Abstract
Turkey has a wide range of flora and fauna due to its climatic diversity. Medicinal plants from Turkey have been used since ancient times for their primary health care. In this study, we examined antiproliferative activities of the extracts from Crataegus monogyna, Vitis vinifera, Glycrrhiza glabra, Alnus glutinosa L. gaertn, and Alcea rosea against rat brain tumor (C6) and human cervical cancer (HeLa) cell lines. The results were compared with the standard anticancer drugs 5-Flurouracil (5-FU) and Cisplatin. C. monogyna, V. vinifera and A. rosea exhibited better antiproliferative activity than 5-FU and cisplatin at 100-75 µg/mL concentrations, against C6 cell lines. On the other hand, C. monogyna and V. vinifera extracts showed considerable antiproliferative activity against HeLa cells compared with 5-FU and cisplatin at 100-75 µg/mL. It can be suggested that, C. monogyna, A. glutinosa L. gaertn, V. vinifera and A. rosea extracts could be developed as an anticancer drug.
GRAPHICAL ABSTRACT
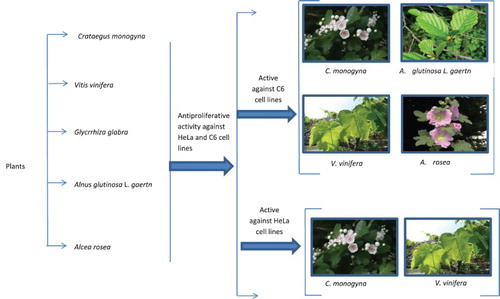
Introduction
Cancer is one of the leading causes of death and rapidly becoming global pandemic (Jemal et al. Citation2009). Many factors play an important role in the development of cancer, such as lifestyle, environment, and nutrition. Cervical cancer is the second most common type of cancer among women worldwide. There were more than half a million new cases of cervical cancer diagnosed in 2010 (De Sanjose et al. Citation2010; Forouzanfar et al. Citation2011). Over the years, the cancer treatment methods have undergone revolutionary changes. On the other hand, chemotherapy of solid tumors is still limited by the lack of anticancer drugs; therefore, new medicines have yet to be developed to cure cancer (Ferguson et al. Citation2004).
Natural products have attracted considerable attention from synthetic community for reasons of wide range of plant diversity along with thousands of natural compounds. Previous studies have demonstrated the role and importance of herbs and spices in the prevention of cardiovascular diseases, carcinogenesis, inflammation, atherosclerosis, and so on. (Hossain et al. Citation2008). It is known that many medicinal plants contain effective chemopreventive and antitumor substances (Borrelli et al. Citation2004). But less than 1% of known 250,000 plant species are investigated for their secondary metabolites and biological activity (Farnsworth Citation1988).
In this present work, five medicinal plants from Turkey, namely Crataegus monogyna (Rosaceae), Vitis vinifera L. (Vitaceae), Glycrrhiza glabra (Fabaceae), Alnus glutinosa L. gaertn (Betulaceae), and Alcea rosea (Malvaceae), were investigated for their antiproliferative activities against C6 and HeLa cancer cell lines. The inhibition potential of plant extracts is aimed to be determined in terms of cell proliferation.
Materials and methods
Plant materials
A. rosea, C. monogyna, V. vinifera L., G. glabra, and A. glutinosa L. gaertn were collected from Tokat and Amasya at their flowering seasons and authenticated by Dr Bedrettin Selvi (Departmant of Biology, Gaziosmanpasa University, Tokat, Turkey). All species were dried in a dark place, at room temperature, until obtaining a stabile weight.
Preparation of extracts
Plant extract have been prepared according to literature method (Chon et al. Citation2009). 10 g of dried and grounded plant material was soaked in 200 mL methanol for three days at room temperature. The mixture was filtered and the solvent was evaporated under vacuum. Stock solution of the samples, 5-florouracil (5-FU) and cisplatin were prepared in sterile dimethyl sulfoxide (DMSO) and were diluted with Dulbecco's modified eagle's medium (DMEM; 1:20). The final concentration of DMSO was kept below 1% in all tests. The stock solutions were stored at ±4°C until usage.
