Abstract
The long-term administration of insulin requires the development of new delivery routes. Using a base developed in-house, called HAMIN®, an insulin suppository containing 100 units (U) of insulin was formulated. The suppository was subjected to stability testing at various temperatures and the assay value was monitored. Other physical factors such as hardness, disintegration time, thermal analysis and dissolution were also tested. The suppository released more than 80% of its drug content in 30 min, and was stable for up to 11 months at −20°C. The suppository effect was studied on 11 New Zealand white rabbits, with body weight ranging from 1.6 to 2.1 kg. The results show that there was a marked reduction in glucose content when the suppository was inserted. The average drop in glucose content was 2.7 mmol/L in 15 min from the time of insertion. The maximum drop in glucose content reached 3.9 mmol/L in 2 h. Plasma insulin level, quantified using an enzyme-linked immunosorbent assay, showed a value of more than 100 µIU insulin/ml blood after 30 min. Although the insulin bioavailability was expectedly low, the rate at which the hypoglycaemic effect took place and the percentage of glucose reduction were comparable to results after subcutaneous injection.
1. Introduction
Insulin resistance is a condition where the insulin produced cannot exert the expected biological effect to attain sufficient glycaemic control (Reavem Citation1995). The large increase in diabetic cases has led to a rise in studies on various control measures. Different classes of drugs, such as biguanides, sulfonylureas, alpha-glucoside inhibitors and thiazolidinediones, each designed to treat diabetes from a different perspective, have been identified to control the severity of the condition (Mohammed Salman & Naseeruddin Inamdar Citation2012; Bhandari et al. Citation2013). However, no treatments have been found that could replace insulin to regulate the uptake of blood glucose into cells and muscle tissues.
Reduced endogenous insulin is commonly supplemented with isolated or recombinant insulin sources via subcutaneous or intravenous injections (Al-Tabakha & Arida Citation2008). Although these delivery routes provide the best bioavailability, the need for continuous administration of the hormone is a problem because of the pain and anxiety that attend the use of hypodermic needles. Various other delivery routes have been explored to replace or supplement subcutaneous insulin injection over the past 30 years (Wilson Citation2011). Many have shown positive results in terms of hypoglycaemic effect but were unable to match the consistency and effectiveness of subcutaneous or intravenous injections. The closest alternative route, which led to successful registration with the US Food and Drug Administration (FDA) but was followed by withdrawal by the company, was Exubera, insulin delivered through an inhaler into the lungs (Patel et al. Citation2005).
Various other delivery modes of insulin using liposomal formulations, iontophoresis and phonophoresis have been studied (Jani et al. Citation2012). These new technologies have been reported as being effective but too expensive to be commercially viable. Acid degradation, pH sensitivity, high variation in bioavailability and low bioavailability are some of the challenges posed by alternative delivery routes (Shivanand Citation2010). While acknowledging the need to present insulin medication in a more painless way, almost all modes of delivery have so far fallen short of providing an equivalent therapeutic dose.
Studies on insulin delivered via the rectal route in humans found its bioavailability to be low (4–10%) compared to subcutaneous injection (Owens Citation2002). Being a large molecule, the absorption of insulin through intracellular and cellular pathways is restricted. Various types of bases and additives such as phenols, sodium chloride and zinc ions have been studied with the objective of increasing the bioavailability of insulin from suppositories (Brange & Langkjaer Citation1992). The use of such chemicals increases the bioavailability, while causing a higher degradation rate of insulin and some inconvenience to the users. However, any active absorption from the rectal passage provides direct access into the systemic circulation, bypassing the hepatic circulation where the insulin will be extracted (Schade & Valentine Citation2002).
Malaysian palm oil accounts for more than 24% of the global trade in oil and fat. The thermostability and lack of polymorphism shown by palm oil give it advantages over the traditionally used theobroma oil in the production of suppository bases. The authors have engaged in studies on a newly developed palm oil base, called HAMIN®, to investigate the possibility of using it as an insulin suppository base and to gauge the hypoglycaemic effect in experimental animals given high doses of insulin. The palm kernel oil suppository base was prepared using HAMIN, which is a mixture of hydrogenated palm kernel oil and hydrogenated palm kernel stearin (Noordin & Chung Citation2007). Drugs such as paracetamol and aspirin have shown positive results using this base as a suppository (Noordin & Chung Citation2004). The base has undergone allergy testing in an Organisation for Economic Co-operation and Development (OECD)-accredited laboratory in the UK and been found safe for use in in vivo studies (Halal Focus).
