ABSTRACT
Cold active lipases (CLPs) are gaining importance nowadays as they are increasingly used in fine chemical synthesis, bioremediation, food processing and as detergent additive. These enzymes exhibit high catalytic activity at low temperatures and flexibility to act at low water medium. Since they are active at low temperatures consume less energy and also stabilize fragile compounds in the reaction medium. CLPs are commonly obtained from psychrophilic microorganisms which thrive in cold habitats. Compared to mesophilic and thermophilic lipases, only a few CLPs were studied and industrially exploited so far. CLPs (C. antarctica lipase-A and C. antarctica lipase-B) from Candida antarctica isolated from Antarctic region are the well studied and industrially employed, and many are being followed up. This review updates the CLPs reported recently and the industrial applications of CLPs.
Introduction
Microorganisms are capable of growing in unusual environmental conditions, such as high temperatures of volcanic hot springs, low temperatures of polar regions, high pressures of deep seas, very high salt concentrations, and very high and low pH values (Fujiwara Citation2002). Microbes growing at temperatures below 20°C and above 45°C are classified into extremophiles and categorized as psychrophiles and thermophiles, respectively (Cavicchioli et al. Citation2002). Psychrophiles are one of the most underutilized resources in the world. In order to thrive at low temperatures, psychrophiles possess enzymes that have a high specific activity at low temperatures and are collectively termed as cold active enzymes. For several industrial applications cold active enzymes provide economic and ecological advantages over their counterpart which operate at higher temperatures (Ohgiya et al. Citation1999; Marchi et al. Citation2007).
Lipases (EC 3.1.1.3: Triacylglycerol acyl hydrolases) constitute the third most important category of enzymes, next to carbohydrases and proteases. They are unique in hydrolysing and synthesizing fatty acid esters in aqueous and non-aqueous media. Cold active lipases (CLPs) demonstrate high specific activity in the temperature range of 0−30°C (Feller et al. Citation1996). These lipases have developed specific structural features which provide them flexibility around the active site. Consequently they display low enthalpy, low affinity towards substrates and high specific activity at low temperatures (Joseph et al. Citation2008).
In industries, enzymes are steadily replacing chemical reactions since they are greener in approach. Enzymes produce fewer by-products, consume less energy, reduce environmental pollution and add improved value to the products. Consequently, it is not surprising to notice the blooming global enzyme market despite the economic slowdown. According to recent BCC research (a leading market research company) conducted in 2014, the global market for industrial enzymes is expected to reach $7.1 billion by 2018, registering a five-year compound annual growth rate (CAGR) of 8.2% (BCC research 2014, in report BIO030H – Global markets for enzymes in industrial applications). The global market size of lipases in particular is projected to reach $590.5 million by 2020, at a CAGR of 6.5% between 2015 and 2020 (Research and Markets 2015, in report – Lipase market by source, application and geography – Global forecasts to 2020 for the $590.5 million industry).
The research on cold active lipolytic enzymes is gaining importance, as many articles were published recently. Most of these studies were focused on isolation, purification and characterization of the enzymes from various sources; a few were on isolation of lipase gene, cloning and expression in heterologous hosts and some on sequencing of the protein (Joseph et al. Citation2008). Commercial applications of these lipases are based on their high catalytic activity at low temperatures and they are employed in such industries as detergent, leather, food, pharmaceuticals, fine chemical synthesis and bioremediation (Joseph et al. Citation2008). One of the key features of these enzymes is consumption of less energy due to low working temperatures.
Several of the cold-adapted lipases studied so far were from psychrotrophic and psychrophilic microorganisms isolated from Antarctic and polar regions, deep-sea environments and refrigerated food samples (Dieckelmann et al. Citation1998; Xiang et al. Citation2004; de Maria et al. Citation2005; Jinwei et al. Citation2007). But these enzymes are unstable even at moderate temperatures, as they are adapted to act at low temperatures (Joseph et al. Citation2008). Their thermal stability could be increased through immobilization, directed evolution, protein engineering and chemical modification by adding polysaccharides (Zhang Citation2003; Siddiqui & Cavicchioli Citation2005; Lafranconi et al. Citation2008). CLPs obtained from microbes native of the tropical region exhibit good thermal stability than their counterparts from the alpine region (Cai et al. Citation2009; Kavitha & Shanthi Citation2013). Therefore they are better choice for industrial applications.