Cell culture and cell proliferation assay
Antiproliferative effects of the plants were investigated against HeLa and C6 cell lines using proliferation BrdU enzyme-linked immuno sorbent assay (ELISA) (Demirtas et al. Citation2009; Demirtas & Sahin Citation2013). HeLa and C6 cells were cultured in DMEM, Sigma, supplemented with 10% (v/v) fetal bovine serum (Sigma, Germany) and PenStrep solution (Sigma, Germany). Cultured cells were detached from the flasks with trypsin-EDTA (Sigma, Germany). After centrifugation of the cells, pellets were resuspended to 3 × 105 cells/mL in DMEM. Cells were plated in 96-well plates (COSTAR, Corning, USA) at a density of 3 × 103 cells/well and incubated at 37°C with 5% CO2 overnight for attachment. All the materials used in experiment were dissolved in sterile DMSO. Tests were carried out in triplicate for each experiment. Cells were treated with crude extracts at final concentrations of 5, 10, 20, 30, 40, 50, 75 and 100 µg/mL. Controls, negative and positive control wells were treated with culture medium, sterile DMSO, 5-FU and cisplatin, respectively. Treated cells were incubated at 37°C with 5% CO2 for 24 h. Cell proliferation was measured by using BrdU Cell Proliferation ELISA (Roche, Germany), a colorimetric immunoassay based on BrdU incorporation into the cellular DNA according to manufacturer's procedure. Briefly, cells were pulsed with BrdU labeling reagent for 4 h followed by fixation in FixDenat solution for 30 min at room temperature. Thereafter, cells were incubated with 1:100 dilution of anti-BrdU-POD for 1.30 h at room temperature. Finally, the immune reaction was detected by adding the substrate solution and the color developed was read at 450 nm with a microplate reader.
Statistical analysis
The results of investigation in vitro are presented as means ± SD of three independent measurements. Statistical comparisons were tested with one-way ANOVA. The P values of <.01 were considered statistically significant. All statistical calculations were performed using SPSS (Version 13.5) software.
Results and discussion
Herbal medicine plays an important role in the prevention and treatment of cancer (Xiong et al. Citation2015). Traditional medicinal herbs such as C. monogyna (Ozyurek et al. Citation2012; Rodrigues et al. Citation2012), A. glutinosa L. gaertn, G. glabra, A. rosea and V. vinifera L. (Karaman & Kocabas Citation2001) have been used in the treatment of different diseases in Turkey. In this study, different parts of six plant species () were tested for their antiproliferative activity against C6 and HeLa cancer cell lines. The antiproliferative potential of the plant extracts was compared with positive control drugs, 5-FU and Cisplatin. In terms of antiproliferative activity against C6 cell lines, C. monogyna extract exhibited the highest performance with its lowest IC50 value (29.8 µg/mL) among other extracts (). C. monogyna (common hawthorn) is widely used in traditional medicine for its beneficial effects (Carvalho Citation2010). Recent studies revealed that, secondary metabolites are responsible from the bioactivity of the plants and the biological effect is mostly the result of synergy or additive effect of the other types of natural compounds (Ramful et al. Citation2011). Therefore, the higher antiproliferative activity of C. monogyna extract (87.33% at 100 µg/mL) can be attributed to its secondary metabolite content, mainly phenolic compounds. Main phenolics of C. monogyna were determined as quercetin derivatives and phenolic acids (Rodrigues et al. Citation2012). It is known that quercetin has inhibitory effect on various tumor cells (Kandaswami et al. Citation2005). In addition, phenolic acids of C. monogyna extract, such as gallic and caffeic acid derivatives, had shown antiproliferative effect against several cancer lines including HeLa cells (Gomes et al. Citation2003). Another study reported a systematic comparison of four different hawthorn parts relating their human inhibitory activity on human tumor cell lines. It was found that the flower buds extract of C. monogyna represents GI50 (cell growth inhibition) value of 63.55 µg/mL for HeLa cells (Rodrigues et al. Citation2012).
Table 1. Selected plants used in the study for screening of antiproliferative activity.
Table 2. Antiproliferative activities (IC50 = µgmL−1) of the plant extracts against C6 and HeLa cell lines.
