 48 h after the injection, the fasting blood glucose level was estimated using a glucometer (Gluco Plus Fast Touch; GlucoPlus, Quebec, Canada). Animals with glucose levels above 13.9 mmol/l were considered diabetic (Ravi et al. Citation2004) and included in the study.
Experimental design
The rats were divided into five groups of six rats each:
• Group 1: Normal control rats were administered with 1 ml of distilled water once daily for 7 days.
• Group 2: Diabetic control rats were administered with 1 ml of distilled water once daily for 7 days.
• Group 3: Diabetic rats were treated with GGCP spice mixture aqueous extract (200 mg/kg body weight/day) for 7 days.
• Group 4: Diabetic rats were treated with GGCP spice mixture aqueous extract (500 mg/kg body weight/day) for 7 days.
• Group 5: Diabetic rats were treated with glibenclamide (5 mg/kg body weight/day) for 7 days. The extracts and drug were administered once daily by oral gavage for 7 days. After 7 days the animals were fasted for 12 h and anaesthetized by intraperitoneal injection of pentobarbital sodium (45 mg/kg). Blood was collected by cardiac puncture into appropriate Vacutainers for haematological and biochemical assays, then the rats were killed and dissected. The pancreas, liver, kidneys, heart and lungs were removed, washed with cold saline and weighed.
Estimation of fasting blood glucose
Throughout the 7 days of treatment, fasting blood glucose levels were measured in tail blood samples using a glucometer on days 0, 1, 3, 5 and 7.
Measurement of haematological parameters
The automated Horiba ABX 80 Diagnostics (ABX Pentra, Montpellier, France) analyser was used to estimate the red blood cell (RBC) count, haemoglobin, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC). Other parameters estimated were glycated haemoglobin, platelets, white blood cell (WBC) and white blood cell differential counts.
Biochemical analyses
The serum total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), total protein and albumin levels, as well as the concentrations of creatinine, urea, calcium and globulin, were measured using an automatic analyser. In addition, the activities of alkaline phosphatise (ALP), aspartate and alanine transaminases (AST, ALT), gamma-glutamyl transferase (GGT), creatine kinase (CK) and lactate dehydrogenase were estimated using Randox assay kits (Wroblewski & La Due Citation1956; Wright et al. Citation1972; Tietz et al. Citation1994; Tietz Citation1995).
Histopathological analysis
Blocks of the pancreas, liver and kidney samples were fixed in 10% buffered formalin, and then dehydrated by successively passing through a gradient mixture of ethanol and water. The samples were rinsed in xylene and embedded in paraffin. Thin sections were cut using a rotary microtome and then stained with hematoxylin and eosin dye and examined under the light microscope.
Statistical analysis
All statistical analyses were performed using MINITAB student version 12. The results are expressed as means ± SD (n = 6). A single-factor parametric one-way analysis of variance was used to determine significant differences among treatment means. Differences were separated using Duncan's multiple range test. A p value less than 0.05 was considered significant.
Results and discussion
Effect of GGCP on body and organ weights
The initial and final body weights in the various experimental groups are shown in . The normal control rats showed steady body weight gain throughout the 7 days of treatment, while the diabetic control rats showed significant (p < 0.01) weight loss. Treatment with the GGCP extract (200 and 500 mg/kg) and glibenclamide for 7 days led to a significant increase in body weight. The combined spice prevented loss of body weight in comparison to the standard drug glibenclamide. Relative liver weight was significantly (p < 0.001) higher in diabetic rats than in control rats (). The GGCP extract at 500 mg/kg body weight also showed an effect equal to glibenclamide in lowering the liver weight. Similar observations to those reported in the present study were made by Shetti et al. (Citation2012) and Sunmonu and Afolayan (Citation2013) using Phyllantus amarus and Artemisia afra, respectively, for the treatment of diabetes.
Figure 1. Effect of garlic, ginger and cayenne pepper (GGCP) on body weight change in control and alloxan-induced diabetic rats. Values are significantly different at p < 0.05. D200 = diabetic + 200 mg/kg spice mixture; D500 = diabetic + 500 mg/kg spice mixture; DG (Dglinben) = diabetic + 5 mg/kg glibenclamide.