2. Method
2.1. Preparation of suppositories
A fat base was chosen to develop the insulin suppository, as a fat-based suppository gives a general melting range of 30–40°C, readily melts on warming and rapidly hardens on cooling, is miscible with other ingredients and is non-irritating. The base was melted in a glass beaker at a slow rate on a laboratory-scale hotplate using a water bath and a magnetic stirrer. Human recombinant insulin (Sigma-Aldrich, WGK, Germany) was incorporated into the base. The temperature of the hotplate and the water bath was set to 40°C and was monitored carefully to avoid heat degradation of the hormone. The recombinant human insulin consists of two polypeptide chains linked by disulfide bonds, similar to the native hormone produced by the beta-cells in the human pancreas (Sigma-Aldrich). The base was pipetted into the plastic moulds. Each suppository was designed to contain approximately 100 units (U) of insulin. The suppository was cooled at 25°C for 48 h before being stored in a freezer at a temperature below −20°C. Placebo suppositories were developed in similar manner without the incorporation of insulin. The suppositories were packed in a 1.5 ml disposable plastic suppository mould. They were incubated at 8°C and 30°C, and tested at 0 and 1 month. Estimated shelf-life was calculated using the Arrhenius equation and the 1 month stability data. A lower strength suppository was prepared for the animal studies (12 U/suppository).
2.2. High-performance liquid chromatography method for insulin analysis
A high-performance liquid chromatography (HPLC) method was developed for the detection and quantitation of insulin. A Waters Alliance 2695 Separation Module HPLC system equipped with a 2996 Photo Diode Array Detector (owned by Pharmaniaga Research Centre, Malaysia) was used. The column used was a Waters Insulin-HMWP, with dimensions of 7.8 × 300 mm, a pore size 125 Å and a 2 µm ethylene bridged hybrid silica stationary phase (Koza et al. Citation2012). The mobile phase is an acidic degassed premix of glacial acetic acid (15%), acetonitrile (20%) and 0.1% arginine solution (65%) run at a flow rate of 0.4 ml/min. Ultraviolet detection at 276 nm was performed. Insulin from bovine pancreas and human recombinant pancreas (Sigma) was used as reference material. The injection volume was set to 100 µl. The temperature of the HPLC system was maintained at 25°C. The insulin reference standard was diluted in 0.01 N HCl to obtain a concentration of 5 U/ml. The sample was melted in a volumetric flask containing 0.01 N HCl, in a water bath to provide sufficient dissolution for the suppository to release the hormones in solution, to produce similar concentrations.
2.3. Uniformity of dosage
The uniformity of dosage test was performed on 10 suppositories individually by dissolving each suppository in a separate volumetric flask containing 0.01 N HCl in a water bath. The water bath was maintained at 50°C for rapid dissolution of the suppositories. The volumetric flask containing the suppository in 0.01 N HCl was stirred until the suppository visually dissolved. The solution was immediately taken out to avoid any degradation of the insulin, cooled, filtered using a 0.45 µm filter and chromatographed using HPLC.
2.4. Dissolution method
A dissolution test was performed to measure the release of insulin from the suppositories over time. As the suppositories were light, sinkers were used to immerse them in 500 ml of medium (0.01 N HCl) at 37°C. The paddle speed was set to 100 rpm. Dissolution samples were withdrawn at 15, 30, 60, 120, 180, 240 and 300 min.
2.5. Thermal analysis
Thermal analysis was carried out on human recombinant insulin, pure HAMIN and insulin suppositories. A Mettler Toledo differential scanning calorimeter (DSC) was used. Nitrogen gas at a flow rate of 50 ml/min was used. Three test samples of pure insulin, pure HAMIN and insulin suppositories were weighed and loaded into the perforated pan of the DSC. The pan was closed with a cover and crimped. The sample was then placed in the DSC heating chamber. The programme was set to heat the bovine insulin from −20°C to 320°C at a rate of 20 K/min. The DSC had been calibrated before the study, using indium and zinc.