The steadily growing interest in microbial CLPs is reflected by an increasing number of articles published. This review aimed to update the more recent studies carried out on microbial CLPs.
Structural features and cold adaption
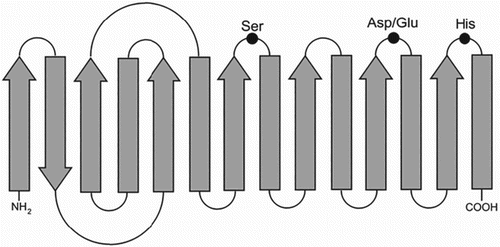
Like all other lipases, CLPs also possess the canonical α/β hydrolase fold (Ollis et al. Citation1992) (). The active site contains the catalytic triad, Ser105 (nucleophile)-His224 (basic residue)-Asp/Glu187 (acidic residue) (Ollis et al. Citation1992). In almost all lipases, the active site is covered by a lid which opens in the presence of an interface to facilitate contact with substrate (Grochulski et al. Citation1994; Cygler & Schrag Citation1997).
CLPs are structurally modified to have high flexibility to accommodate substrates at low temperatures. The correlation between the molecular structure and cold adaption of CLPs is elucidated by comparing with mesophilic and thermophilic lipases using site-directed mutagenesis and crystal studies (Narinx et al. Citation1997; Wintrode et al. Citation2000). The molecular structure of lipase from Pseudomonas immobilis and Pseudomonas fragi IFO 3458 when compared with their mesophilic counterpart revealed the features for cold adaption (Arpigny et al. Citation1997; Alquati et al. Citation2002), which are very low content of arginine residues in comparison to lysine residues, low content of proline residues, weak hydrophobic core, very less number of salt bridges and very less number of aromatic–aromatic interactions (Arpigny et al. Citation1997; Gerday et al. Citation1997).
Distribution of arginine residues of cold active enzymes is different from that of mesophilic enzymes. A few of them stabilizes at intramolecular salt bridges and a many of them occupy the surface and may confer the conformational flexibility (Alquati et al. Citation2002; Siddiqui & Cavicchioli Citation2006; Feller Citation2013). Additional structural features responsible for cold adaption are high content and aggregation of glycine residues (for local mobility), low content of ion pairs and weak charge-dipole interactions in α helices (Georlette et al. Citation2004; Gomes & Steiner Citation2004). Increased flexibility of CLPs may be correlated with their ease in accommodating substrates at low temperatures (Joseph et al. Citation2008; de Pascale et al. Citation2012) and their ability to catalyze hydrolysis in low water medium (Tutino et al. Citation2009).
Another feature associated with cold adaption is the production of trehalose and exopolysaccharides which act as a cryoprotectant to prevent precipitation and denaturation of proteins, including the cold active enzymes (Krembs et al. Citation2002; Ewert & Deming Citation2013). A shift in the acyl chain length specificity and enhanced thermostability of the enzyme when glycine was substituted with proline was reported (Kulakovaa et al. Citation2004). A mutation introduced in the lid region of catalytic triad of CLPs from P. fragi improved substrate selectivity and thermostability (Santarossa et al. Citation2005). The catalytic portion of the cold active lipase is observed to possess high plasticity responsible for low activation energy and low thermal stability and the same is conferred by several structural adaptations discussed above.
Recently, modern techniques are increasingly employed to understand the structure–function relationship of CLPs. Gene cloning in heterologous overexpressing hosts (Parra et al. Citation2008; Xuezheng et al. Citation2010; Novototskaya-Vlasova et al. Citation2013), crystal studies (Uppenberg et al. Citation1995; Juhl et al. Citation2010; Durmaz et al. Citation2013) and molecular modelling studies (Maraite et al. Citation2013; Mohamad Ali et al. Citation2013) strongly supports the structural attributes of cold-adapted lipases for cold habitation discussed above.
Thermal stability
As the CLPs are structurally modified for cold adaption, they are inherently heat unstable and undergo rapid inactivation at moderate and even low temperatures (Georlette et al. Citation2004; Siddiqui & Cavicchioli Citation2006). Thermal denaturation is a common cause of enzyme inactivation in industrial applications; therefore an industrial enzyme necessarily needs to be thermostable (Ruslan et al. Citation2012). The psychrophilic yeast, Candida antarctica expresses two lipases, namely C. antarctica lipase-A and C. antarctica lipase-B (CAL-A and CAL-B) with different physiochemical properties. The most surprising aspect of CAL-A is its high thermostability. Till date it is considered to be the most thermostable lipase known which is capable of active at >90°C (de Maria et al. Citation2005). CAL-B is less thermostable and smaller in size than CAL-A (Patkar et al. Citation1993).