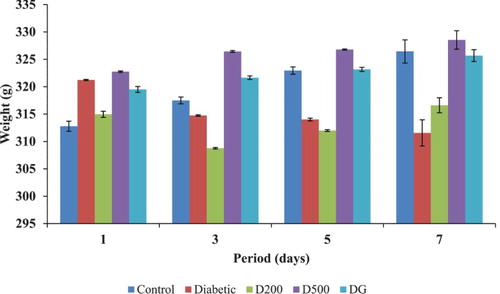
Table 1. Effect of garlic, ginger and cayenne pepper (GGCP) on organ weight (g/100 g) in control and alloxan-induced diabetic rats (n = 6, mean ± SD).
Table 2. Effect of garlic, ginger and cayenne pepper (GGCP) on haematological indices in control and alloxan-induced diabetic rats (n = 6, mean ± SD).
Effect of GGCP on serum glucose levels
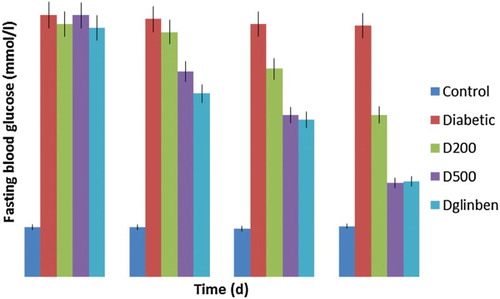
The effect of GGCP extract and glibenclamide on hyperglycaemia was measured using fasting blood glucose. Diabetic control rats showed severe (p < 0.01) hyperglycaemia compared to normal control rats (). This was completely prevented by treatment with GGCP extract at doses of 200 and 500 mg/kg body weight. The GGCP combination significantly (p < 0.01) reduced the blood glucose in alloxan-induced diabetic rats. The hypoglycaemic activity of the spices at 200 mg/kg and 500 mg/kg and glibenclamide was 35.38%, 62.54% and 61.97%, respectively. The hypoglycaemic action of the spice extract may be attributed to improved insulin sensitivity or inhibition of endogenous glucose production (Kuroda et al. Citation2003). In addition, the active constituents in the spice mixture, i.e. alkaloids, tannins, polyphenols and other antioxidants (Otunola and Afolayan), which are known to be bioactive antidiabetic principles, modulate various metabolic cascades which directly or indirectly lower the level of glucose in the system. This result agrees with previous reports by Srivastava et al. (Citation2012) and Petchi et al. (Citation2014) on the antidiabetic activity of polyherbal formulations on induced diabetic rats.
Figure 2. Effect of garlic, ginger and cayenne pepper (GGCP) on fasting blood glucose in control and alloxan-induced diabetic rats. Values are significantly different at p < 0.05. D200 = diabetic + 200 mg/kg spice mixture; D500 = diabetic + 500 mg/kg spice mixture; DG (Dglinben) = diabetic + 5 mg/kg glibenclamide.
Effect of GGCP on serum lipid profile
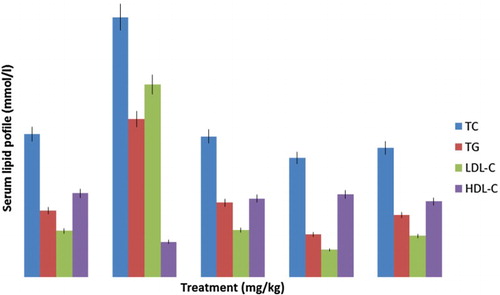
Diabetic control rats exhibited a significant elevation in the levels of TC, TG and LDL-C, as well as a significant reduction in HDL-C compared to control rats (). Treatment with the GGCP extract at 200 mg/kg and 500 mg/kg doses for 7 days caused a marked reduction in the level of TC, TG and LDL-C, but elevated the HDL-C concentration to control levels in all the treated rats.
