ABSTRACT
EU legislation restricted many chemicals for root-knot nematodes (Meloidogyne spp., RKNs) control. Alternative ways of plant protection were hence investigated, based on the use of microbial formulations exploiting plant parasites antagonism. RKNs are severe and widespread pests causing extensive damage to crops in greenhouse and field. Several antagonistic microorganisms are suitable for biocontrol, including the nematophagous hyphomycete Pochonia chlamydosporia that parasitizes eggs to acquire additional nourishment and face competition with other soil microorganisms. A commercial product (POCHAR) was developed by Microspore based on P. chlamydosporia with other microbial inocula that can be applied through irrigation. The aim of this study was to test POCHAR’s efficacy against RKNs on potato and its promotion effect on tomato in two field trials. Moreover, the research included trials to evaluate the best method for open-field application in crops not managed through drip irrigation, opening up the possibility to treat large areas without major technological needs. RKNs control with POCHAR represented a viable alternative to chemicals. In conclusion, the organic approach developed through the bioformulated product highlighted effective RKNs management, with a potential to sustain both plant nutrition and the related root protection needs.
Introduction
During the last decade the EU legislation restricted many chemicals and fumigants applied for control and management of root-knot nematodes (Meloidogyne spp., RKNs). The goals underpinning this trend mainly relate to the reduction in climate warming effects and to avoid soil or water contamination. The demand for alternative methods of plant defense increased, allowing the intensification of studies concerning the identification of agronomic measures and the application of microbial biocontrol formulations, exploiting the community of soil biological antagonists.
RKNs are severe, widespread pests causing extensive damage to vegetables and ornamental plants in greenhouse and field crops. However, several antagonistic microorganisms are suitable for biocontrol, including the nematophagous hyphomycetes Pochonia chlamydosporia and Arthrobotrys spp. The former is a parasitic fungus provided with enzymes adapted to digest the egg layers of different plant-parasitic nematode genera (Kerry Citation2000; Lopez-Llorca et al. Citation2010; Manzanilla-Lopez et al. Citation2013; Rosso et al. Citation2014). Furthermore, P. chlamydosporia is also a root endophyte, improving growth of a range of host plant species and sustaining their defense reaction to different pathogens (Maciá-Vicente et al. Citation2009; Ciancio et al. Citation2013). Arthrobotrys spp. are specialized nematophagous species which form traps of adhesive hyphae that capture and destroy motile nematode stages in soil. Their predatory activity aims at acquiring additional nutrients to face competition with microorganisms colonizing their same soil and rhizosphere microcosms (Persmark & Jansson Citation1997).
A number of previous studies have shown that both fungi may act as efficient biocontrol agents of nematodes (Kerry Citation2000; Manzanilla-Lopez et al. Citation2013). An industrial product based on selected isolates of P. chlamydosporia and A. oligospora (each at 108 CFU/g) that can be applied through irrigation (Pochar™, Microspore, Larino, Italy) was developed and marketed in Italy. It aims at amending soil by introducing useful fungi that stabilize the RKNs population dynamics to restore the soil ecological balance, in particular in conditions in which the microflora has been affected by applications of fumigants or other sterilizing agents. Moreover, inocula of the mycorrhiza Glomus sp. (0.1 mg/kg) and of plant growth-promoting rhizobacteria (Bacillus spp., 10 CFU/mg) are also present in the formulation. The aim of this study was to test effectiveness of Pochar against RKNs attacking potato and the P. chlamydosporia growth effects on non-parasitized tomato plants, in order to evaluate the best conditions for its open-field applications.
Materials and methods
The formulation was tested in two open-field trials, the first one on a potato crop with sprinkler irrigation and the second on an uninfested tomato crop. The first trial was carried out in a farm with a sandy soil and a previous history of high levels of RKN (M. incognita) infestations, located at Bosco Mesola (Ferrara, Italy). The test parcels had a uniform water and soil profile, and were sown with commercial potato var. Hermes. The presence of the RKN population was identified by examination of females. It was the only significant plant-parasitic nematode present in soil before planting, at very low J2 or eggs density levels. The trial was set up in two adjacent strips, with six plots of 900 m2, of which three replicated plots were used for treatment with Pochar, leaving three other plots as control, without any application. A randomized block design was used. The experimental treatments, as indicated by the manufacturer, consisted of two applications, initially adding the product at sowing, the last week of March 2014 by drilling, using a liquid distributor added to the seed drill. For the second application, performed at first sprouts emergence six weeks later, the product was applied to leaves or soil with a boom sprayer, followed by irrigation. The monthly volume of irrigation water ranged from 700 to 1100 mc/Ha, mainly concentrated in the period May–July. The product was mixed with a fertilizer (Nutryaction®, N = 1%, organic C = 40%) and tap water (5 L) and left for 12 hours prior spraying. The infestation levels were assessed twice (9 June and 25 July 2014) for each parcel and treatment. At each sampling 30 sub-samples per plot were randomly selected. The samples were analyzed by extracting second stage juveniles (J2) of M. incognita from soil with wool-paper filtering, for subsequent counting with a stereoscope in the suspension obtained. At harvesting (18 August 2014), the tubers were inspected, randomly collecting 100 samples for each plot, counting those with clear symptoms of nematode attacks, as well as the healthy ones.