Several strategies have been used to improve the thermostability of CLPs. They are enzymatic or chemical modifications, use of additives, immobilization, directed evolution and protein engineering (Zhang Citation2003; Eijsink et al. Citation2004; Siddiqui & Cavicchioli Citation2005; Lafranconi et al. Citation2008). Directed evolution with random mutagenesis based on error-prone PCR (ep-PCR) and iterative saturation mutagenesis guided by rational design are more frequently employed nowadays to enhance the thermostability (Bassegoda et al. Citation2012). The factors commonly considered to increase thermal stability are the hydrophobicity, number of hydrogen bonds, amino acid composition, amino acid distribution and interactions in the protein (Vielle & Zeikus Citation2001). The structural features of thermophilic and mesophilic lipases are compared with that of CLPs in order to arrive for stabilizing mutations (Suhre & Claverie Citation2003). But it was very hard to correlate thermostability with specific amino acids interactions (Nawani & Kaur Citation2007). Therefore directed evolution through random mutagenesis and tedious and time-consuming screening for maximum thermostability had been more effective (Eijsink et al. Citation2004; Jaeger & Eggert Citation2004; Reyes-Duarte et al. Citation2005).
Improved variant of Lip-A from Bacillus subtilis after ep-PCR exhibited an increase of 15°C in the melting temperature and 20°C in optimum temperature compared to wild-type lipase (Ahmad et al. Citation2008). In the case of cold active lipase from P. fragi, a variant obtained after two rounds of evolution displayed a fivefold increase in half-life at 42°C and a shift of 10°C in the temperature optimum (Lafranconi et al. Citation2008). Directed evolution was applied to generate two mutants of CAL-B with >20-fold increase in half-life at 70°C (Zhang et al. Citation2003).
Saturation mutagenesis combined with B-factor criterion (B-FITTER) to target the amino acid positions to modify (Reetz et al. Citation2006), several mutants with enhanced thermostability were developed. Two thermostable variants of Lip-A from B. subtilis (Reetz & Carballeira Citation2007), a double mutant from Rhizomucor miehei lipase (Zhang et al. Citation2012) and a mutant of Lip-C from Pseudomonas sp. 42A2 (Cesarini et al. Citation2012), are a few to specify.
The underlying mechanism of thermostabilization in directed evolution was studied in Pseudomonas aeruginosa lipase using circular dichroism spectroscopy which revealed that the secondary structure was retained in mutant up to 70–80°C, whereas the wild-type protein structure was completely distorted above 35°C (Sharma et al. Citation2012). In another study using circular dichroism, X-ray structure analysis and nuclear magnetic resonance spectroscopy on B. subtilis Lip-A, it was observed that mutation of surface residues hinder the tendency of Lip-A to undergo precipitation under thermal stress (Augustyniak et al. Citation2012).
The protein engineering strategy was adapted to enhance the thermostability where the disulphide and other bonds are modified to decrease the entropy of the unfolded form of proteins or to decrease the unfolding rate of irreversibly denatured proteins (Siadat et al. Citation2006). CAL-B and Geobacillus zalihae T1 lipase were successfully engineered by mutating five amino acid pairs to cysteine and by introducing an ion-pair in the inter-loop (Le et al. Citation2012; Ruslan et al. Citation2012).
Sources of CLPs
CLPs are generally present in psychrophillic and psychrotrophic microorganisms capable of surviving at low temperatures close to 5°C. A number of cold active lipase-producing microorganisms are reported so far, but only a few bacteria and yeast were commercially exploited. Most of them were isolated from Antarctic and polar regions that exhibit constant cold habitat at 0 ± 2°C. The bacteria from these regions known to produce cold active lipase include, Moraxella sp. TA144 (Feller et al. Citation1991), Psychrobacter immobilis B10 (Arpigny et al. Citation1997), Psychrobacter sp. Ant300 (Kulakovaa et al. Citation2004), Psychrobacter sp. 7195 (Jinwei et al. Citation2007) and Pseudoalteromonas haloplanktis TAC125 (de Pascale et al. Citation2008). More recently, Halomonas sp. BRI 8 was reported to be isolated from Antarctic seawater sample (Jadhav et al. Citation2013). Apart from Antarctic and polar regions, the other cold regions such as glaciers and high mountain tops also harbour cold active lipase-producing microorganisms. Microbacterium luteolum and Bacillus sphaericus MTCC 7526 were isolated from the Gangothri glacier of western Himalayas (Joseph et al. Citation2012; Joseph & Ramteke Citation2013). Acinetobacter sp. 6 and Psychrobacter cryohalolentis K5T were isolated from Siberian tundra soil and cryopeg, respectively (Suzuki et al. Citation2001; Novototskaya-Vlasova et al. Citation2013). Pseudomonas sp. B11-1 isolated from Alaskan soil was documented to cold active lipase (Choo et al. Citation1998).