2.6. Animal studies procedure
All experiments were performed in accordance with the relevant regulations and approved guidelines. The animal research protocol [FARMAKO/28/08/2013/SN(R)] was approved by the University of Malaya Institutional Animal Care and Use Committee (IACUC) to study the effect of an insulin suppository in an animal model. The suppository effect was studied on 11 New Zealand white rabbits, with body weight ranging from 1.6 to 2.1 kg, obtained from East Asia Rabbit Corporation (Malaysia). After a 2 week acclimatization period, eight rabbits were given an insulin suppository (12 U/suppository) and three rabbits a placebo suppository. All the rabbits were well fed and none of them was starved. A suitable restraining box was used to hold the rabbit firmly. The hair on the ears was shaved to improve the visibility of the veins. A diluted alcohol swab was used to disinfect the area and a blood sample was withdrawn using a butterfly needle. The blood was sampled at 0 (pre-dose), 30, 60 and 120 min. About 1–2 ml of blood was collected from the marginal ear veins into a plain tube. The blood was centrifuged at 3500 rpm and the plasma was separated into two portions, one for the determination of glucose and the other for the determination of insulin by enzyme-linked immunosorbent assay (ELISA). The sample for glucose testing was sent to an independent laboratory (Gribbles Pathology, Malaysia).
2.7. Insulin quantitative analysis by enzyme-linked immunosorbent assay method
The insulin was quantitated using an insulin immunoassay kit (IBL International, Hamburg, Germany). The kit contained a microtitre plate layered with a monoclonal antibody. First, 50 μl of each concentration of insulin standard solution or the sample solution ranging from 0–500 µIU/ml was pipetted into the wells of a microplate. Then, 50 μl of anti-insulin horseradish peroxidase conjugate was pipetted into the same well. The microplate was incubated for 30 min at room temperature on a horizontal shaker. After decanting the supernatant, each well was washed three times with 0.4 ml of washing solution. The washing solution was carefully removed by inverting the plate and tapping it firmly on clean blotting paper. Then, 200 μl of freshly prepared substrate solution was pipetted into each well within 15 min. The plate was then incubated for 15 min at room temperature on a shaker, avoiding direct sunlight. Finally, the reaction was stopped by adding 50 μl of stopping reagent and briefly mixing the contents by gently shaking the plate.
A microplate optical density reader was used to measure the absorbance of the samples at 450 nm and 490 nm. Measurements were taken within 60 min from the time the stop solution was added.
3. Results
3.1. Physical attributes
The hardness of the suppositories was tested using an automated hardness tester, and the average value obtained was 2.1 kP. The disintegration of the suppositories was tested in water at 37°C, using discs. The suppositories dissolved within 13 min.
3.2. Validation of high-performance liquid chromatography method
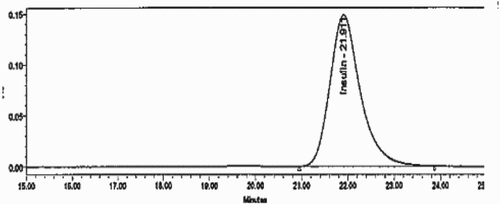
The assay method using HPLC was validated from 0.02 mg/ml to 0.5 mg/ml. The insulin peak eluted at about 22 min (). Five concentrations of insulin were studied for the standard and the placebo spiked with reference standard. The y-intercept from the plotted graph was 1.3% for standard and 0.5% for sample (spiked placebo) preparation. The coefficient of determination was about 1.00. The precision and accuracy were obtained using three replicate samples at three concentrations ranging from 0.02 mg/ml to 0.5 mg/ml. The relative standard deviation (RSD) was 1.9% and the percentage accuracy was about 100.5 ± 1.3% at a 95% confidence level.
3.3. High-performance liquid chromatography assay results
HPLC was used to measure insulin concentrations in samples obtained from stability studies. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guideline on stability studies for products intended to be refrigerated recommends accelerated storage conditions of 25 ± 2°C/65% relative humidity (RH) and long-term storage at 5 ± 3°C. However, in this study the accelerated condition was set to 30°C/75% RH. The assay results obtained at 1 month were about 3.21 mg and 3.00 mg at temperatures of 5°C and 30°C, respectively, compared to the initial assay value of 3.33 mg (). Using the Arrhenius equation, the calculated shelf-life at −20°C was about 14 months. Practically, the assay at 11 months was about 91.4% for samples stored below −20°C.
