ABSTRACT
In this work, the time-dependent effect of exogenous salicylic acid (SA) applied before cold stress was investigated on the regulation of antioxidative respond mechanisms in two barley (Hordeum vulgare L.) cultivars (Akhisar and Tokak) differed in cold tolerance. SA (0.1 mM) was applied to 7-days old barley seedlings growing under control conditions (20/18°C). After this application, the seedlings were transferred to cold conditions (7/5°C) at different times (7, 14, 21 and 28 days) for 3 days. Then, the contents of malondialdehyde (MDA), hydrogen peroxide (H2O2) and the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) were determined in the leaves (control, cold and SA + cold) from the seedlings harvested at 10, 17, 24 and 31 days. Moreover, the effect of cold stress was evaluated on the endogenous SA level at the both cultivars by comparing to control plants. The MDA content increased in the cold treatment while it decreased in the SA treatment at all the days studied at the both cultivars. The SA + cold treatment could have a variable effect on H2O2 content at the tolerant barley (Tokak) while decreased its content at the sensitive cultivar (Akhisar). The SA + cold treatment could increase the activities of POX and SOD at both cultivars, but it increased the CAT activity at the tolerant cultivar while decreased at the sensitive cultivar. In addition, the content of endogenous SA was decreased by cold stress at all the days studied at the barley cultivars as compared to control plants. The results show that the SA treatment could be effective on the regulation of the parameters studied at cold conditions until 31 days after SA application, and the importance of exogenous SA treatment before cold stress. It was concluded that exogenous and endogenous SA could play an ameliorating role on cold tolerance by regulating reactive oxygen species and the activities of antioxidative enzymes in both cold-sensitive and cold-tolerant cultivars of barley.
Introduction
Cold stress caused by low temperatures has important implications on the growth and development of crop plants (Janda et al. Citation2003; Tasgin et al. Citation2006). Although plants have different responses in their resistance mechanism to low temperatures (Lewitt Citation1980), cold-sensitive plants suffer from metabolic decomposition when they are exposed to cold stress and characteristically exhibit structural injuries (Atici and Nalbantoglu Citation2003). Cold-resistant or over-wintering ones can withstand low temperatures that are lethal to many sensitive plants. The defence mechanisms against cold stress are quite complicated as both biochemical and physiological; however, it is well known that one of the most important among them is the antioxidative system. Because, similar to other stresses, reactive oxygen species (ROS) such as superoxide anion (), H2O2 and hydroxyl radicals (·OH) are produced during response to cold stress (Mutlu et al. Citation2009a, Citation2009b; Hu et al. Citation2010). Toxic levels of ROS can impair membrane permeability (Dias et al. Citation2011) by inducing lipid peroxidation (Mutlu et al. Citation2011; Esim et al. Citation2014), cause damage to DNA and proteins (Bertrand et al. Citation2011; Kekec et al. Citation2013) and ultimately lead to programmed cell death. These symptoms during cold stress have been evaluated as oxidative damage (Wang et al. Citation2009a). Therefore, an effective antioxidative system in cells is a key component of the resistant mechanisms against oxidative damage. Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) have a main role in scavenging of the ROS. During cold stress in plants, the levels of decreased ROS have a critical role for living cells because ROS seriously disrupt normal metabolism through oxidative damage in the absence of any protective mechanism (Rout and Shaw Citation2001).
Researches for signal molecules mediating plant stress response are an important step in our better understanding of how plants acclimatize to their adverse environments. Salicylic acid (SA) is also accepted as a signal molecule or a plant hormone synthesized for the activation of plant defence (Khan et al. Citation2015). Roles of exogenous/endogenous SA on plant growth and development, flowering, ion uptake, stomatal regulation and photosynthesis have been investigated in some researches (Popova et al. Citation2009; Kadioglu et al. Citation2011).
In addition, several studies have evaluated the roles of exogenous SA in modulating plant response to several abiotic and biotic stresses such as ultraviolet light (Mahdavian et al. Citation2008), drought (Kadioglu et al. Citation2011; Saruhan et al. Citation2012), salt (Mutlu et al. Citation2009a; Mutlu and Atici Citation2013; Khan et al. Citation2014; Jayakannan et al. Citation2015), heat (Khan et al. Citation2013), heavy metals (Guo et al. Citation2009; Popova et al. Citation2009) and plant pathogenesis (Wang et al. Citation2007). For example, exogenous SA treatment could improve chilling tolerance in maize (Janda et al. Citation1999; Horvath et al. Citation2002), tomato (Ding et al. Citation2002), banana (Kang et al. Citation2003), winter wheat (Tasgin et al. Citation2006; Esim and Atici Citation2015), guayule (Sundar et al. Citation2004), red globe grape (Li et al. Citation2005), Brassica juncea (Setia et al. Citation2006), radish (Biao, Citation2006), cucumber (Xia et al. Citation2007; Lei et al. Citation2010), grass (Wang et al. Citation2009a) and barley (Mutlu et al. Citation2013a, Citation2013b). Recently, some researchers carried out on winter wheat (Tasgin et al. Citation2006) and watermelon (Yang et al. Citation2008) demonstrated that exogenous SA treatment could be involved in cold tolerance by regulating the activities of antioxidant enzymes. The action mode of exogenous SA on the antioxidative system during response to cold and the time-dependent effect of SA when it was applied to a plant before cold exposure are still unclear although recent studies provide intensive data about the role of exogenous SA in the regulation of environmental stress response, including cold stress (Korkmaz, Citation2005; Shi et al. Citation2006; Guo et al. Citation2009; Mutlu et al. Citation2009a; Xia et al. Citation2009; Hu et al. Citation2010; Dias et al. Citation2011; Zhang et al. Citation2011b; Luo et al. Citation2012). On the other hand, there are inadequate and conflicting results in the literature about the effects of exogenous SA applied to a plant before cold stress treatment on the enzymatic antioxidative system. In the present study, therefore, effects of exogenous SA applied before cold treatment on lipid peroxidation (as malondialdehyde (MDA) content), H2O2 content and the activities of antioxidative enzymes (SOD, CAT and POX) were investigated by depending on the time of application in two barley (Hordeum vulgare L.) cultivars differing at cold tolerance. In addition, contents of endogenous SA during cold response were also determined to evaluate together with the antioxidant system parameters.