The next major source for cold active lipase-producing bacteria is deep-sea and marine environment. Aeromonas sp. LPB4 (Lee et al. Citation2003), Pseudoalteromonas sp. wp27 and Psychrobacter sp. wp37 (Zeng et al. Citation2004) were from deep-sea sediments where temperature is below 3°C. Aeromonas hydrophila and Pseudomonas sp. MSI057 were isolated from marine environment and marine sponge, respectively (Pemberton et al. Citation1997; Kiran et al. Citation2008). Recently, Janibacter sp. HTCC2649 isolated from marine environment was described to produce cold active lipase (Yuan et al. Citation2014). Constant refrigeration of food stuff results in evolution of psychrotolerant microorganisms producing cold active enzymes. Pseudomonas fluorescens (Dieckelmann et al. Citation1998) and Staphylococcus epidermidis (Joseph et al. Citation2006) were isolated from refrigerated milk and fish samples.
Apart from bacteria, psychrophilic fungi and yeast were also reported to produce cold active lipase. C. antarctica isolated from Antarctic habitat is a well-known source for two industrially important CLPs, CAL-A and CAL-B (de Maria et al. Citation2005). Other fungi and yeast reported to produce cold active lipase include Candida lipolytica, Geotrichum candidum and Pencillium roqueforti isolated from frozen food samples (Alford & Pierce Citation1961).
One of the major advantages of these enzymes is consumption of less energy as they act at low working temperatures. But several of these enzymes are naturally not thermostable to withstand the temperatures of tropical and temperate climates in order to be exploited for industrial applications. Their thermal stability can be increased by immobilization, directed evolution, protein engineering and chemical modifications (Zhang et al. Citation2003; Siddiqui & Cavicchioli Citation2005; Joseph et al. Citation2008; Lafranconi et al. Citation2008). CLPs obtained from microbes native of tropical region generally exhibit good thermal stability. So far there are only two reports on such organisms. Geotrichum sp., a mesophilic yeast and Pseudomonas sp. VITCLP4, a mesophilic bacterium isolated from subtropical region and tropical seacoast, respectively, were documented to produce CLPs (Cai et al. Citation2009; Kavitha & Shanthi Citation2013). A comprehensive list of various cold active lipase-producing microorganisms reported recently is presented in .
Table 1. Microorganisms producing cold active lipase.
As previously mentioned, the major setback for industrial exploitation of CLPs is their thermoinstability. Therefore attempts have been made to screen mesophilic organisms for CLPs since they are expected to be inherently stable at moderate to high temperatures. Geotrichum sp., mesophilic yeast isolated from subtropical region was reported to produce two CLPs stable at room temperature (Cai et al. Citation2009).
Industrial applications of CLPs
CLPs are structurally modified to accommodate substrates at low temperatures. One of the key features of these enzymes is consumption of less energy due to low working temperatures. They have true enzyme potentialities for various industrial applications, such as leather processing, medical and pharmaceutical preparations, fine chemical synthesis, detergent additive, food processing, environmental bioremediation, biotransformation, preparation of cosmetics and gene expression in heterologous hosts to block inclusion bodies (Feller et al. Citation1996). A list of industrial applications of CLPs is presented in . CLPs are advantageous because they are active under low water conditions due to inherent greater flexibility, whereas mesophilic and thermophilic enzymes show higher rigidity. The other advantages include low cost in production, wide variety, stability to organic solvents, specificity in action, mild reaction condition and low energy consumption.
Table 2. Industrial applications of cold active lipases.