C. monogyna, V. vinifera, A. glutinosa L. gaertn, and A. rosea possessed considerable antiproliferative activity against C6 cell lines (P < .01, ). Inhibition of A. glutinosa and V. vinifera plant extracts against C6 cell lines was found to be 84.33% and 84.00%, at 100 µg/mL concentration, respectively. Not only V. vinifera, but also C. monogyna and A. glutinosa extract exhibited higher antiproliferative activity than standard drugs against HeLa cells at 75 µg/mL concentration (P < .01, ). Previous study showed that A. glutinosa (stem bark) exhibits inhibitory activity against the human LoVo colon cancer, PC3 prostate cancer, and U373 glioblastoma cell lines (Frederich et al. Citation2009). Main chemical constituents of A. glutinosa were reported as hirsutanonol, oregonin, genkwanin, rhododendron, and glutinic acid (Guz et al. Citation2002; O'Rourke et al. Citation2005) which can be attributed its biological activity. Genkwanin, rhododendrin, and glutinic acid are the main constituents of A. glutinosa L. gaertn (O'Rourke et al. Citation2005). It can be suggested that the main constituents of the plant extracts including anthocyanins and polyphenols play an important role in the inhibition of C6 and HeLa cancer cell lines.
Figure 1. The antiproliferative activities of the plant extracts against C6 cell lines. The results were expressed as percentage of cell proliferation inhibition compared with standard drugs. Data were presented as mean ± SD (n = 3).
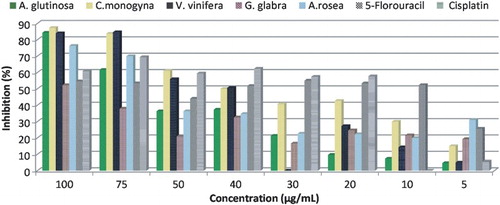
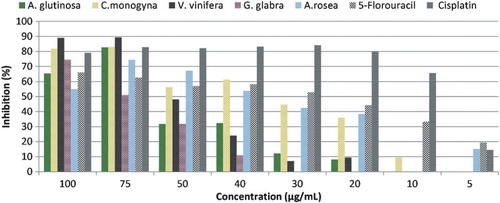
Figure 2. The antiproliferative activities of the plant extracts against HeLa cell lines. The results were expressed as percentage of cell proliferation inhibition compared with standard drugs. Data were presented as mean ± SD (n = 3).
Considering the antiproliferative activity described by the popular use of C. monogyna, the results of antiproliferative activity tests against HeLa and C6 cell lines supported the ethnomedicinal use of this plant. C. monogyna had higher antiproliferative activity against C6 cells than positive controls, 5-FU and Cisplatin at 50–100 µg/mL concentrations (P < .01, ).
For HeLa cells, the highest inhibition value (89.33%) was obtained with V. vinifera extract at 75 µg/mL concentration (P < .01, ). On the other hand, V. vinifera extract effect decreases in lower concentrations (5–50 µg/mL). Different parts of V. vinifera have been widely investigated against different types of cancer (Kaliora et al. Citation2008; Amico et al. Citation2009; Lazze et al. Citation2009; Sung & Lee Citation2010; Nechita et al. Citation2012; Apostolou et al. Citation2013; Esfahanian et al. Citation2013; Espino et al. Citation2013; Kountouri et al. Citation2013; Giovannelli et al. Citation2014; Liang et al. Citation2014; Sahpazidou et al. Citation2014) and it has been reported that V. vinifera has an antiproliferative effect against many cancer cell lines. Naturally occurring bioactive component of V. vinifera that is responsible from the cardioprotective and cancer chemopreventive activities (Jang et al. Citation1997) is identified as resveratrol (3,4′,5-trihydroxystilbene) (Frankel et al. Citation1993).
Finally, we investigated the antiproliferative activity of G. glabra extract, which exhibited considerable antiproliferative activity against HeLa cell lines () at the highest concentration (100 µg/mL). It is reported that G. glabra showed biological activity similar to that of anti-microtubule drugs which are widely used for the treatment of malignancy (DiPaola et al. Citation1999; Rafi et al. Citation2002). The plant-derived phytochemicals are able to inhibit the proliferation and apoptosis in tumor cells and therefore there is an increasing interest in plant secondary metabolites and their antiproliferative activities (Alesiani et al. Citation2010).