Table 1. Assay results for insulin suppositories incubated under refrigerated and stressed conditions.
3.4. Uniformity of dosage and dissolution results
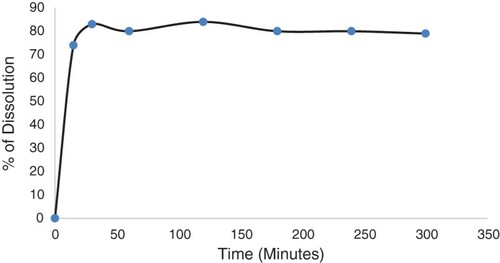
The uniformity of dosage test showed that the amount of insulin was consistent and homogeneously distributed in all suppositories tested. The percentage RSD obtained from all 10 suppositories tested was 5.8%. The dissolution procedure of the suppositories was initially developed in a basket at various speeds. In the basket, the suppositories melted into droplets but stayed within the mesh of the basket. The base adhesion to the mesh was greater than its solubility in water. The suppositories melted slowly up to 5 h at 100 rpm but only managed to release 14% of insulin in the medium when using the basket. It appeared that the medium was not able to penetrate the molten base and dissolve the hormone completely. Polysorbate, a non-ionic surfactant, was used in the dissolution medium to increase the release of insulin from the melted droplets. This increased the dissolved insulin percentage to 80% using a paddle and sinker. The sample was filtered using a 0.45 µm nylon filter and chromatographed using similar HPLC conditions to those described above. shows the dissolution profile of the insulin suppository.
3.5. Thermal analysis
3.5.1. Thermal characteristics of HAMIN
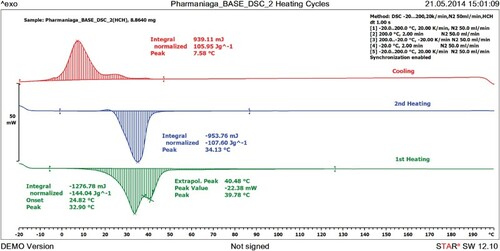
shows the DSC curve for HAMIN when heated from −20°C to 200°C and cooled back to −20°C at a rate of 20 K/min. Melted HAMIN was cooled to −20°C and remelted to 200°C. The purposes of the heating and cooling processes are to reveal the crystallization properties of HAMIN and to identify the presence of polymorphism of HAMIN. The curve shows a melting peak at 32.90°C and ΔH of −144.04 J/g. HAMIN showed two polymorphic forms in the first heating and only a single form when remelted using DSC. There was no significant difference between the melting points of the first and the second melts (p > 0.05).
After the remelting of HAMIN, the peak reached its highest point at 34.13°C. This melting peak shows that the base remains solid at room temperature and will melt at body temperature.
3.5.2. Thermal characteristics of human recombinant insulin
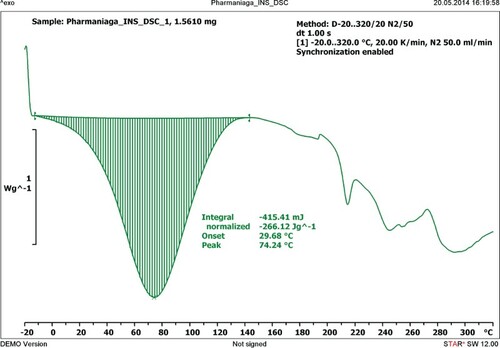
describes the thermal characteristics of human recombinant insulin when heated using the DSC from −20°C to 320°C at a rate of 20 K/min. The thermal analysis of insulin showed a single degradation peak at 74.24°C and ΔH of −266.12 J/g.
3.5.3. Thermal characteristics of HAMIN insulin suppository
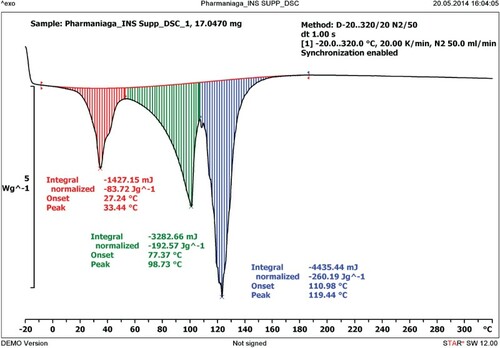
describes the thermal characteristics of HAMIN insulin suppository when heated using the DSC from a temperature of −20°C to 320°C at a rate of 20 K/min. The thermal behaviour of the insulin was portrayed by two distinct peaks. The peak seen at 33.44°C shows the melting point of HAMIN when incorporated with insulin. The degradation peak of insulin in the suppository has also shifted to 98.73°C compared to 74.24°C. Thus, the insulin melting peak increased when incorporated with HAMIN. This result shows that insulin combined with HAMIN was more resistant to heat than insulin alone.