Applications in the detergent industry
The detergent industry is the largest market for industrial enzymes (Ahuja et al. Citation2004). Lipases improve the washing capability of detergents towards the fatty food stains from fabrics which are difficult to go off during normal washing conditions (Andree et al. Citation1980). An ideal detergent enzyme should be stable at alkaline pH and active in the presence of surfactants (Jurado et al. Citation2007). It should withstand oxidizing and chelating agents, which are used in detergents as active oxygen bleach and builder (Wang et al. Citation1995). The enzyme also needs to be effective at lower concentration and have broad substrate specificity (Wang et al. Citation1995).
Traditionally cloths are washed in hot and warm water. Increasing use of synthetic fibres which cannot tolerate temperatures above 50−60°C and the energy crisis has changed the washing habits towards lower washing temperatures of 30−40°C (Nielsen et al. Citation1981). The use of cold-adapted lipase in detergents would be of great advantage since they specifically allow washing under cold conditions which in turn decrease energy utilization and wear and tear of cloth fibres (Feller & Gerday Citation2003). The other advantages include reduced environmental load of detergent products, reduced use of chemicals in detergents, biodegradable, no negative impact on disposal of domestic waste and no risk for aquatic organisms.
Recombinant cold active lipase from C. antarctica is successfully used in detergent formulation (Uppenberg et al. Citation1994b). CLPs from Microbacterium phyllosphaerae and B. sphaericus were reported to be efficient in the removal of lipid stains in the presence of commercial detergents from fabrics (Joseph & Ramteke Citation2013). Lipomax and Lumafest (Genencor International) contain lipase from Pseudomonas sp. (Jaeger & Reetz Citation1998). Incorporation of enzymes in detergent formulations makes them eco-friendly and also reduces laundering temperatures to warm and cold, saving energy (Hemachander & Puvanakrishnan Citation2000).
Medical and pharmaceutical applications
Cold-adapted lipases are used in the manufacture of single-isomer chiral drugs (Gotor-Fernandez et al. Citation2006a). Cold active lipase from C. antarctica or Pseudomonas sp. is used to act on stereospecific N-acylamines to form optically active amines as intermediates in the preparation of pharmaceuticals (Smidt et al. Citation1996). CAL-B is widely employed in the manufacture and segregation of a large number of nitrogenated compounds intended to be used in the synthesis of pharmaceuticals (Gotor-Fernandez et al. Citation2006b).
Fine chemical synthesis
Some fine chemicals synthesized from fats and oils by chemical processes can be synthesized using CLPs with good specificity in milder reaction conditions (Sih & Wu Citation1989; Vulfson Citation1994). Kinetics of acyl transfer reactions in organic media catalysed by CAL-B was reported by Martinelle and Hult (Citation1995). Anderson et al. (Citation1998) demonstrated the applications of CAL-B in organic synthesis. CAL-B was also used in the synthesis of optically active alcohols (Rotticci et al. Citation2001). Gustavsson et al. (Citation2004) applied CAL-B to catalyse ring opening polymerization of epsilon-caprolactone in proximity to cellulose fibre surface. Ong et al. (Citation2006) demonstrated the improved performance of free CAL-B in a mixed solvent system for enantioselective esterification of (R)-ketoprofen, leaving the target product (S)-ketoprofen in the unreacted state.
Highly thermostable CAL-A exhibits chemo selectivity for amine groups; it was used in the asymmetric synthesis of amino acids/amino esters (de Maria et al. Citation2005). CAL-B was successfully used in the ethyl esterification of docosahexaenoic acid to form ethyl docosahexaenoate in an organic solvent-free system (Shimada et al. Citation2001). Tan et al. (Citation1996) reported the use of cold active lipase from P. fluorescens P38 in the synthesis of butyl caprylate in n-heptane at low temperatures.
Applications in the food industry
In order to preserve the heat-sensitive micro- and macronutrients present in food ingredients, food industries prefer reactions that occur at low temperatures. Thus cold active enzymes are widely employed in food industries in place of traditional chemical processes. Tan et al. (Citation1996) reported the use of cold active lipase from P. fluorescens P38 in the synthesis of the flavouring compound, butyl caprylate in n-heptane at low temperatures. Lipases from C. antarctica (CAL-B), Candida cylindracea AY30, Hansinuela lanuginosa, Pseudomonas sp. and G. candidum were used in the esterification of functionalized phenols to form antioxidants in order to be used in sunflower oil (Buisman et al. Citation1998; Pandey et al. Citation1999).