For the tomato experiment, a field trial was performed in spring 2013 on tomato cv Regina, in a field located at Fasano (Brindisi, Italy), to check the growth promotion effects of the same P. chlamydosporia isolate (DSM 26985) present in Pochar, in the absence of RKNs. The soil was cultivated with tomato in the previous seasons and no nematode attack was reported. It was, however, checked to confirm the absence of RKNs at the end of the trial, by examining tomato roots for galls and by extracting nematodes from 20 soil samples. The Cobb’s sieving and decanting technique was used, followed by examination at 50× with transmission light microscopy of the suspension obtained. The roots of tomato plants collected in the preceding crop were checked for P. chlamydosporia and other nematophagous fungi in vitro on 1.0% water agar, and no species of interest were found, apart of a non-pathogenic Fusarium solani.
The plants were inoculated at transplant with 10 g of dry chlamydospores of DSM 26985 plus substrate with mycelial matter, introduced in three points around the plant base, corresponding to a dose of 5 × 103 chlamydospores · g−1 of soil and a total of 3 × 106 chlamydospores per plant. For treatment A the fungus was added at transplant only. The second treatment B was as A, but with further chlamydospores additions repeated every 15 days. Since a fertilization effect may be produced by adding organic fungal mass to soil, the control C was set as B, but with the same product autoclaved. To check for the effect of the applications frequencies, a further treatment D was considered, as B but with the fungus addition performed every 30 days. Data from both trials were analyzed with analysis of variance or Student’s t-test.
Results
In the first sampling on potato the J2 densities were undetected in late spring either in treated or untreated plots. However, the M. incognita J2 were found in the second late summer sampling, with a significant reduction of nematodes (F = 78.4, P = .02) in the treated plots, compared to the untreated controls (Figure (a)). The damage assessment on harvested tubers confirmed this result, since a higher proportion of galls and histological damages were scored on tubers collected from untreated plots. Galled tubers ranged from 5–24 in untreated plots, to 0–4 in those treated. On average, an almost 10-fold and significantly higher (F = 41.7, P = .04) percent of tubers resulted damaged and were not commercially suitable in the untreated plots, compared to those in the treated parcels (Figure (b)). The local temperature and relative humidity data for the period March–August 2014 are given in Figure .
Figure 1. Effect of treatments with P. chlamydosporia on the densities of M. incognita in soil (a) and percent of damaged tubers at harvest (b).
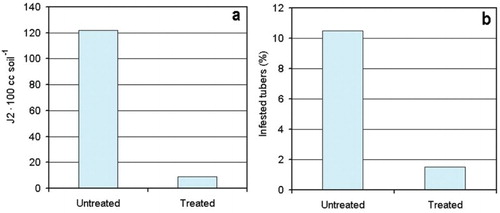
Figure 2. Relative humidity (a) and mean, maximum and minimum temperatures (b) registered at the station closest to the Ferrara field trial (March–August 2014).
For the second field trial carried out on tomato at Fasano, the temperature and relative humidity data for the period March–June 2013 are given in Figure . The same P. chlamydosporia isolate that was present in Pochar showed a promotion effect of the fungus on the plant growth, likely related to its capacity to nourish roots as an endophyte and to activate plant defense.
Figure 3. Relative humidity (a) and mean, maximum and minimum temperatures (b) registered at the station closest to the Fasano field trial (March–June 2013).
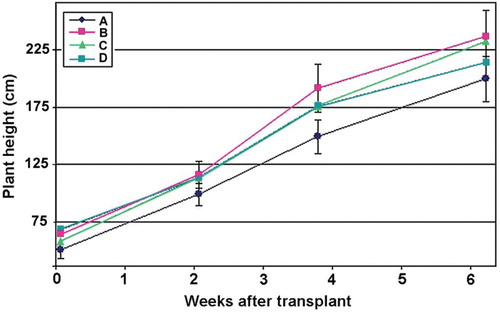
The fungus addition at 15 days intervals showed the highest increase in plants heights, significantly higher than the single application (Figure ).
Figure 4. Effect of treatments with P. chlamydosporia on tomato plants after transplant. For treatments description see text. Vertical bars = SE.
The fertilization effect induced by the organic matter added with the treatments was evident when considering the plants and roots weights in the treatment with the autoclaved product (Figure (a)).