3.6. In vivo studies in rabbits
3.6.1. Glucose assay
The plasma glucose content was analysed using the Sysmex Automated Chemistry Analyzer. It was found that the average plasma glucose level dropped from 5.8 mmol/l to 3.1 mmol/l in the first 15 min. It further dropped to 2.5, 2.2 and 1.9 mmol/l in samples withdrawn at 30 min, 1 h and 2 h, respectively, in rabbits given an insulin suppository. There was no significant drop in plasma glucose level in rabbits given a placebo suppository. The glucose level ranged from 5.2 to 5.6 mmol/l for the same sampling time interval.
3.6.2. Insulin assay
Using the standard solutions provided in the insulin immunoassay kit, a calibration curve was plotted using five concentration of insulin ranging from 6.25 to 100 µIU/ml. The coefficient of determination (R2) value for the curve was above 0.98. In rabbits given an insulin suppository, the insulin content in the plasma increased from 6.08 to 7.60 µIU/ml in 15 min. It shot up to more than 100 µIU/ml within the next 15 min, and the value stayed the same in the 1 h interval sample. At 2 h, the amount of plasma insulin had reduced to 77.01 µIU/ml. The calculated area under the curve (AUC) and the maximum concentration (Cmax) observed after the administration of insulin suppositories were 9219.8 and 100 µIU/ml, respectively.
The insulin concentrations measured in all rabbits treated with placebo ranged from 5.3 to 6.9 µIU/ml at all intervals tested. The calculated AUC and observed Cmax were 757.5 and 6.9 µIU/ml, respectively.
4. Discussion
In this study, a new formulation insulin suppository has been developed and fully tested for its physical and thermal characteristics, and also tested in in vivo studies in rabbits. The HAMIN insulin suppository passed all the tests for its suitability as a suppository preparation of insulin. The results showed that insulin suppositories developed using HAMIN base have potential formulation stability at −20°C. Characterization of the thermal properties using DSC showed that insulin interacts with HAMIN when both of them are incorporated together as a suppository. This interaction give desirable properties to insulin, such as enhanced heat stability.
The dissolution data show that the suppository dissolved and released 80% of its content within 15 min at 37°C. The in vivo study conducted in rabbits shows that the suppositories melted rapidly in the temperature range of 38.5–39.6°C in the rabbit's rectal cavity (Merck Manuals).
The stability data show that the formulation has to be stored well below 8°C to reduce the drop in assay content of insulin. Further studies were conducted on this preparation to measure the insulin bioavailability in plasma and to assess the ability of this preparation to deliver a therapeutic concentration of insulin in vivo.
Since none of the rabbits developed diabetes with streptozotocin administered in the range of 65–100 mg/kg body weight, the insulin content of each suppository was increased to 12 U to see a marked glucose-lowering effect. A further increase in streptozotocin to 130 mg/kg body weight resulted in mortality.
The glucose reduction was an almost instantaneous response, as trace amounts of insulin started to trigger the absorption of glucose. An amount of 1.5 µIU/ml reduced about 48.6 mg/dl (−47%) of glucose in just 15 min. However, as the insulin content kept on increasing, the plasma glucose level did not show corresponding reductions in the following intervals. This indicates that increasing insulin levels in the blood circulation could not effect a proportional uptake of glucose by cells and tissues. It appears that there is a limiting factor to a continuous uptake of glucose into cells or a mechanism to maintain a minimal blood glucose level, even when a high concentration of insulin is found in the bloodstream. It was noted that one of the eight rabbits given an insulin suppository had seizures and became unconscious owing to the severe and rapid reduction in the glucose level. The total amount of glucose uptake caused by the insulin suppository was 702.6 mg in 2 h.