Environmental applications
Biodegradation of petroleum hydrocarbons in cold environments including Alpine soils is due to indigenous cold-adapted microorganisms which are able to degrade these contaminants by producing cold active enzymes. CLPs have great potential in the field of wastewater treatment, bioremediation in oil-contaminated cold environment and active compounds synthesis in the cold condition (Buchon et al. Citation2000). Suzuki et al. (Citation2001) reported a cold active lipase from a psychrotroph, Acinetobacter sp. six efficiently hydrolysed triglycerides in soybean oil at 4°C and has the potential for in situ bioremediation or bioaugmentation of oil-contaminated cold environments. Belousova and Shkidchenko (Citation2004) reported the isolation of 30 strains capable of oil degradation at 4–6°C.
Applications in the leather industry
Among the end users of technical enzymes, the leather industry is on top (Sarrouh et al. Citation2012). This is due to environmental pollution by the chemicals used and implementation of global environmental regulations. Pre-tanning and tanning steps contribute to 80–90% of the total pollution (Thanikaivelan et al. Citation2004). With respect to biological oxygen demand, chemical oxygen demand and total dissolved solids (TDS), approximately 70% of the pollutants are generated from pre-tanning operations (Ramasami et al. Citation1999). Dehairing and chrome-tanning steps result in the release of hydrogen sulphide and chromium plus sulphate ions, respectively (Rao et al. Citation1997; Marsal et al. Citation1999). Degreasing leads to discharge of solvents and surfactants (Christner Citation1992).
In leather processing, lipases are used in the removal of natural fat present in animal skin. A separate degreasing step is required for animal skins with high fat content. Fat is present in sebaceous gland, hair follicle, between collagen fibres and connective tissue fibres in subcutaneous layer of the skin. The level of fat in skin varies with such factors as breed, age and sex. It is approximately 2−4% in cattle, 12−15% in goat and 30% in sheep skin and mainly comprises 56% triglycerol, 23% glycerol, 6% phospholipid, 5% cholesterol and 10% fatty acids (Christner Citation1992).
Insufficient removal of natural fat during processing prevents the chemicals from penetrating into the leather which leads to negative impacts on the quality of finished leather, such as hardness without sufficient internal softness, fatty spew formation, stained appearance due to chrome soap formation, weak bonding of the finishing layer and bad odour. Traditionally excess fat was removed using solvents and emulsifiers but they add to pollution. Alternatively, lipases of microbial origin can be used in degreasing to reduce pollution. Research on the use of lipase for degreasing dated a few decades back. Use of acid lipase of fungal origin in degreasing on pickled pelts was reported in 1978 (Yeshodha et al. Citation1978). Acid lipase from Rhizopus nodosus along with commercial degreaser was used in degreasing in 1982 (Muthukumaran & Dhar Citation1982). Recently, with the availability of commercial lipases, the effectiveness of acid and alkaline lipases of commercial origin in degreasing at various stages of leather processing was also reported (Afsar & Cetinkaya Citation2008).
Conclusions and future prospects
Cold active enzymes are now available for various commercial exploitations, such as organic synthesis, bioremediation, leather processing, biotransformation and biocatalysis. The advantage of CLPs is that they are active under low water conditions due to inherent greater flexibility, whereas mesophilic and thermophilic enzymes show higher rigidity, low cost in production, wide variety, stability to organic solvents, specificity in action, mild reaction condition and low energy consumption. Therefore CLPs are very promising enzymes to replace the conventional enzyme processes of the biotechnological industries. But the specific effort needs to be taken to overcome certain aspects, such as low stability and low biodiversity. With the availability of newer techniques, such as site-directed mutagenesis and metagenomics, it is highly possible to come up with many tailor-made microbial CLPs from diverse sources for several industrial and biotechnological applications.
Disclosure statement
No potential conflict of interest was reported by the author.
ORCiD
M. Kavitha http://orcid.org/0000-0002-1862-8037
References
- Abdou AM. 2003. Purification and partial characterization of psychrotrophic Serratia marcescens lipase. J Dairy Sci. 86:127–132. doi: 10.3168/jds.S0022-0302(03)73591-7
- Afsar A, Cetinkaya F. 2008. A research on increasing the effectiveness of degreasing process by using enzymes. Tekstil Ve Konfeksiyon. 18:278–283.