Results and discussion
Effects of cold and SA + cold on MDA content
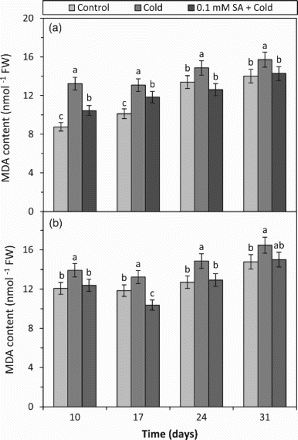
The damage in cell membranes of living cells was evaluated by measuring the level of MDA content in the barley leaves. MDA is a product of peroxidation of unsaturated fatty acids in phospholipids and responsible for cell membrane damage. An increase in MDA content expresses an increase at the lipid peroxidation level of cell membranes (Xu et al. Citation2006; Baloglu et al. Citation2012). In the present study, cold treatment alone increased the MDA content in both cultivars (tolerant and sensitive) of barley at all days studied (10, 17, 24 and 31) in comparison to respective controls (Figure ). This result can show that cold treatment can cause an important damage on membrane lipids by increasing the levels of the ROS, such as superoxide anion () and H2O2. Certain researchers also reported that cold stress increased the generation of ROS, including H2O2 in plants, and as a result of this the MDA content could be also increased (Senaratna et al. Citation2000; Kang et al. Citation2003). In addition, it has been suggested that this effect of cold stress can disrupt the permeability of the cell membrane structure of the plant (Dias et al. Citation2011). In the plants applied SA and then exposed to cold (SA + cold treatment), the MDA content was determined significantly to be decreased at all days (10, 17, 24 and 31) in comparison to respective cold values (Figure ). The result shows that the exogenous SA treatment applied before cold stress can contribute to the improving of cold tolerance by reducing the MDA content in the both barley plants. Certain studies also showed that the MDA content decreased in the SA treatment during cold stress in watermelon (Yang et al. Citation2008) and bamboo (Luo et al. Citation2012). SA also alleviates abiotic stress-induced oxidative damage as indicated by a downregulation in lipid peroxidation (MDA level) in plants (Posmyk et al. Citation2005; Huang et al. Citation2008; Yang et al. Citation2008; Luo et al. Citation2012). In addition to these, the MDA results showed that exogenous SA applied before cold stress could previously prepare barley plants against cold damage and this protective effect could be seen for 24 days after it was applied.
Figure 1. The effects of cold and SA + cold treatments on the MDA content in the leaves of two barley cultivars differing at cold tolerance. (a) Cold-tolerant cultivar; Tokak and (b) cold-sensitive cultivar; Akhisar): each datum is the average of six independent samples (n = 6). Values in a group followed by the same letter are not statistically different at P < .05 level as determined by Duncan's Multiple Range Test. Each value in the graph shows average of three experiments. Vertical bars represent ± SE.
Effects of cold and SA + cold on H2O2 content
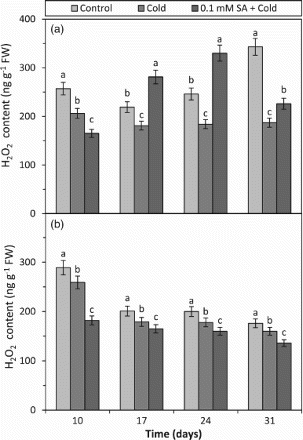
In the both barley cultivars, H2O2 content was reduced by cold alone at all the days (10, 17, 24 and 31) as compared to respective controls (Figure ). However, reduction ratios in H2O2 content were statistically more meaningful in cold-tolerant variety. When the values of SA + cold treatment were compared to values of the cold treatment, H2O2 content significantly increased at 17, 24 and 31 days of tolerant plant and decreased at all the days of sensitive plant (Figure ). The results obtained from the both cultivars are in agreement with some earlier reports. It was determined that exogenous SA increased the accumulation of H2O2 in Cucumis sativus leaves under cold conditions (Zhang et al. Citation2011b), but displayed a lower H2O2 content in eggplant under chilling stress (Chen et al. Citation2011). The increase and decrease in the H2O2 content can be explained by the change in antioxidant enzyme activities. Therefore, the H2O2 results are connected with the results of antioxidant enzyme activities obtained in the present study. In addition to these, the H2O2 results showed that exogenous SA applied before cold stress could previously prepare sensitive barley plant against cold damage and this protective effect could be seen for 24 days after it was applied.
Figure 2. The effects of cold and SA + cold treatments on H2O2 content in the leaves of two barley cultivars differing at cold tolerance. (a) Cold-tolerant cultivar; Tokak and (b) cold-sensitive cultivar; Akhisar. Each datum is the average of six independent samples (n = 6). Values in a group followed by the same letter are not statistically different at P < .05 level as determined by Duncan's Multiple Range Test. Each value in the graph shows the average of three experiments. Vertical bars represent ± SE.