Yoshimitsu et al. (Citation1981) reported that the plasma insulin availability at 30 min was 30 ± 5.6 µU/ml, when 0.7–0.9 U/kg of insulin was administered in humans. This is equivalent to a glucose drop of 10 mg/dl in 45 min (Yoshimitsu et al. Citation1981). In the present study, the glucose reduction at 30 min was about 59.4 mg/dl.
In a study on humans administered subcutaneously with regular human insulin of 0.15 U/kg, maximum concentrations of insulin reached 36 µIU/ml (GLOBALRPh). Using 12 U insulin per suppository, the maximum concentration of insulin observed was greater than 100 µIU/ml. However, the time taken to reach the maximum concentration of insulin for the subcutaneous injection and the suppository was 40–50 min and 30 min, respectively (Sanlioglu et al. Citation2013). This shows that the suppository was able to match the rapid-acting analogues of insulin in term of response, but required a much higher dose to cause the same effect owing to poor bioavailability.
Given the size of insulin, at concentrations of 100 U and the rectal pH of 7.9, the hormone mostly exists in hexamer form (Brange et al. Citation1990). It dissociates and is absorbed into the capillaries in its dimeric or monomeric form. The absorption of the poorly solubilized insulin at rectal pH is achieved through the transcellular and paracellular routes (Muranishi et al. Citation1993). In the transcellular route, the lipophilicity of the suppository formulation plays an essential role, whereas a simple drug diffusion process enables the drug to be absorbed through the paracellular route. However, the diffusion rate of the insulin will be slow owing to the low coefficient partition of the molecule (Shi et al. Citation2006). Insulin absorption from the colon and the duodenal wall was studied by Bendayan et al. (Citation1994). The study found that biologically active insulin is absorbed by the intestinal mucosa and transferred to the blood circulation. Multiple routes of transfer, such as through the endosomal compartment, Golgi apparatus, membrane interdigitations, interstitial space and endothelial plasmalemmal vesicles, were identified (Bendayan et al. Citation1994).
The absence of a similar glucose reduction pattern in rabbits given a placebo suppository confirms that the insulin source is the suppository and not the endogenous insulin. The total plasma glucose content was reduced by more than 50% in 2 h using the 100 U insulin suppository compared to the AUC in rabbits given a placebo suppository.
Many studies have used various types of surfactants and absorption enhancers to increase the bioavailability of insulin from suppositories. Some claim to have produced a comparable hypoglycaemic effect to subcutaneous injection at short time intervals, but over a longer duration they were unable to match either the hypoglycaemic effect or the insulin bioavailability of the subcutaneous injection (Hosny et al. Citation2003).
5. Conclusion
This study shows that the palm oil base HAMIN has good characteristics and is suitable for use as an insulin suppository to deliver the required therapeutic dose through the human intestinal membrane. The insulin suppository has all the desirable features for use as an alternative to subcutaneous insulin injection, especially in patients with a needle phobia. Although the high-dose insulin suppository did not increase plasma insulin content, the rate at which the hypoglycaemic effect occurred and the percentage of glucose reduction were comparable to those seen after subcutaneous injection. The results of this study could be used in the development of insulin suppositories, which may improve compliance in diabetic patients administering subcutaneous insulin injections. Indirectly, this method will also increase the demand for and use of palm oil in the pharmaceutical industry.2
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Al-Tabakha MM, Arida AI. 2008. Recent challenges in insulin delivery systems: a review. Indian J Pharm Sci. 70:278–286. doi: https://doi.org/10.4103/0250-474X.42968
- Bendayan M, Ziv E, Gingras D, Ben-Sasson R, Bar-On H, Kidron M. 1994. Biochemical and morpho-cytochemical evidence for the intestinal absorption of insulin in control and diabetic rats. Comparison between the effectiveness of duodenal and colon mucosa. Diabetologia. 37:119–26. doi: https://doi.org/10.1007/s001250050081
- Bhandari U, Chaudhari HS, Khanna G, Najmi AK. 2013. Antidiabetic effects of Embelia ribes in high fat diet and low dose streptozotocin induced type 2 diabetic rats. Front Life Sci. 7:186–196. doi: https://doi.org/10.1080/21553769.2014.881304
- Brange J, Langkjaer L. 1992. Chemical stability of insulin, influence of excipients, formulation, and pH. Acta Pharm Nord. 4:149–158.