- Ahmad S, Kamal MZ, Sankaranarayanan R, Rao NM. 2008. Thermostable Bacillus subtilis lipases: in vitro evolution and structural insight. J Mol Biol. 381:324–340. doi: 10.1016/j.jmb.2008.05.063
- Ahuja SK, Ferreira GM, Moreira AR. 2004. Utilization of enzyme for environmental applications. Crit Rev Biotechnol. 24:125–154. doi: 10.1080/07388550490493726
- Alford JA, Pierce DA. 1961. Lipolytic activity of microorganisms at low and intermediate temperatures. III. Activity of microbial lipases at temperatures below 0°C. J Food Sci. 26:518–524. doi: 10.1111/j.1365-2621.1961.tb00399.x
- Ali MSM, Fuzi SFM, Ganasen M, Rahman RNZRA, Basri M, Salleh AB. 2013. Structural adaptation of cold-active RTX lipase from Pseudomonas sp. strain AMS8 revealed via homology and molecular dynamics simulation approaches. Biomed Res Int. 2013:925373. doi: 10.1155/2013/134914.
- Alquati C, De Gioia L, Santarossa G, Alberghina L, Fantucci P, Lotti M. 2002. The cold-active lipase of Pseudomonas fragi. Heterologous expression, biochemical characterization and molecular modeling. Eur J Biochem. 269:3321–3328. doi: 10.1046/j.1432-1033.2002.03012.x
- Anderson EM, Larsson KM, Kirk O. 1998. One biocatalyst – many applications: the use of Candida antarctica B-lipase in organic synthesis. Biocatal Biotransfor. 16:181–204. doi: 10.3109/10242429809003198
- Andree H, Muller WR, Schmid RD. 1980. Lipases as detergent components. J Appl Biochem. 2:218–219.
- Arpigny JL, Lamotte J, Gerday C. 1997. Molecular adaptation to cold of an Antarctic bacterial lipase. J Mol Catal, B Enzym. 3:29–35. doi: 10.1016/S1381-1177(96)00041-0
- Augustyniak W, Brzezinska AA, Pijning T, Wienk H, Boelens R, Dijkstra BW, Reetz MT. 2012. Biophysical characterization of mutants of Bacillus subtilis lipase evolved for thermostability: factors contributing to increased activity retention. Protein Sci. 21:487–497. doi: 10.1002/pro.2031
- Bassegoda A, Cesarini S, Diaz P. 2012. Lipase improvement: goals and strategies. Comput Struct Biotechnol J. 2:1–8. doi: 10.5936/csbj.201209005
- Belousova NI, Shkidchenko AN. 2004. Low-temperature microbial degradation of oil products differing in the extent of condensation. Appl Biochem Microbiol. 40:262–265. doi: 10.1023/B:ABIM.0000025949.91999.b4
- Bofill C, Prim N, Mormeneo M, Manresa A, Javier Pastor FI, Diaz P. 2010. Differential behaviour of Pseudomonas sp. 42A2 LipC, a lipase showing greater versatility than its counterpart LipA. Biochimie. 92:307–316. doi: 10.1016/j.biochi.2009.11.005
- Buchon L, Laurent P, Gounot AM, Guespin-Michel JF. 2000. Temperature dependence of extracellular enzyme production by psychotrophic and psychrophilic bacteria. Biotechnol Lett. 22:1577–1581. doi: 10.1023/A:1005641119076
- Buisman GJH, van Helteren CTW, Kramer GFH, Veldsink JW, Derksen JTP, Cuperus FP. 1998. Enzymatic esterifications of functionalized phenols for the synthesis of lipophilic antioxidants. Biotechnol Lett. 20:131–136. doi: 10.1023/A:1005368222340
- Cai Y, Wang L, Liao X, Ding Y, Sun J. 2009. Purification and partial characterization of two new cold-adapted lipases from mesophilic Geotrichum sp. SYBC WU-3. Process Biochem. 44:786–790. doi: 10.1016/j.procbio.2009.03.011
- Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR. 2002. Low-temperature extremophiles and their applications. Curr Opin Biotechnol. 13:253–261. doi: 10.1016/S0958-1669(02)00317-8
- Cesarini S, Bofill C, Pastor FIJ, Reetz MT, Diaz P. 2012. A thermostable variant of Pseudomonas aeruginosa cold-adapted Lip C obtained by rational design and saturation mutagenesis. Process Biochem. 47:2064–2071. doi: 10.1016/j.procbio.2012.07.023
- Chang HM, Liao HF, Lee CC, Shieh CJ. 2005. Optimized synthesis of lipase-catalyzed biodiesel by Novozym 435. J Chem Tech Biotech. 80:307–312. doi: 10.1002/jctb.1166
- Chen R, Guo L, Dang H. 2010. Gene cloning, expression and characterization of a cold-adapted lipase from a psychrophilic deep-sea bacterium Psychrobacter sp. C18. World J Microbiol Biotechnol. 27:431–441. doi: 10.1007/s11274-010-0475-7
- Choo DW, Kurihara T, Suzuki T, Soda K, Esaki N. 1998. A cold-adapted lipase of an Alaskan psychrotroph, Pseudomonas sp. strain B11-1: gene cloning and enzyme purification and characterization. Appl Environ Microbiol. 64:486–491.