Figure 3. Effect of garlic, ginger and cayenne pepper (GGCP) on lipid profile in control and alloxan-induced diabetic rats. Values are significantly different at p < 0.05. TC = total cholesterol; TG = triglycerides; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol.
Dyslipidaemia and hyperglycaemia generally coexist in diabetic subjects, and abnormalities of the lipid profile are one of the most common complications of diabetes. According to Khan et al. (Citation2007), impaired lipid metabolism resulting from uncontrolled hyperglycaemia is a risk factor for coronary heart disease and myocardial infection. Insulin deficiency and increased blood glucose levels lead to hyperglycaemia and hypercholesterolaemia, as observed in this study. However, the elevated lipid levels were restored to near normal in the spice-treated group, with a concomitant increase in HDL-C levels. Our observations in this study are similar to the report of Kumar et al. (Citation2011), who treated some diabetic rats with an extract of Callistemon lanceolatus leaves.
Effect of GGCP on haematological parameters
The assessment of haematological parameters could be used to reveal the deleterious effect of foreign compounds including plant extracts on the blood constituents of animals. In this study, diabetic control rats exhibited significant reductions in RBC, haemoglobin, haematocrit, MCV, MCH and MCHC indices (). This agrees with existing literature that anaemia is a common pathophysiology associated with diabetes mellitus (Akindele et al. Citation2012; Colak et al. Citation2014). However, alloxan-induced diabetic rats had a significantly increased WBC count and its differentials such as basophils, monocytes, eosinophils, lymphocytes and neutrophils compared to normal and GGCP-treated rats. This indicates impairment of haematological functions in the diabetic rats and may be attributed to a defence reaction against alloxan-induced diabetes. According to Elkind et al. (Citation2001), relative elevation of WBCs in humans is associated with carotid atherosclerosis, cardiovascular disease and cerebrovascular disease.
Platelet aggregation occurs in diabetic patients with long-term poor glycaemic control due to a lack or deficiency of insulin (Jarald et al. Citation2008). The reduction of platelet levels in diabetic rats induced with alloxan was confirmed in this study in relation to normal control rats. Long-term reduction of this parameter may result in internal and external haemorrhage and finally death. However, administration of the combined spice extract and glibenclamide markedly improved the platelet count. This indicates the ability of the plant extract to stimulate the biosynthesis of clotting factors for blood coagulation or clotting, especially during severe bleeding or haemorrhage (Adebayo et al. Citation2005).
Effect of GGCP on cellular toxicity markers
Diabetic control rats exhibited reduced concentrations of total protein, albumin, globulin and bilirubin. The creatinine, urea, total protein, albumin and urate content were elevated, while significant increases (p < 0.01) in the activities of serum ALP, AST, ALT, GGT, CK and cholinesterase were observed (). The negative alterations in toxicity markers reflect the significant impact on liver and kidney functions caused by alloxan-induced diabetes. The combined spice at 500 mg/kg body weight was found to be equipotent to glibenclamide in restoring the levels of these markers to normal.
Table 3. Effect of garlic, ginger and cayenne pepper (GGCP) on some markers of cellular toxicity in control and alloxan-induced diabetic rats (n = 6, mean ± SD).
The increased serum levels of urea, creatinine and urate in untreated diabetic rats observed in this study could be due to insulin deficiency and the consequent inability of glucose to reach extrahepatic tissues which activate gluconeogenesis as an alternative source of glucose (Gastaldelli et al. Citation2000). Also, because of the increased proteolysis needed to sustain this route, deamination of glucogenic amino acids released into the plasma consequently leads to increased urea in the blood. Creatinine is a metabolite of creatine and its concentration in serum is proportional to the body muscle mass. Elevated levels of urea, creatinine and urate in the serum could therefore signify kidney impairment. The significant reduction in the levels of theses markers is therefore indicative of the extract's potential to protect the cell against diabetic injury.