Previous studies on A. rosea plant were focused on its biological activities such as hepatotoxicity (Hussain et al. Citation2014), tyrosinase inhibitory activity (Namjooyan et al. Citation2011), antibacterial activity (Seyyednejad et al. Citation2010). Studies on antiproliferative activity of A. rosea plant are not sufficient.
The finding from our study showed that C. monogyna, V. vinifera, and A. glutinosa L. gaertn exhibited high antiproliferative activity against C6 and HeLa cancer cell lines at the concentration of 75 and100 µg/mL. This may indicate that extracts of the plants may have potential antiproliferative activity for different cancer cell lines.
Conclusions
In this study, we report antiproliferative activities of several plant extracts, which are used in Turkish folk medicine, against HeLa and C6 cell lines. According to the results obtained, it is feasible to speculate that C. monogyna, V. vinifera, A. glutinosa L. gaertn, and A. rosea plant extracts could be a promising alternative natural source of chemotherapy agents.
Acknowledgements
The authors thank Dr Ali Karagoz and Prof. Nazlı Arda from Istanbul University, Department of Molecular Biology, for help in providing C6 (Rat Brain tumor cells) and HeLa (human cervix carcinoma) cells. The authors are also grateful to Dr Nihal Deligonul for the grammatical revision of the manuscript. This work is funded by Gaziosmanpasa University Scientific Research Projects (BAP-File No.2010/39), Tokat, Turkey.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Acero N, Muñoz-Mingarro D. 2012. Effect on tumor necrosis factor-α production and antioxidant ability of black alder, as factors related to its anti-inflammatory properties. J Med Food. 15:542–548.
- Aghili Khorasani MH, Makhzan-Al-Adviah. 1992. Research institute for islamic and complementary medicine. Tehran: Islamic Publishing and Educational Organization.
- Alesiani D, Canini A, D'Abrosca B, DellaGreca M, Fiorentino A, Mastellone C, Monaco P, Pacifico S. 2010. Antioxidant and antiproliferative activities of phytochemicals from Quince (Cydonia vulgaris) peels. Food Chem. 118:199–207.
- Amico V, Barresi V, Chillemi R, Condorelli DF, Sciuto S, Spatafora C, Tringali C. 2009. Bioassay-guided isolation and antiproliferative compounds from grape (Vitis vinifera) stems. Nat Prod Commun. 4:27–34.
- Apostolou A, Stagos D, Galitsiou E, Spyrou A, Haroutounian S, Portesis N, Trizoglou I, Hayes AW, Tsatsakis AM, Kouretas D. 2013. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem Toxicol. 61:60–68.
- Baytop T. 1999. Bitkiler ile tedavi. İstanbul: Nobel Tıp Kitabevleri.
- Bombardelli E, Morazzonni P. 1995. Vitis vinifera. L. Fitoterapia. 66:291–317.
- Borrelli F, Capasso R, Russo A, Ernst E. 2004. Systematic review: green tea and gastrointestinal cancer risk. Aliment Pharm Therap. 19:497–510.
- Carvalho AM. 2010. Plantas y sabiduria popular del Parque Natural de Montesinha. Un estudio etnobotánico en Portugal. Biblioteca de Ciencias, 35. Madrid: Consejo Superior de Investigaciones Cientificas (CSIC).
- Chon SU, Kim YM, Park YJ, Heo BG, Park YS, Gorinstein S. 2009. Antioxidant and antiproliferative effects of methanol extracts from raw and fermented parts of mulberry plant (Morus alba L.). Eur Food Res Technol. 230:231–237.
- Demirtas I, Sahin A. 2013. Bioactive volatile content of the stem and root of Centaurea carduiformis DC. subsp. carduiformis var. carduiformis. J Chem. 2013:1–6.
- Demirtas I, Sahin A, Ayhan B, Tekin S, Telci I. 2009. Antiproliferative effects of the methanolic extracts of Sideritis libanotica Labill. subsp. linearis. Rec Nat Prod. 3:104–109.