Effects of cold and SA + cold on antioxidant enzyme activities
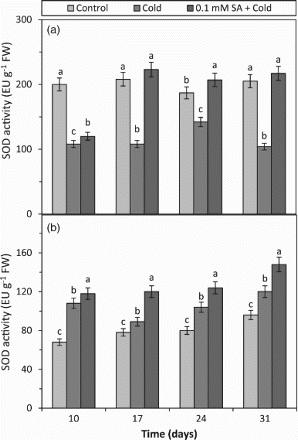
Antioxidant enzymes including SOD, CAT and POX are the most efficient protective mechanisms against oxidative stress (Senaratna et al. Citation2000; Kang et al. Citation2003; Patykowski and Urbanek, Citation2003; Hu et al. Citation2010). They control the level of ROS and eliminate their detrimental effects. SOD transforms to H2O2 by acting as the first line of defence against ROS and H2O2 also is scavenged by CAT and POX to water (Rout and Shaw Citation2001). The present findings showed that SOD activity decreased in cold-tolerant barley and increased in cold-sensitive barley by the cold treatment at all the days (10, 17, 24 and 31) as compared to respective controls (Figure ). The decrease in SOD activity can cause the accumulation of
in plant cells during oxidative stress, confirming that the reduced SOD activity or the excessive production of
is one of the fundamental factors in metabolic deterioration during cold stress (Mutlu et al. Citation2013b). In the SA + cold-treated plants, SOD activity was increased by SA in both barley cultivars at all the days (10, 17, 24 and 31) in comparison to respective cold treatments (Figure ). Although there are no reports elucidating SOD activity in plants treated with SA before cold exposure and the time-dependent effect of SA treatment on SOD activity, it has been shown that under cold conditions, the activity of SOD was increased by the SA treatment in the root of the sensitive cultivar of rice plant (Wang et al. Citation2009b). The results imply that SA can play a significant role in response to cold conditions by regulating the impaired activity of SOD by cold stress in barley leaves. Conversely, the effect of SA on the activation of SOD in both cultivars may facilitate the integrity of membrane structures of the cell, due to the fact that SOD is involved in the deactivation of the lipid peroxidation processes by decreasing the level of superoxide anion (O2•−) and by increasing transforms of
to H2O2. Therefore, SOD acts as the first line of defence against ROS by dismutating superoxide to H2O2 and hence decreasing the risk of hydroxyl radical formation from superoxide (Apel and Hirt, Citation2004). When the results of H2O2 content and SOD activity obtained from the cold-tolerant barley treated with SA + cold are connected, it is seen that the H2O2 contents and SOD activities were increased by the SA treatment (Figures (a) and (a)). In the light of the result, it can be interpreted that the activation of SOD induced by the SA treatment may contribute to its anti-stress effects on plants (Mutlu et al. Citation2009a). In addition to these, the data obtained from SOD activity showed that exogenous SA applied before cold stress could previously prepare barley plants against cold damage and this protective effect could be seen for 24 days after it was applied.
Figure 3. The effects of cold and SA + cold treatments on SOD activity in the leaves of two barley cultivars differing at cold tolerance. (a) Cold-tolerant cultivar; Tokak and (b) cold-sensitive cultivar; Akhisar. Each datum is the average of six independent samples (n = 6). Values in a group followed by the same letter are not statistically different at P <.05 level as determined by Duncan's Multiple Range Test. Each value in the graph shows the average of three experiments. Vertical bars represent ± SE.
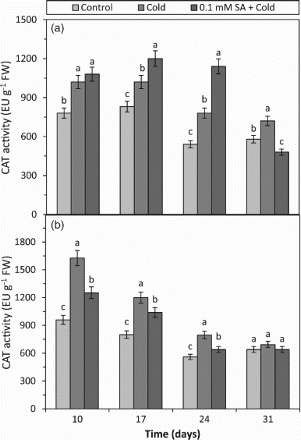
The cold treatment alone increased CAT activity in the both cultivars at all the days (10, 17, 24 and 31) in comparison to respective controls (Figure ). In addition, under the cold condition, the CAT activity in the sensitive cultivar gradually decreased depending on days. CAT can involve the removal of H2O2 in the cell (Patykowski and Urbanek, Citation2003; Mutlu et al. Citation2009a). Certain researchers have suggested that the stress-tolerant genotypes have a better radical scavenging ability (Zhang et al. Citation2011a). It has been shown that the cold treatment increases the CAT activity in cold-tolerant cultivars and decreases it in salt-sensitive plants day by day. These data suggest that the CAT activity can be significantly affected by cold stress. The stimulation of CAT activity by biotic and abiotic stresses is a phenomenon that occurs in many different kinds of plant species (Vanacker et al. Citation1998; Hernandez et al. Citation2000; Patykowski and Urbanek, Citation2003; Tasgin et al. Citation2006; Mutlu et al. Citation2009a). When the results of H2O2 content and CAT activity obtained from the both cold-treated barley plants are connected, it is seen that the decrease in the H2O2 content was parallel with the increase in the CAT activity (Figure and ). It can be interpreted that an increase in CAT activity will lead to a decrease in the H2O2 content. Therefore, our results suggest that the increase in the CAT activity in both cultivars can be a result of the scavenging of H2O2 produced excessively in cell during cold stress, and CAT is an important H2O2 scavenging enzyme leading to the cold tolerance of barley plants. However, certain research studies reported reduced CAT activity in plants exposed to low temperatures and bright light due to photo-inactivation of CAT (Feierabend et al. Citation1992). In addition, it is concluded that exposure to cold temperatures causes an increase in CAT activity (Farooq et al. Citation2008), which supports our results. Finally, Farooq et al. (Citation2008) suggested that the higher CAT activity in leaves under cold stress the more efficient the scavenging system is, which may result in better protection against ROS during stress. At the SA + cold-treated barley plants, the CAT activity was increased by SA at 17 and 24 days while only slightly reduced on day 31 in the cold-tolerant cultivar and the CAT activity was reduced by SA at 10, 17 and 24 days in the cold-sensitive cultivar (Figure ). Our results show that the exogenous SA treatment changes the CAT activity in the barley cultivars under the cold treatment and plays a regulating role in plants during the oxidative burst caused by cold stress. Although Tasgin et al. (Citation2006) determined that the SA treatment caused a decrease in the CAT activity in the winter wheat under cold conditions, in the present study; CAT activities were induced by 0.1 mM SA application in tolerant cultivar. Zhang et al. (Citation2011b) also found that the exogenous application of SA increased the CAT activities in C. sativus, while the activity reduced in two rice cultivars under the cold condition (Wang et al. Citation2009b). However, the contradictory findings in our study and the literature related to the influence of SA on CAT can be based on dose and vary according to plant species (Wang et al. Citation2009b) or can be attributed to its role in plant growth regulation being a hormone-like substance. Conversely, the SA treatment can stimulate the accumulation of ROS such as H2O2 in a plant cell (Minibaeva and Gordon, Citation2003). Low concentrations of especially H2O2 are known to act as a signal molecule initiating several protective resistance mechanisms against pathogens, chilling and heat stress (Lamb, Citation1994). If H2O2 accumulation induced by SA was excessive, serious oxidative stress can be occurred and unrecoverable membrane damage can be appeared (Rao et al. Citation1997). We conclude that the increasing CAT activity can be considered as an important mechanism in the cell defence strategy. Therefore, a significant increase in the cellular activity of CAT can be observed in only low SA concentration (0.1 mM) in the tolerant cultivars grown under cold conditions. In an inconvenient concentration, SA itself also can be a stress factor. In addition to these, the CAT results showed that exogenous SA applied before cold stress could previously prepare tolerant barley plant against cold damage and this protective effect could be seen for 17 days after it was applied.