The increase in ALP activity observed in this study may be a result of hepatic damage, which led to the leakage of the enzymes from the tissues to the serum (Appidi et al. Citation2009). AST and GGT are sensitive indicators of hepatocellular damage. Their elevation in diabetic rats, as observed in this study, is indicative of severe damage to the liver. The reduction in the activities of these enzymes towards control levels in the GGCP-treated diabetic rats indicates that the activity of the liver was preserved by the spice extract. The elevated cholinesterase activity in diabetic rats could be a response to hypertriglyceridaemia or a reflection of diabetic microangiopathy (Dave & Katyare Citation2002).
The elevated cellular toxicity markers in diabetic control rats are reflections of the significant alteration of liver function as a result of alloxan induction. The combined spice at 500 mg/kg body weight was found to be equipotent to glibenclamide in restoring the elevated markers to normal. Thus, it is possible to suggest that this combined spice extract might play an important role in reducing the risk of kidney and liver problems as well as cardiovascular diseases via lowering of serum urea, uric acid and creatinine caused by diabetes.
Histopathological evaluation
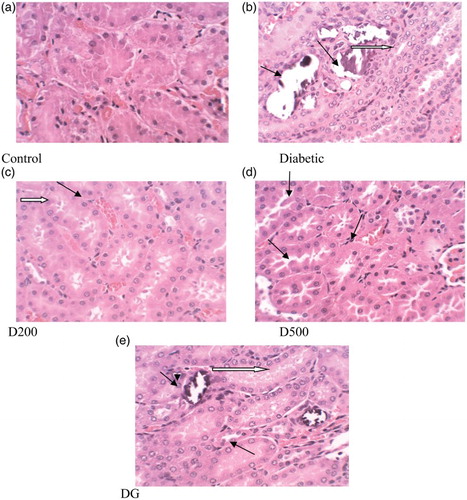
shows photomicrographs of the renal tissues of rats. The histology of control rats showed normal renal tubules, glomeruli and corpuscles (a), while the kidney of the diabetic rats exhibited acute cellular swelling, tubular degeneration and tubular necrosis (b). Long-term damage, dysfunction and failure of the kidneys are major complications of diabetes mellitus. Increased glucose levels in the blood have been shown to lead to oxidative stress, which is considered as one of the causative factors of diabetes-associated kidney disorders such as apoptosis, tubular atrophy and necrosis (Kumar et al. Citation2004; El-Midaoui & de Champlain Citation2005). Administration of the GGCP extract or glibenclamide improved the architecture of the kidney and by extension restored its functionality. The groups administered the 500 mg/kg extract demonstrated a distinct regenerative capacity over 200 mg/kg and glibenclamide.
Figure 4. Histopathological changes (× 400) in kidney of control and alloxan-induced diabetic rats. Control: normal renal corpuscle and tubules. Diabetic: cellular degeneration and swelling (black arrow), glomerular atrophy and widening of Bowman's space (white arrow). D200: mild cellular degeneration (black arrow) and congestion (white arrow). D500: mild atrophy and accumulation of protein in glomerular spaces (black arrow). DG: mild cellular degeneration (black arrow) and fatty changes in glomerular epithelia (white arrow).
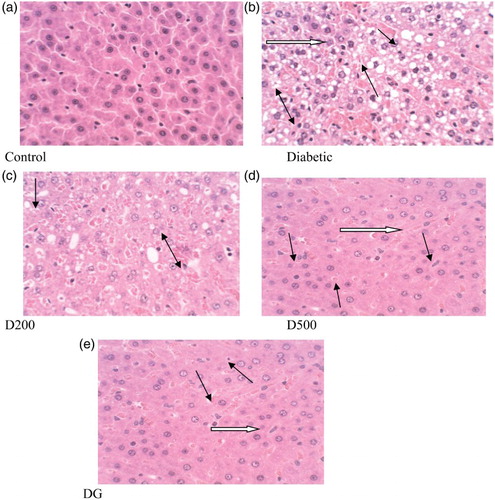
Histopathology of the liver (a) in normal control animals revealed normal hepatic cells with well-preserved sinusoids, nucleus, portal tracts and central vein. In contrast, in diabetic control animals the lobular architecture was maintained, but there was also vacuolation of the hepatocytes, fatty deposits, sinusoidal dilation and congestion, mild portal inflammation, severe degeneration and necrosis. The observed degeneration of the hepatocytes in untreated diabetic rats could be attributed to insulin deficiency and suppression of mitochondrial β-oxidation of fatty acids, leading to deposition of triglycerides in the hepatocytes (Huang et al. Citation2010). However, diabetic rats treated with combined spice extract or glibenclamide showed hepatocytes with nearly normal appearance and minimal necrosis. The hepatoprotective activity of the combined spices may be due to the combined free-radical scavenging activities of polyphenols, especially flavonoids, in the spices (Atta et al. Citation2010).