- DiPaola RS, Rafi MM, Vyas V, Toppmeyer D, Rubin E, Patel J, Goodin S, Medina M, Medina P, Zamek R, et al. 1999. Phase I clinical and pharmacologic study of 13-cis-retinoic acid, interferon alfa, and paclitaxel in patients with prostate cancer and other advanced malignancies. J Clin Oncol. 17:2213–2218.
- Esfahanian Z, Behbahani M, Shanehsaz M, Hessami MJ, Nejaban MA. 2013. Evaluation of anticancer activity of fruit and leave extracts from virüs infected and healthy cultivars of Vitis vinifera. Cell J. 15:116–123.
- Espino J, Gonzales-Gomez D, Moreno D, Fernandez-Leon MF, Rodriguez AB, Pariente JA, Delgado-Adamez J. 2013. Tempranillo-derived grape seed extract induces apoptotic cell death and cell growth arrest in human promyelocytic leukemia HL-60 cells. Food Funct. 4:1759–1766.
- Farnsworth NR. 1988. Screening plants for new medicines. Washington, DC: National Academy Press.
- Ferguson PJ, Kurowska E, Freeman DJ, Chambers AF, Koropatnick DJ. 2004. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. Nutr Cancer. 134:1529–1535.
- Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. 2011. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. The Lancet. 378:1461–1484.
- Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. 1993. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. The Lancet. 341:454–457.
- Frederich M, Marcowycz A, Cieckiewicz E, Megalizzi V, Angenot L, Kiss R. 2009. In vitro anticancer potential of tree extracts from the walloon region forest. Planta Med. 75:1634–1637.
- Giovannelli L, Innocenti M, Santamaria AR, Bigagli E, Pasqua G, Mulinacci N. 2014. Antitumoural activity of viniferin-enriched extracts from Vitis vinifera L. cell cultures. Nat Prod Res. 28:2006–2016.
- Gomes CA, Cruz TG, Andrade JL, Milhazes N, Borges F, Marques MPM. 2003. Anticancer activity of phenolic acids of natural or synthetic origin: a structure–activity study. J Med Chem. 46:5395–5401.
- Guz NR, Lorenz P, Metraux JP. 2002. Oregonin from the bark of European alnus species. Biochem Syst Ecol. 30:471–474.
- Hartwell JL. 1967. Plants used against cancer. A survey. Lloydia (1967–1971); p. 30–34.
- Hossain MB, Brunton NP, Barry-Ryan C, Martin-Diana AB, Wilkinson M. 2008. Antioxidant activity of spice extracts and phenolics comparison to synthetic antioxidants. Rasayan J Chem. 1:751–756.
- Hussain L, Akash MSH, Tahir M, Rehman K, Ahmed KZ. 2014. Hepatoprotective effects of methanolic extract of Alcea rosea against acetaminophen-induced hepatotoxicity in mice. Bangl J Pharmacol. 9:322–327.
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 275:218–220.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. 2009. Cancer statistics. CA Cancer J Clin. 59:225–249.
- Kaliora AC, Kountouri AM, Karathanos VT, Koumbi L, Papadopoulos NG, Andrikopolulos NK. 2008. Effect of greek raisins (Vitis vinifera L.) from different origins on gastric cancer cell growth. Nutr Cancer. 60:792–799.
- Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. 2005. The antitumor activities of flavonoids. In Vivo. 19:895–90.
- Karaman S, Kocabas YZ. 2001. Traditional medicinal plants of K. Maras (Turkey). Sci. 1:126–128.
- Kountouri AM, Gioxari A, Karvela E, Kaliora AC, Karvelas M, Karathanos VT. 2013. Chemopreventive properties of raisins originating from Greece in colon cancer cells. Food Funct. 4:366–372.
- Lardos A, Kreuter MH. 2000. Red vine leaf. In: Kreuter MH, editor. Phytopharm and phytochem products. Flachsmann, AG: Zurich; p. 1–7.
- Lazze MC, Pizzala R, Pecharroman FJG, Garnica PG, Rodriguez JMA, Fabris N, Bianchi L. 2009. Grape waste extract obtained by supercritical fluid extraction contains bioactive antioxidant molecules and induces antiproliferative effects in human colon adenocarcinoma cells. J Med Food. 12:561–568.