Figure 4. The effects of cold and SA + cold treatments on the CAT activity in the leaves of two barley cultivars differing at cold tolerance. (a) Cold-tolerant cultivar; Tokak and (b) cold-sensitive cultivar; Akhisar. Each datum is the average of six independent samples (n = 6). Values in a group followed by the same letter are not statistically different at P < .05 level as determined by Duncan's Multiple Range Test. Each value in the graph shows the average of three experiments. Vertical bars represent ± SE.
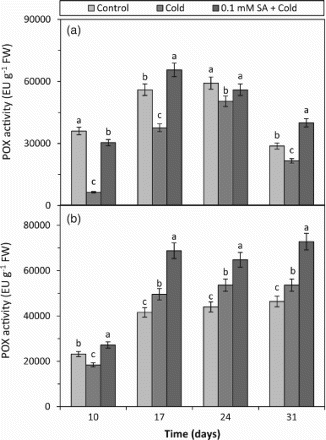
The POX activity was reduced by the cold treatment at all the days (10, 17, 24 and 31) in cold-tolerant barley (Figure ). However, in cold-sensitive barley, the POX activity was increased by the cold treatment at 17 and 24 days while only slightly reduced on day 10 (Figure ). The results suggest that the POX activity can be significantly affected by cold stress. The present study demonstrates that the increase in POX activity due to cold stress in barley leaves is more conspicuous in the cold-sensitive cultivar. This increase in the POX activity can play a crucial role in the detoxification of H2O2 since POX is the primary H2O2-scavenging enzyme in the plant cells. At the SA + cold treatments applied to both cultivars, POX activities were significantly increased compared to respective cold treatments (Figure ). The result shows that exogenous SA treatment contributes to regulation of cold stress tolerance by stimulating the POX activity in barley exposed to the cold condition. A recent study also found that exogenous SA application increased the POX activities in C. sativus under the cold condition (Zhang et al. Citation2011b). SA is also known to be effective in the processes about the biosynthesis of some substances such as lignin and suberin by increasing the POX activity (Sakhabutdinova et al. Citation2004). It is a well-known fact that POX protects cells against the damaging effects of H2O2 during an oxidative-burst response under stress conditions (Levine et al. Citation1994). At a recently reported study, the effect of exogenous application of SA in eggplant under chilling stress determined that pre-treated with SA displayed a lower H2O2 content and higher POX activity (Chen et al. Citation2011). In the present study, when the results of H2O2 content and POX activity obtained from the cold-sensitive barley treated with SA + cold are connected, it was determined that the H2O2 contents decreased and POX activities increased (Figure (b) and 5(b)). In the light of the results, it can be interpreted that the exogenous SA treatment can undertake a significant role in the regulation of response to cold stress of both barley cultivars by regulating the POX activity. However, it is seen that the regulatory effect of SA has been more effective on the cold-sensitive cultivar. In addition to these, the POX results showed that exogenous SA applied before cold stress could previously prepare both tolerant and sensitive barley plant against cold damage and this protective effect could be seen for 24 days after it was applied.
Figure 5. The effects of cold and SA + cold treatments on POX activity in the leaves of two barley cultivars differing at cold tolerance. (a) Cold-tolerant cultivar; Tokak and (b) Cold-sensitive cultivar; Akhisar. Each datum is the average of six independent samples (n = 6). Values in a group followed by the same letter are not statistically different at P < .05 level as determined by Duncan's Multiple Range Test. Each value in the graph shows the average of three experiments. Vertical bars represent ± SE.
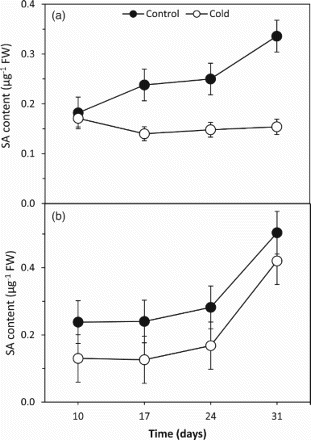
Effects of cold on endogenous SA content
Endogenous SA contents increased depending on time in the both cold-sensitive and cold-tolerant barley cultivars at the control conditions (Figure ). However, the cold treatment decreased endogenous SA content in the both cold-sensitive and cold-tolerant barley cultivars in comparison to respective controls (Figure ). Only in the cold-sensitive cultivar, SA contents gradually increased depending on days at the both control and cold treatments. This can be an important difference at antioxidative response to cold stress since cold tolerance of the varieties is different. Although the endogenous SA contents were decreased by the cold treatment in the present study, endogenous SA levels were increased by UV light, ozone (Yalpani et al. Citation1994), salt (Hernandez et al. Citation2000) and drought stress (Kadioglu et al. Citation2011). In addition, increases in the endogenous SA content following exogenous SA treatment were also determined in some plants (Talieva and Kondrateva, Citation2002; Popova et al. Citation2009; Kadioglu et al. Citation2011). It can be seen that both stress factors and exogenous SA treatment can be effective on endogenous SA content. Thus, in the present study, endogenous SA can play a key role in providing tolerance to the plant exposed to cold stress by affecting contents of MDA and H2O2 and activities of SOD, CAT and POX enzymes.