Figure 5. Histopathological changes (× 400) in liver of control and alloxan-induced diabetic rats. Control: normal histological structure of rat liver. Diabetic: degeneration (white arrow), fatty deposition (black arrow), cellular swelling and necrotic cells (double black arrow). D200: mild granular degeneration and swelling (black arrow), narrowed sinusoidal capillaries (double black arrow). D500: reduced fatty change (black arrow) and mild sinusoidal congestion (white arrow), mild granular degeneration (double black arrow). DG: degenerative cells reduced (black arrow) moderate hyperplasia (white arrow).
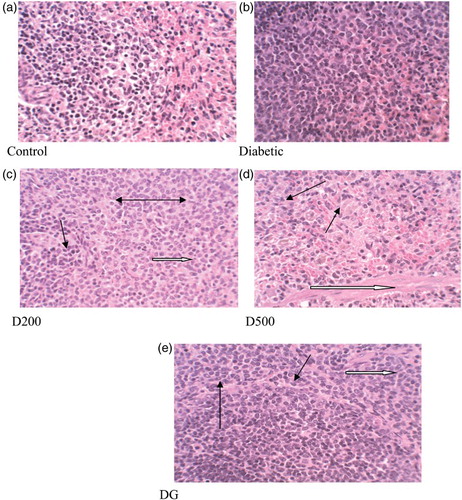
The pancreas of control rats exhibited characteristic features of normal acini and islets of Langerhans (a). In contrast, histopathological examination of diabetic control rats showed pancreatic hyperplasia, necrosis, haemosiderosis and degeneration (b). The pancreas of diabetic rats treated with the combined spices extract and glibenclamide showed minimal necrosis, mild to moderate hyperplasia and degeneration. The noticeable reduction in the extent of these injuries in the diabetic rats treated with GGCP extract could be due to suppression of further damage to the pancreas, prevention of β-cell death and/or recovery of partly injured β-cells. Treatment of diabetic rats with different spice extracts has been reported to reverse degeneration of the pancreas (Jelodar et al. Citation2005; Abdollahi et al. Citation2011).
Figure 6. Histopathological changes (× 400) in pancreas of control and alloxan-induced diabetic rats. Control: normal pancreatic islets of Langerhans and acini. Diabetic: degeneration and shrinkage (white arrow), vacuolar change (black arrow), necrotic cells (black double-headed arrow), haemosiderosis; D200: reduced necrosis (black arrow), degeneration (white arrow) and irregular spaces (double arrow). D500: reduced necrosis (white arrow), degeneration (black arrows). DG: reduced cellular degeneration and vacuolar swelling (black arrows), mild haemosiderosis (white arrow).
Conclusion
The results of this study show that aqueous extract of a combination of garlic, ginger and cayenne pepper has strong hypoglycaemic, hypolipidaemic and organ-protective potential in alloxan-induced diabetic rats. Oral administration of aqueous extract of GGCP modulated the body and organ weights of diabetic rats, reduced hyperglycaemia, attenuated blood and cellular toxicity parameters, and repaired damage to liver, kidney and pancreatic tissues of diabetic rats. Further studies on this combination of spices, mechanisms of action and clinical trials to validate these data, which may lead to the development of more potent antidiabetic formulations, will be carried out in the future.
Acknowledgements
This research was supported by the National Research Foundation (NRF) and Medical Research Council (MRC), both of South Africa.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aaron C. 1996. Garlic & life. N Am Rev. 281:14–24.