- Liang ZC, Cheng LL, Zhong GY, Liu RH. 2014. Antioxidant and antiproliferative activities of twenty-four Vitis vinifera grapes. PloS. 9:1–10. e105146.
- Mert T, Fatal T, Kıvcak H, Tansel Ozturk H. 2010. Antimicrobial and cytotoxic activities of the extracts obtained from the flowers of Alcea rosea L. Hacettepe Univ J Fac Pharm. 30:17–24.
- Namjooyan F, Moosavi H, Taherian A. 2011. Review of natural and synthetic tyrosinase inhibitors. Planta Med. 77:PL 67.
- Nechita A, Cotea VV, Nechita CB, Pincu RR, Mihai CT, Colibaba CL. 2012. Study of cytostaic and cytotoxic activity of several polyphenolic extracts obtained from Vitis vinifera. Not Bot Horti Agrobot Cluj-Napoca. 40:216–221.
- O'Rourke C, Byres M, Delazar A, Kumarasamy Y, Nahar L, Stewart F. 2005. Hirsutanonol, oregonin and genkwanin from the seeds of Alnus glutinosa (Betulaceae). Biochem Syst Ecol. 33:749–752.
- Ozyurek M, Bener M, Guclu K, Donmez AA, Suzgec-Selcuk S, Pirildar S, Mericli AH, Apak R. 2012. Evaluation of antioxidant activity of Crataegus species collected from different regions of Turkey. Rec Nat Prod. 6:263–277.
- Rafi MM, Vastano BC, Zhu N, Ho CT, Ghai G, Rosen RT, Gallo MA, DiPaola RS. 2002. Novel polyphenol molecule isolated from licorice root (Glycrrhiza glabra) induces apoptosis, G2/M cell cycle arrest, and Bcl-2 phosphorylation in tumor cell lines. J Agric Food Chem. 50:677–684.
- Ramful D, Aumjaud B, Neergheen VS, Soobrattee MA, Googoolye K, Aruoma OI, Bahotun T. 2011. Polyphenolic content and antioxidant activity of Eugenia pollicina leaf extract in vitro and in model emulsion systems. Food Res Int. 44:1190–1196.
- Rodrigues S, Calhelha RC, Barreira JCM, Duenas M, Carvalho AM, Abreu RMV, Santos-Buelga C, Ferreira ICFR. 2012. Crataegus monogyna buds and fruits phenolic extracts: growth inhibitory activity on human tumor cell lines andd chemical characterization by HPLC-DAD-ESI/MS. Food Res Int. 49:516–523.
- Sahpazidou D, Geromichalos GD, Stagos D, Apostolou A, Haroutounian SA, Tsatsakis AM, Tzanakakis GN, Hayes AW, Kouretas D. 2014. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicol Lett. 230:218–224.
- Sanjose DS, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. 2010. Human papillomavirus genotype attribution in invasive cervical canser: a retrospective cross-sectional worldwide study. Lancet Oncol. 11:1048–1056.
- Saxena S. 2005. Glycyrrhiza glabra: Medicine over the millennium. Nat Prod Radiance. 4:358–367.
- Seyyednejad SM, Koochak H, Darabpour E, Motamedi A. 2010. A survey on Hibiscus rosa-sinensis, Alcea rosea L., and Malva neglecta Wallr as antibacterial agents. APJTM. 3:351–355.
- Sung J, Lee J. 2010. Antioxidant and antiproliferative activities of grape seeds from different cultivars. Food Sci Biotechnol. 19:321–326.
- Tadic VM, Dobric S, Markovic GM, Dordevic SM, Arsic IA, Menkovic NR, Stevic T. 2008. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J Agric Food Chem. 56:7700–7709.
- Wang DF, Shang JY, Yu QH. 1989. Analgesic and anti-inflammatory effects of the flower of Althaea rosea (L.) Cav. Zhongguo Zhong Yao Za Zhi. 14:46–48.
- Xiong Y, Wu X, Rao L. 2015. Tetrastigma hemsleyanum (Sanyeqing) root tuber extracts induces apoptosis in human cervical carcinoma HeLa cells. J Ethnopharmacol. 165:46–53.
- Zargari A. 1992. Alcea rosea L. In: Medicinal plants. Tehran: Tehran University Press; p. 360–363.