Figure 6. The effects of cold treatment on endogenous SA content in the leaves of two barley cultivars differing at cold tolerance. (a) Cold-tolerant cultivar; Tokak and (b) Cold-sensitive cultivar; Akhisar. Each datum is the average of six independent samples (n = 6). Values in a group followed by the same letter are not statistically different at P < .05 level as determined by Duncan's Multiple Range Test. Each value in the graph shows the average of three experiments. Vertical bars represent ± SE.
Conclusion
The exogenous SA treatment (0.1 mM) applied before cold stress could decrease the lipid peroxidation level of cell membranes increased by the cold treatment at the both cultivar of barley. But the SA treatment could not have a distinctive effect on H2O2 content at the tolerant barley while decreased its content at the sensitive cultivar. The SA treatment could increase the activities of POX and SOD at both cultivars, but it increased the CAT activity at the tolerant cultivar while decreased at the sensitive cultivar. It was evaluated that the SA treatment could be effective on the regulation of the parameters studied at cold conditions until 31 days after SA application. In addition, the content of endogenous SA was decreased by cold stress at all the days studied at the barley cultivars as compared control plants. This shows the importance of exogenous SA treatment before cold stress. It was concluded that exogenous and endogenous SA could play an ameliorating role on cold tolerance by regulating ROS and the activities of antioxidative enzymes in both cold-sensitive and cold-tolerant cultivars of barley.
Materials and methods
Plant material
In this study, cold-tolerant (Tokak) and cold-sensitive (Akhisar) two barley (Hordeum vulgare) cultivars were used as plant material. The plant seeds were provided from the Eastern Anatolian Agricultural Research Institute, Erzurum, Turkey. Seeds were planted in 15 cm-pots including sand at the same rate. They were maintained in a 20/18°C (day/night), 75% relative humidity and a photon flux density of 400 µmol m−2 s−1 photosynthetic active radiation growth chamber with a 12-h photoperiod for 7 days to initiate germination. The solution of SA was prepared in concentration 0.1 mM with distilled water and Tween-20 (50 µL/100 mL) added to its solution to improve penetration. After the 7 days, the SA solution was sprayed on the leaves of barley plants and distilled water (including Tween-20) in same pH was sprayed on the control plant leaves. Seedlings were watered regularly with half strength Hoagland solution. Some of the plants (with and without SA treatment) were transferred to cold conditions (7/5°C) for 3 days at different times (7, 14, 21 and 28 days) after SA treatment. Then, the leaves of 10, 17, 24 and 31-days old seedlings were harvested at the end of the treatments (control, cold and SA + cold) and they were used as research material.
Determination of MDA content
The level of MDA being a product of lipid peroxidation was measured using the method of Heath and Packer (Citation1968) with slight modifications (Ananieva et al. Citation2002). Leaves (0.5 g) were homogenized in 3 mL 0.1% TCA and centrifuged at 15,000 × g at 4°C for 30 min. To 0.5 mL aliquot of the supernatant, 1 mL reagent (0.5% Thiobarbituric acid (TBA) in 20% TCA, w/v) was added. For a negative control, 0.5 mL 0.1% TCA and 1 mL reagent were added. The test-tubes were heated at 95°C for 30 min and then quickly cooled in an ice bath. After cooling and centrifugation to give a clear supernatant, the absorbance of the supernatant at 532 nm was read and the value for the non-specific absorption at 600 nm was subtracted. The level of MDA was estimated by using the mmol/L extinction coefficient of 155 mmol/L−1 cm−1.
Determination of hydrogen peroxide (H2O2) content
Hydrogen peroxide concentrations were measured by monitoring the absorbance at 410 nm of the titanium-peroxide complex according to He et al. (Citation2005). One mL of supernatant extracted with cold acetone was added to 0.1 mL 20% titanium reagent and 0.2 mL 17 mol ammonia solution. The solution was centrifuged at 3000 × g at 4°C for 10 min and the supernatant was discarded. The pellet was dissolved in 3 mL 1 mol sulphuric acid. The absorbance of the solution was measured at 410 nm. Absorbance values were calibrated to a standard curve generated with known concentrations of H2O2.
Extraction of antioxidative enzymes
Fresh leaves (0.5 g) were homogenized with a mortar and pestle in ice-cold 0.2 M phosphate buffer (pH 7) including 1% PVP and 1 mM EDTA. The homogenate was centrifuged at 12,000 × g at 4°C for 15 min. The supernatant was used to determine SOD, CAT and POX activities.
Determination of enzyme activities
SOD, CAT and POX enzyme activities in the fractions were measured spectrophotometrically. The SOD (EC 1.15.1.1) activity was estimated by recording the decrease in optical density of nitro-blue tetrazolium dye by the enzyme (Beauchamp and Fridovich, Citation1971). The reaction mixture (3 mL) contained 2 µM riboflavin, 13 mM methionine, 75 µM Nitroblue Tetrazolium Chloride (NBT), 0.1 mM EDTA, 50 mM phosphate buffer (pH 7.8), 50 mM sodium carbonate and 0.05 mL enzyme fraction. The reaction was started by adding riboflavin solution and placing the tubes under two 30 W fluorescent lamps for 15 min. A complete reaction mixture without enzyme, which gave the maximal colour served as a control. The reaction was stopped by switching off the light and putting the tubes in the dark. A non-irradiated complete reaction mixture served as a blank. The absorbance was recorded at 560 nm, and one unit of enzyme activity was taken as the amount of enzyme that reduced the absorbance reading to 50% in comparison with tubes lacking enzyme. The CAT (EC 1.11.1.6) activity was measured by monitoring the decrease in absorbance at 240 nm in 50 mM phosphate buffer (pH 7.5) containing 20 mM H2O2. One unit of CAT activity was defined as the amount of enzyme that used 1 µmol H2O2/minute (Upadhyaya et al. Citation1985). The POX (EC 1.11.1.7) activity was measured by monitoring the increase in absorbance at 470 nm in 50 mM phosphate buffer (pH 5.5) containing 1 mM guaiacol and 0.5 mM H2O2. One unit of POX activity was defined as the amount of enzyme that caused an increase in absorbance of 0.01/minutes (Upadhyaya et al. Citation1985).