- Abdollahi M, Zuki ABZ, Goh YM, Rajion MA, Rezaeizadeh A, Noordin MM. 2011. Effects of Momordica charantia on pancreatic histopathological changes associated streptocotozin-induced diabetes in neonatal rats. Histol Histopathol. 26:13–21.
- Adebayo OJ, Adesokan AA, Olatunji LA, Buoro DO, Soladoye AO. 2005. Effect of ethanolic extract of Bougainvillea spectabilis leaves on haematological and serum lipid variables in rats. Biokemistri. 17:45–50. doi: 10.4314/biokem.v17i1.32588
- Ahuja KDK, Robertson IK, Geraghty DP, Ball MJ. 2006. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am J Clin Nutr. 84:63–69.
- Akindele OA, Babatunde AI, Chinedu FM, Samuel OA, Oluwasola CA, Oluseyi AA. 2012. Rat model of food induced non-obese-type 2 diabetes mellitus; comparative pathophysology and histopathology. Intern J Physiol, Pathophysiol Pharmacol. 4:51–58.
- Amagase H. 2006. Clarifying the real bioactive constituents of garlic. J Nutr Suppl. 136:716–725.
- Antonious GF, Kochar TS, Jarret RL, Synder JC. 2006. Antioxidants in hot pepper: variations among accessions. J Sci Health Biol. 41:1237–1243. doi: 10.1080/03601230600857114
- Appidi JR, Yakubu MT, Grierson DS, Afolayan AJ. 2009. Toxicological evaluation of aqueous extracts of Hermannia incana Cav leaves in male Wistar rats. Afr J Biotechnol. 8:2016–2020.
- Atta AH, Elkoly TA, Mouneir SM, Kamel G, Alwabel NA, Zaher S. 2010. Hepatoprotective effect of methanol extracts of Zingiber officinale and Circhonium intybus. Indian J Pharmaceut Sci. 72:564–570. doi: 10.4103/0250-474X.78521
- Barrie SA, Jonathan ND, Wright MD, Pizzorno ND. 1987. Effects of garlic oil on platelet aggregation, serum lipids and blood pressure in humans. J Orthomol Med. 2:15–21.
- Bayan L, Koulivand PH, Gorji A. 2014. Garlic: a review of potential therapeutic effects. Avicenna J Phytomed. 4:1–14. doi: 10.4103/2231-0770.127413
- Bode AM, Dong Z. 2011. The amazing and mighty ginger. In: Benzie IFF, Wachtel-Galor S, editors. Herbal medicine: biomolecular and clinical aspects. 2nd ed. Boca Raton, FL: CRC Press; Chapter 7. Available from: http://www.ncbi.nlm.nih.gov/books/NBK92775/
- Broadhurst CL, Polansky MM, Anderson RA. 2000. Insulin-like biological activity of culinary and medicinal plant aqueous extracts in vitro. J Agric Food Chem. 48:849–52. doi: 10.1021/jf9904517
- Colak S, Geyi Koghu F, Aslan A, Deniz GY. 2014. Effects of lichen extracts on haematological parameters of rats with experimental insulin-dependent diabetes mellitus. Toxicol Ind Health. 30:878–887. doi: 10.1177/0748233712466130
- Colín-González AL, Santana RA, Silva-Islas CA, Chánez-Cárdenas ME, Santamaría A, Maldonado PD. 2012. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid Med Cell Longev. 50:209–230.
- Dave KR, Katyare SS. 2002. Effect of alloxan-induced diabetes on serum and cardiac butyrylcholinesterases in the rat. J Endocrinol. 175:241–250. doi: 10.1677/joe.0.1750241
- Eidi A, Eidi M, Meki AR. 2006. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 13:624–629. doi: 10.1016/j.phymed.2005.09.010
- Elkind MS, Cheng J, Boden-Albala B, Paik NC, Sacco RL. 2001. Elevated white blood cell count and carotid plaque thickness: the Northern Manhattan stroke study. Stroke. 32:842–849. doi: 10.1161/01.STR.32.4.842
- El-Midaoui A, de Champlain J. 2005. Effects of glucose and insulin on the development of oxidative stress and hypertension in animal models of type 1 and type 2 diabetes. J Hypertens. 23:581–588. doi: 10.1097/01.hjh.0000160215.78973.ba
- Gall A, Shenkute Z. 2009. Ethiopian traditional and herbal medications with conventional drugs and their interactions with conventional drugs. Ethnomed.org/clin/pharm/Ethiopean–he.