Figure 5. Effect of P. chlamydosporia on the tomato dry plant and root weights (a), fruit weight (b), number of fruits (c) and average fruit weight (d) at harvest. For treatments description see text. Vertical bars = SE.
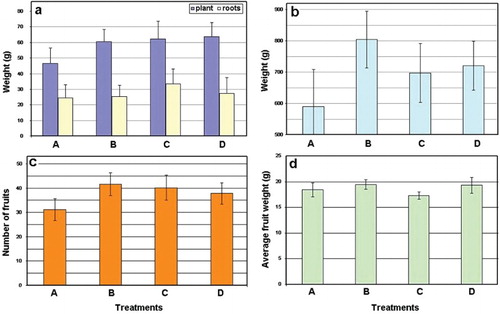
Treatment with the fungus added at 15 days intervals showed the highest fruit weights and numbers (Figure (b) and 5(c)) significantly different from the addition at transplant only (treatments A) (P = .0428), with a higher average fruit weight when compared to the autoclaved control (Figure (d)).
Conclusions
The use of Pochar in open-field conditions for RKNs control on potato appeared effective in controlling RKN. Cooler climate and higher rainfalls, however, induced a delay in nematode infestations. RKN control was effective in the late season when nematode populations reached their peak (from June onwards), as few juveniles were found during the early stages of the production cycle. Later analyses (data not shown) indicated lower densities of adult nematodes in roots from the treated plots, with a reduced incidence of galls on tubers at harvest.
In addition to the effectiveness of the product, the field trial showed that the application method in the open field, with soil/leaf treatments followed by irrigation, was effective and allowed fungus survival. This practice may be suitable in field crops that are not subject to drip irrigation, thus enabling the fungus application in large areas without additional technology with simple spraying equipment needed. The control of nematodes with Pochar appears as a viable alternative to chemicals and provides an additional useful aid to agronomic practices, enabling the management of RKN infestations in potato in integrated or organic farming. The fungus sustained growth of tomato plants when added at 15 days intervals, with significantly higher fruit weights for treatment B, when compared to the single transplant application (A). The autoclaved product showed a fruit weight increase, less pronounced than treatment B, indicating the presence also of an effect of the biomass introduced in soil.
In conclusion, field applications showed a potential of the fungi tested on potato and tomato, confirming the effects already reported for P. chlamydosporia on barley and other crops (Kerry Citation2000; Lopez-Llorca et al. Citation2010; Manzanilla-Lopez et al. Citation2013). Application via irrigation appears feasible and capable to maintain the fungus viability. The organic approach developed through the bio-formulation highlights an effective RKNs bio-management potential, sustaining either plant nutrition and root protection needs.
Acknowledgements
We gratefully thank APPE (Emilia Potato Producers Association, Ferrara, Italy) for the assistance provided in the field trials. AC and MC acknowledge Mr N. Salatino for the technical assistance provided with the field sampling.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ciancio A, Colagiero M, Ferrara M, Nigro F, Pentimone I, Rosso LC. 2013. Transcriptome changes in tomato roots during colonization by the endophytic fungus Pochonia chlamydosporia. Abstr. 5th Congress, Federation of the European Microbiologists Societies (FEMS), July 21–25, 2013, Leipzig, Germany.
- Kerry BR. 2000. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu Rev Phytopathol. 38:423–424. doi: 10.1146/annurev.phyto.38.1.423
- Lopez-Llorca LV, Gómez-Vidal S, Monfort E, Larriba E, Casado-Vela J, Elortza F, Jansson HB, Salinas J, Martín-Nieto J. 2010. Expression of serine proteases in egg-parasitic nematophagous fungi during barley root colonization. Fungal Genet Biol 47:342–351. doi: 10.1016/j.fgb.2010.01.004
- Maciá-Vicente J, Rosso LC, Ciancio A, Jansson H-B, Lopez-Llorca LV. 2009. Colonisation of barley roots by endophytic Fusarium equiseti and Pochonia chlamydosporia: effects on plant growth and disease. Ann Appl Biol. 155:391–401. doi: 10.1111/j.1744-7348.2009.00352.x
- Manzanilla-Lopez RH, Esteves I, Finetti-Sialer MM, Hirsch PR, Ward E, Devonshire J, Hidalgo-Diaz L. 2013. Pochonia chlamydosporia: advances and challenges to improve its performance as a biological control agent of sedentary endo-parasitic nematodes. J Nematol. 45:1–7.
- Persmark L, Jansson HB. 1997. Nematophagous fungi in the rhizosphere of agricultural crops. FEMS Microbiol Ecol. 22:303–312. doi: 10.1111/j.1574-6941.1997.tb00382.x
- Rosso LC, Colagiero M, Salatino N, Ciancio A. 2014. Effect of trophic conditions on gene expression of Pochonia chlamydosporia. Ann Appl Biol. 164:232–243. doi: 10.1111/aab.12099