Extraction and determination of endogenous SA content
SA was extracted from fresh plant tissue according to the method of Raskin et al. (Citation1989) with some modifications. Leaves (0.5 g) were homogenized in 3 ml 90% methanol by a homogenizer and centrifuged at 12,000 × g for 15 min. Supernatants were evaporated by a rotary evaporator at 68°C for 5 min. The residue was dissolved in 2.5 mL, 5% trichloroacetic acid and centrifuged at 12,000 × g for 10 min. Five ml mixture of ethylacetate, cyclopentane and ispropanole (100:99:1, v:v:v) was added to supernatant. The supernatant was evaporated at 90°C for 10 min. The residue was dissolved in 2.5 ml of 20% methanol, filtered by 45 µm syringe filter and injected (100 µl) to high-performance liquid chromatography (HPLC) system. HPLC system consisted of an LC 20 AT/Prominence, Shimadzu, Japan equipped with a quaternary HPLC pump, micro vacuum degasser, thermostated column compartment, refractive detector, fluorescence detector, standard micro and preparative autosampler. The SA analysis was performed on Lichrosorb SI-60 (250 9 4.0 mm i.d. 5 µm particle size, Teknorama, Barcelona, Spain) column, containing methanol/water, 50:50, (v/v) the in mobile phase, operating at 22°C with a flow rate 1 ml/min. The measurements were performed using 100 µl loop, Shimadzu RF10-AXL fluorescence detector (excitation/emission detection at 313/405 nm). SA was identified by comparison of their retention times to those of authentic standards. With standard solutions, calibration curves for SA were made, which was later used for assessing the concentrations corresponding to the different peaks in the chromatograms. The areas of peaks of compound were quantified by the Shimadzu LC solution Software (Kadioglu et al. Citation2011).
Statistical analysis
All experiments were designed in a randomized complete block. The data obtained from the six samples at random were used for statistical analyses (n = 6) and the average of the six values are stated in figures. The data were evaluated using variance analyses (ANOVA) in SPSS 19.0 for Microsoft Windows and means were compared by Duncan's Multiple Range Test at 0.05 level of confidence.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ananieva EA, Alexieva VS, Popova LP. 2002. Treatment with salicylic acid decreases the effects of paraquat on photosynthesis. J Plant Physiol. 159:685–693. doi: 10.1078/0176-1617-0706
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701
- Atici O, Nalbantoglu B. 2003. Antifreeze proteins in higher plants. Phytochemistry. 64:1187–1196. doi: 10.1016/S0031-9422(03)00420-5
- Baloglu MC, Kavas M, Aydin G, Oktem HA, Yucel AM. 2012. Antioxidative and physiological responses of two sunflower (Helianthus annuus) cultivars under PEG-mediated drought stress. Turk J Bot. 36:707–714.
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 44:276–287. doi: 10.1016/0003-2697(71)90370-8
- Bertrand P, Severine J, Jerome S, Pinelli E. 2011. Lead-induced DNA damage in Vicia faba root cells: potential involvement of oxidative stress. Mutat Res-Gen Tox En. 726:123–128. doi: 10.1016/j.mrgentox.2011.09.001
- Biao WN. 2006. Alleviating effects of exogenous salicylic acid (SA) on chilling stress in radish. J Southwest Agric Univ. 28:782–785.
- Chen S, Zimei L, Cui J, Jiangang D, Xia X, Liu D, Yu J. 2011. Alleviation of chilling-induced oxidative damage by salicylic acid pretreatment and related gene expression in eggplant seedlings. Plant Growth Regulat. 65:101–108. doi: 10.1007/s10725-011-9579-9
- Dias MC, Pinto G, Santos C. 2011. Acclimatization of micropropagated plantlets induces an antioxidative burst: a case study with Ulmus minor Mill. Photosynthetica. 49:259–266. doi: 10.1007/s11099-011-0028-9
- Ding CK, Wang CY, Gross KC, Smith DL. 2002. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 214:895–901. doi: 10.1007/s00425-001-0698-9
- Esim N, Atici O. 2015. Effects of exogenous nitric oxide and salicylic acid on chilling-induced oxidative stress in wheat (Triticum aestivum). Front Life Sci. 8:124–130. doi: 10.1080/21553769.2014.998299
- Esim N, Atici O, Mutlu S. 2014. Effects of exogenous nitric oxide in wheat seedlings under chilling stress. Toxicol Ind Health. 30:268–274. doi: 10.1177/0748233712457444
- Farooq M, Aziz T, Basra SMA, Cheema MA, Rehamn H. 2008. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J Agron Crop Sci. 194:161–168. doi: 10.1111/j.1439-037X.2008.00300.x
- Feierabend J, Schaan C, Hertwig B. 1992. Photoinactivation of catalase occurs under both high- and low- temperature stress conditions and accompanies photoinhibition of PSII. Plant Physiol. 100:1554–1561. doi: 10.1104/pp.100.3.1554
- Guo B, Liang YC, Zhu YG. 2009. Does salicylic acid regulate antioxidant defense system, cell death, cadmium uptake and partitioning to acquire cadmium tolerance in rice? J Plant Physiol. 166:20–31. doi: 10.1016/j.jplph.2008.01.002
- He YL, Liu YL, Cao WX, Huai MF, Xu BG, Huang BG. 2005. Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in Kentucky bluegrass. Crop Sci. 45:988–995. doi: 10.2135/cropsci2003.0678
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198. doi: 10.1016/0003-9861(68)90654-1