- Gastaldelli A, Simona B, Pettiti M, Toschi E, Camastra S, Natali A, Landau BR, Ferrannini E. 2000. Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans: a quantitative study. Diabetes. 49:1367–1373. doi: 10.2337/diabetes.49.8.1367
- Grzanna R, Lindmark L, Frondoza CG. 2005. Ginger – an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 8:125–132. doi:10.1089/jmf.2005.8.125
- Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. 2010. Depletion of liver kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 59:347–357. doi: 10.2337/db09-0016
- Jarald E, Joshi SB, Jain DC. 2008. Diabetes and herbal medicines. Iran J Pharmacol Ther. 7:97–106.
- Jelodar GA, Maleki M, Motadayen MH, Sirus S. 2005. Effect of fenugreek, onion and garlic on blood glucose and histopathology of pancreas of alloxan-induced diabetic rats. Indian J Med Sci. 59:64–69. doi: 10.4103/0019-5359.13905
- Jolayemi AT, Ojewole JAO. 2013. Comparative anti-inflammatory properties of capsaicin and ethyl-acetate extract of Capsicum frutescens linn [Solanaceae] in rats. Afr Health Sci. 13:357–361. doi:10.4314/ahs.v13i2.23
- Khan HA, Sobki SH, Khan SA. 2007. Association between glycaemic control and serum lipid profile in type 2 diabetic patients: HbA1c predicts dyslipidemia. Clin Exp Med. 7:24–29. doi: 10.1007/s10238-007-0121-3
- Kulkarni JS, Metha AA, Santani DD, Ramesh KG. 2002. Effects of chronic treatment with cromakalim and glinbenclamide in alloxan-induced diabetic rats. Pharmacol Res. 46:101–105. doi: 10.1016/S1043-6618(02)00078-6
- Kumar S, Kumar V, Prakash O. 2011. Antidiabetic, hypolipidemic and antioxidant activities of Callistemon lanceolatus leaves extract. J Herbs Spice Med Plant. 17:144–153. doi: 10.1080/10496475.2011.583139
- Kumar D, Zimpellmann J, Robertson S, Burns KD. 2004. Tubular and interstitial cell apoptosis in the streptozotocin-diabetic rat kidney. Nephron. 96:e77–e88.
- Kumari KD, Suresh KP, Samarasinghe K, Handunnetti SM, Samaranayake TSP. 2013. Evaluation of a traditional Sri Lankan herbal beverage (water extract of dried flowers of Aegle marmelos, Bael fruit) in type II diabetic patients. J Diabetes Metab. 4:6 http://dx.doi.org/10.4172/2155-6156.S1.023
- Kuroda M, Mimaki Y, Sashida Y, Mae T, Kishida H, Nishiyama T. 2003. Phenolics with PPAR-ligand-binding activity obtained from liquorice (Glycyrhizza uralens roots) and ameliorative effects of glycerine on genetically diabetic KK-Ayʸ mice. Bioorg Med Chem Letters. 13:4267–4272. doi: 10.1016/j.bmcl.2003.09.052
- Liu C-T, Sheen L-Y, Lii C-K. 2007. Does garlic have a role as an antidiabetic agent? Mol Nutr Food Res. 51:1353–1364. doi: 10.1002/mnfr.200700082
- Moller DE, Flier JS. 1991. Insulin resistance – mechanisms, syndromes, and implications. N Engl J Med. 325:938–948. doi: 10.1056/NEJM199109263251307
- Morakinyo AO, Akindele AJ, Ahmed Z. 2011. Modulation of antioxidant enzymes and inflammatory cytokines: possible mechanism of anti-diabetic effect of ginger extracts. Afr J Biomed Res. 14:195–202.