- Brange J, Owens DR, Kang S, Volund A. 1990. Monomeric insulin and their experimental and clinical implications. Diabetes Care. 13:923–954. doi: https://doi.org/10.2337/diacare.13.9.923
- GLOBALRPh. Home page. Available from: http://www.globalrph.com/rapid-acting-analogues.htm
- Halal Focus. Home page. Available from: http://halalfocus.net/malaysia-palm-oil-base-for-pharmaceutical-cosmetic-products
- Hosny EA, Al-Marzouki ZM, Metwally ME, Souaida MY, Alshaik AR. 2003. Evaluation of efficiency of insulin suppository formulations containing sodium salicylate or sodium cholate in insulin dependent diabetic patients. Boll Chim Farm. 142:361–6.
- Jani P, Manseta P, Patel S. 2012. Pharmaceutical approaches related to systemic delivery of protein and peptide drugs: an overview. Int J Pharm Sci Rev Res. 12 Article-007:42–52.
- Koza S, Hong P, Fontain KJ. 2012. Size exclusion ultra performance liquid chromatography for the analysis of covalent high molecular weight insulin. Available from: http://goo.gl/EGT4v3
- Merck Manuals. Home page. Available from: http://www.merckmanuals.com/vet/appendixes/reference_guides/normal_rectal_temperature_ranges.html
- Mohammed Salman I, Naseeruddin Inamdar Md. 2012. Effect of gliclazide on cardiovascular risk factors involved in split-dose streptozotocin induced neonatal rat model: a chronic study. Int J Basic Clin Pharmacol. 1:196–201. doi: https://doi.org/10.5455/2319-2003.ijbcp003612
- Muranishi S, Yamamoto A. Okada H. 1993. Rectal and vaginal absorption of peptides and proteins. In: Biological Barriers to Protein Delivery. Pharmaceutical Biotechnology. 4: 199–222. doi: https://doi.org/10.1007/978-1-4615-2898-2_9
- Noordin MI, Chung LY. 2004. A rapid micro quantification method of paracetamol in suppositories using differential scanning calorimetry. Drug Dev Ind Pharm. 30:925–930. doi: https://doi.org/10.1081/DDC-200034991
- Noordin MI, Chung LY. 2007. Palm kernel oil blends as suppository bases in the delivery of aspirin. Journal of the University of Malaya Medical Centre (JUMMEC). 10:43–50.
- Owens D R. 2002. New horizons – alternative routes for insulin therapy. Nat Rev Drug Discov. 1:529–540. doi: https://doi.org/10.1038/nrd836
- Patel A A, Coleman CI., White CM. 2005. Focus on: Exubera an orally inhaled insulin. Formulary. Available from: http://goo.gl/6CKxyb
- Reavem GM. 1995. Pathophysiology of insulin resistance in human disease. Physiol Rev. 75:473–486.
- Sanlioglu A D., Altunbas H A, Balci MK, Griffith TS, Sanlioglu S. 2013. Clinical utility of insulin and insulin analogs. Islets. 5:67–78. doi: https://doi.org/10.4161/isl.24590
- Schade DS, Valentine V. 2002. To pump or not to pump. Diabetes Care. 25:2100–2102. doi: https://doi.org/10.2337/diacare.25.11.2100
- Shi K, Cui F, Yu Y, Zhang L, Tao A, Cun D. 2006. Preparation and characterization of a novel insulin–phospholipid complex. Asian J Pharm Sci. 1:168–174. doi: https://doi.org/10.1002/jps.3080010233
- Shivanand P. 2010. Non-invasive insulin delivery systems: challenges and needs for improvement. Int J PharmTech Res. 2:603–614.
- Sigma-Aldrich. Home page. Available from: http://www.sigmaaldrich.com/catalog/product/sigma/91077c?lang=en®ion=MY
- Wilson EJ. 2011. Three generations: the past, present, and future of transdermal drug delivery systems. Available from: http://goo.gl/AfCYwd.
- Yamasaki Y, Shichiri M, Kawamori R, Kikuchi M, Yagi T, Arai S, Tohdo R, Hakui N, Oji N, Abe H. 1981. The effectiveness of rectal administration of insulin suppository on normal and diabetic subjects. Diabetes Care. 4:454–458. doi: https://doi.org/10.2337/diacare.4.4.454