- Hernandez JA, Jimenez A, Mullineauxs PM, Sevilla F. 2000. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ. 23:853–862. doi: 10.1046/j.1365-3040.2000.00602.x
- Horvath E, Janda T, Szalia G, Paldi E. 2002. In vitro salicylic acid inhibition of catalase activity in maize: differences between the isozymes and a possible role in the induction of chilling tolerance. Plant Sci. 163:1129–1135. doi: 10.1016/S0168-9452(02)00324-2
- Hu WH, Wu Y, Zeng JZ, He L, Zeng QM. 2010. Chill-induced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. Photosynthetica. 48:537–544. doi: 10.1007/s11099-010-0071-y
- Huang RH, Liu JH, Lu YM, Xia RX. 2008. Effect of salicylic acid on the antioxidant system in the pulp of ‘Cara cara’ navel orange (Citrus sinensis L. Osbeck) at different storage temperatures. Postharvest Biol Technol. 47:168–175. doi: 10.1016/j.postharvbio.2007.06.018
- Janda T, Szalai G, Rios-Ganzalez K, Veisz O, Paldi E. 2003. Comparative study of frost tolerance and antioxidant activity in cereals. Plant Sci. 164:301–306. doi: 10.1016/S0168-9452(02)00414-4
- Janda T, Szalai G, Tari I, Paldi E. 1999. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta. 208:175–180. doi: 10.1007/s004250050547
- Jayakannan M, Bose J, Babourina O, Rengel Z, Shabala S. 2015. Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regulat. 76:25–40. doi: 10.1007/s10725-015-0028-z
- Kadioglu A, Saruhan N, Saglam A, Terzi R, Acet T. 2011. Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regulat. 64:27–37. doi: 10.1007/s10725-010-9532-3
- Kang G, Wang C, Sun G, Wang Z. 2003. Salicylic acid changes activities of H2O2-metabolizing enzymes and increase the chilling tolerance of banana seedlings. Environ Exp Bot. 50:9–15. doi: 10.1016/S0098-8472(02)00109-0
- Kekec G, Mutlu S, Alpsoy L, Sakcali MS, Atici O. 2013. Genotoxic effects of catmint (Nepeta meyeri Benth.) essential oils on some weed and crop plants. Toxicol Ind Health. 29:504–513. doi: 10.1177/0748233712440135
- Khan MIR, Asgher M, Khan NA. 2014. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol Biochem. 80:67–74. doi: 10.1016/j.plaphy.2014.03.026
- Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. 2015. Salicylic acid-induced a biotic stress tolerance and underlying mechanismsin plants. Front Plant Sci. 6:462. doi:10.3389/fpls.2015.00462
- Khan MIR, Iqbal N, Masood A, Per TS, Khan NA. 2013. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav, 8:11 e26374. doi:10.4161/psb.26374
- Korkmaz A. 2005. Inclusion of acetyl salicylic acid and methyl jasmonate into the priming solution improves low temperature germination and emergence of sweet pepper. Hortic Sci. 40:197–200.
- Lamb CJ. 1994. Plant disease resistance genes in signal perception and transduction. Cell. 76:419–422. doi: 10.1016/0092-8674(94)90106-6
- Lei T, Feng H, Sun X, Dai QL, Zhang F, Liang HG, Lin HH. 2010. The alternative pathway in cucumber seedlings under low temperature stress was enhanced by salicylic acid. Plant Growth Regulat. 60:35–42. doi: 10.1007/s10725-009-9416-6
- Levine A, Tenhaken R, Dixon RA, Lamb CJ. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 79:583–593. doi: 10.1016/0092-8674(94)90544-4
- Lewitt J. 1980. Responses of plants to environmental stresses. New York: Academic Press.
- Li W, Ling WY, Kang L. 2005. Effect of exogenous salicylic acid on the cold resistance of the red globe grape. J Xinjiang Agric Univ. 28:51–54.
- Luo Z, Wu X, Xie Y, Chen C. 2012. Alleviation of chilling injury and browning of postharvest bamboo shoot by salicylic acid treatment. Food Chem. 131:456–461. doi: 10.1016/j.foodchem.2011.09.007
- Mahdavian K, Ghorbanli M, Kalantari KM. 2008. Role of salicylic acid in regulating ultraviolet radiation-induced oxidative stress in pepper leaves. Russ J Plant Physiol. 55:560–563. doi: 10.1134/S1021443708040195
- Minibaeva FV, Gordon LK. 2003. Superoxide production and the activity of extracellular peroxidase in plant tissues under stress conditions. Russ J Plant Physiol. 50:411–416. doi: 10.1023/A:1023842808624
- Mutlu S, Atici O. 2013. Alleviation of high salt toxicity-induced oxidative damage by salicylic acid pretreatment in two wheat cultivars. Toxicol Ind Health. 29:89–96. doi: 10.1177/0748233712451769
- Mutlu S, Atici O, Esim N, Mete E. 2011. Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in germinating seeds of various weed species. Acta Physiol Plant. 33:943–951. doi: 10.1007/s11738-010-0626-3
- Mutlu S, Atici O, Kaya Y. 2009a. Effect of cement dust on the diversity and the antioxidant enzyme activities of plants growing around a cement factory. Fresen Environ Bull. 18(10):1823–1827.
- Mutlu S, Atici O, Nalbantoglu B. 2009b. Effects of salicylic acid and salinity on apoplastic antioxidant enzymes in two wheat cultivars differing in salt tolerance. Biol Plant. 53:334–338. doi: 10.1007/s10535-009-0061-8
- Mutlu S, Karadagoglu O, Atici O, Nalbantoglu B. 2013a. Protective role of salicylic acid applied before cold stress on antioxidative system and protein patterns in barley apoplast. Biol Plant. 57:507–513. doi: 10.1007/s10535-013-0322-4
- Mutlu S, Karadagoglu O, Atici O, Tasgin E, Nalbantoglu B. 2013b. Time-dependent effect of salicylic acid on alleviating cold damage in two barley cultivars differing at cold tolerance. Turk J Bot. 37:343–349.