- Nwose EU. 2009. Pepper soup as an antioxidant nutrition therapy. Med Hypoth. 73:860–861. doi: 10.1016/j.mehy.2009.04.016
- Otunola GA, Oloyede HOB, Oladiji AT, Afolayan AJ. 2012. Hypolipidemic effect of aqueous extracts of selected spices and their mixture on diet-induced hypercholesterolemia in wistar rats. Can J Pure Appl Sci. 6:2063–2071.
- Otunola GA, Oloyede HOB, Oladiji AT, Afolayan AJ. 2014. Selected spices and their combination modulate hypercholesterolemia-induced oxidative stress in experimental rats. Biol Res. 47:5. doi:10.1186/0717-6287-47-5
- Panda A, Jena S, Sahu PK, Nayak S, Padhi P. 2013. Effect of polyherbal mixtures on the treatment of diabetes. ISRN Endocrinol. doi:10.1155/2013/934797.
- Petchi RR, Vijaya C, Parasuraman S. 2014. Antidiabetic activity of polyherbal formulation in streptozotocin–nicotinamide induced diabetic Wistar rats. J Trad Complement Med. 4:108–117. doi:10.4103/2225-4110.126174
- Rackova L, Cupáková M, Ťažký A, Mičová J, Kolek E, Košťálová D. 2013. Redox properties of ginger extracts: perspectives of use of Zingiber officinale Rosc. as an antidiabetic agent. Interdiscip Toxicol. 6:26–33. doi: 10.2478/intox-2013-0005
- Ravi K, Sivagnanam K, Subramanian S. 2004. Anti-diabetic activity of Eugenia jambolana seed kernels on streptozotocin-induced diabetic rats. J Med Food. 7(2):187–191. doi: 10.1089/1096620041224067
- Renault S, De Lucca AJ, Boue S, Bland JM, Vigo CB, Selitrennikoff CP. 2003. CAY-I, a novel antifungal compound from cayenne pepper. Med Mycol. 41:75–82. 10.1080/mmy.41.1.75.82 doi: 10.1080/mmy.41.1.75.82
- Shetti AA, Sanakal RD, Kaliwal BB. 2012. Antidiabetic effect of ethanolic leaf extract of Phyllantus amarus in alloxan induced diabetic mice. Asian J Plant Sci Res. 2:11–15.
- Srivastava S, Lal VK, Pant KK. 2012. Polyherbal formulations based on Indian medicinal plants as antidiabetic phytotherapeutics. Phytopharmacol. 2:1–15.
- Sunmonu TO, Afolayan AJ. 2013. Evaluation of antidiabetic activity and associated toxicity of Artemisia afra aqueous extract in Wistar Rats. Evid based Complement Altern Med. doi:10.1155/2013/929074
- Tietz NW. 1995. Clinical guide to laboratory tests. 3rd ed. Philadelphia, PA, USA: WB Saunders.
- Tietz NW, Prude EL, Sirgard-Anderson O. 1994. Textbook of clinical chemistry. London, UK: WB Saunders.
- Tolan I, Ragoobirsingh D, Morrison EY. 2001. The effect of capsaicin on blood glucose, plasma insulin levels and insulin binding in dog models. Phytother Res. 15:391–4. doi: 10.1002/ptr.750
- Walter HG. 1986. Pepper breeding. In: Bassett MJ, editor. Breeding vegetable crops. NY, USA: AVI Publishing Co. Inc.; pp. 67–134.
- Wright PJ, Leatherwood PD, Plummer DT. 1972. Enzymes in rat urine: alkaline phosphatase. Enzymologia. 42:317–327.
- Wroblewski F, La Due JS. 1956. Serum glutamic pyruvic transaminase SGP-T in hepatic disease: a preliminary report. Ann Intern Med. 45:801–811. doi: 10.7326/0003-4819-45-5-801