- Patykowski J, Urbanek H. 2003. Activity of enzymes related to H2O2 generation and metabolism in leaf apoplastic fraction of tomato leaves infected with Botrytis cinerea. J Plant Physiol. 151:153–161.
- Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Krantev AP, Szalai G, Janda T. 2009. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem. 47:224–231. doi: 10.1016/j.plaphy.2008.11.007
- Posmyk MM, Bailly C, Szafranska K, Janas KM, Corbineau F. 2005. Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedling. J Plant Physiol. 162:403–412. doi: 10.1016/j.jplph.2004.08.004
- Rao MV, Paliyath G, Ormrod P, Murr DP, Watkins CB. 1997. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Plant Physiol. 115:137–149. doi: 10.1104/pp.115.1.137
- Raskin I, Turner-Ivan M, Melander-Wayne R. 1989. Regulation of heat production in the inflorescences of an Arum lily by endogenous salicylic acid. Proc Natl Acad Sci USA. 86:2214–2218. doi: 10.1073/pnas.86.7.2214
- Rout NP, Shaw BP. 2001. Salt tolerance in aquatic macrophytes: possible involvement of the antioxidative enzymes. Plant Sci. 160:415–423. doi: 10.1016/S0168-9452(00)00406-4
- Sakhabutdinova AR, Fatkhutdinova DR, Shakirova FM. 2004. Effect of salicylic acid on the activity of antioxidant enzymes in wheat under conditions of salination. App Biochem Microbiol. 40:501–505. doi: 10.1023/B:ABIM.0000040675.29736.91
- Saruhan N, Saglam A, Kadioglu A. 2012. Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol Plant. 34:97–106. doi: 10.1007/s11738-011-0808-7
- Senaratna T, Touchell D, Bunn E, Dixon K. 2000. Acetyl salicylic acid (aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regulat. 30:157–161. doi: 10.1023/A:1006386800974
- Setia RC, Dhanda A, Setia N. 2006. Chilling tolerance and related antioxidant enzyme activities induced by salicylic acid in leaves of Brassica juncea (L.) Czern and Coss. Environ Ecol. 24:886–889.
- Shi O, Bao Z, Zhu Z, Ying O, Qian O. 2006. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regulat. 48:127–135. doi: 10.1007/s10725-005-5482-6
- Sundar D, Chaitanya KV, Jutur PP, Reddy AR. 2004. Low temperature-induced changes in antioxidative metabolism in rubber-producing shrub, guayule (Parthenium argentatum Gray). Plant Growth Regulat. 44:175–181. doi: 10.1023/B:GROW.0000049417.48603.a4
- Talieva MN, Kondrateva VV. 2002. Influence of exogenous salicylic acid on the level of phytohormones in tissues of Phlox paniculata and Phlox setacea leaves with special reference to resistance against the powdery mildew causative agent Erysiphe cichoracearum DC. f. phlogis Jacz. Biol Bull. 29:551–554. doi: 10.1023/A:1021763924798
- Tasgin E, Atici O, Nalbantoglu B, Popova LP. 2006. Effects of salicylic acid and cold treatments on protein levels and on the activities of antioxidant enzymes in the apoplast of winter wheat leaves. Phytochemistry. 67:710–715. doi: 10.1016/j.phytochem.2006.01.022
- Upadhyaya A, Sankhla D, Davis N, Sankhla N, Smith BN. 1985. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J Plant Physiol. 121:453–461. doi: 10.1016/S0176-1617(85)80081-X
- Vanacker H, Carver TLW, Foyer CH. 1998. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 117:1103–1114. doi: 10.1104/pp.117.3.1103
- Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X. 2007. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr Biol. 17:1784–1790. doi: 10.1016/j.cub.2007.09.025
- Wang DH, Li XX, Su ZK, Ren HX. 2009a. The role of salicylic acid in response of two rice cultivars to chilling stress. Biol Plant. 53:545–552. doi: 10.1007/s10535-009-0099-7
- Wang Y, Yang ZM, Zhang QF, Li JL. 2009b. Enhanced chilling tolerance in Zoysia matrella by pre-treatment with salicylic acid, calcium chloride, hydrogen peroxide or 6-benzylaminopurine. Biol Plant. 53:179–182. doi: 10.1007/s10535-009-0030-2
- Xia DX, Ping SJ, Lin S. 2007. Effects of exogenous salicylic acid and endogenous H2O2 on the physiological parameters of cucumber under cold storage. J China Agric Univ. 12:68–72.
- Xia JC, Zhao H, Liu WZ, Li LG, He YK. 2009. Role of cytokinin and salicylic acid in plant growth at low temperatures. Plant Growth Regulat. 57:211–221. doi: 10.1007/s10725-008-9338-8
- Xu S, Li JL, Zhang XQ, Wei H, Cui LJ. 2006. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot. 56:274–285. doi: 10.1016/j.envexpbot.2005.03.002
- Yalpani N, Enyedi AJ, Leon J, Raskin I. 1994. Ultraviolet light and ozone stimulates accumulation of salicylic acid, pathogen related proteins and virus resistance in tobacco. Planta. 193:372–376. doi: 10.1007/BF00201815
- Yang JH, Gao Y, Li YM, Qi XH, Zhang MF. 2008. Salicylic acid-induced enhancement of cold tolerance through activation of antioxidative capacity in watermelon. Sci Hortic. 118:200–205. doi: 10.1016/j.scienta.2008.06.015
- Zhang Q, Zhang JZ, Chow WS, Sun LL, Chen JW, Chen YJ, Peng CL. 2011a. The influence of low temperature on photosynthesis and antioxidant enzymes in sensitive banana and tolerant plantain (Musa sp.) cultivars. Photosynthetica. 49:201–208. doi: 10.1007/s11099-011-0012-4
- Zhang WP, Jiang B, Lou LN, Lu MH, Yang M, Chen JF. 2011b. Impact of salicylic acid on the antioxidant enzyme system and hydrogen peroxide production in Cucumis sativus under chilling stress. Z Naturforsch C. 66:413–422. doi: 10.5560/ZNC.2011.66c0413