- Christner J. 1992. The use of lipases in the beamhouse process. J Am Leather Chem Assoc. 87:128–139.
- Cygler M, Schrag JD. 1997. Structure as basis for understanding interfacial properties of lipases. Methods Enzymol. 284:3–27. doi: 10.1016/S0076-6879(97)84003-7
- de Maria PD, Carboni-Oerlemans C, Tuin B, Bargeman G, van der Meer A, van Gemert R. 2005. Biotechnological applications of Candida antarctica lipase A: state-of-the-art. J Mol Catal, B Enzym. 37:36–46. doi: 10.1016/j.molcatb.2005.09.001
- de Pascale D, Cusano AM, Autore F, Parrilli E, di Prisco G, Marino G, Tutino ML. 2008. The cold-active Lip1 lipase from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125 is a member of a new bacterial lipolytic enzyme family. Extremophiles. 12:311–323. doi: 10.1007/s00792-008-0163-9
- de Pascale D, de Santi C, Fu J, Landfald B. 2012. The microbial diversity of Polar environments is a fertile ground for bioprospecting. Mar Genomics. 8:15–22. doi: 10.1016/j.margen.2012.04.004
- De Santi C, Tutino ML, Mandrich L, Giuliani M, Parrilli E, Del Vecchio P, de Pascale D. 2010. The hormone-sensitive lipase from Psychrobacter sp. TA144: new insight in the structural/functional characterization. Biochimie. 92:949–957. doi: 10.1016/j.biochi.2010.04.001
- Dieckelmann M, Johnson LA, Beacham IR. 1998. The diversity of lipases from psychrotrophic strains of Pseudomonas: a novel lipase from a highly lipolytic strain of Pseudomonas fluorescens. J Appl Microbiol. 85:527–536. doi: 10.1046/j.1365-2672.1998.853530.x
- Do H, Lee JH, Kwon MH, Song HE, An JY, Eom SH, Lee SG, Kim HJ. 2013. Purification, characterization and preliminary X-ray diffraction analysis of a cold-active lipase (CpsLip) from the psychrophilic bacterium Colwellia psychrerythraea 34H. Acta Crystallogr, Sect F: Struct Biol Cryst Commun. 69:920–924. doi: 10.1107/S1744309113019428
- Durmaz E, Kuyucak S, Sezerman UO. 2013. Modifying the catalytic preference of tributyrin in Bacillus thermocatenulatus lipase through in silico modeling of enzyme-substrate complex. Protein Eng Des Sel. 26:325–333. doi: 10.1093/protein/gzt004
- Eijsink VGH, Bjork A, Gaseidnes S, Sirevag R, Synstad B, van den Burg B, Vriend G. 2004. Rational engineering of enzyme stability. J Biotechnol. 113:105–120. doi: 10.1016/j.jbiotec.2004.03.026
- Ewert M, Deming J. 2013. Sea ice microorganisms: environmental constraints and extracellular responses. Biology (Basel). 2:603–628.
- Feller G. 2013. Psychrophilic enzymes: from folding to function and Biotechnology. Scientifica (Cairo). 2013:512840. doi:10.1155/2013/512840.
- Feller G, Gerday C. 2003. Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol. 1:200–208. doi: 10.1038/nrmicro773
- Feller G, Narinx E, Arpigny JL, Aittaleb M, Baise E, Genicot S, Gerday C. 1996. Enzymes from psychrophilic organisms. FEMS Microbiol Rev. 18:189–202. doi: 10.1111/j.1574-6976.1996.tb00236.x
- Feller G, Thiry M, Arpigny JL, Gerday C. 1991. Cloning and expression in Escherichia coli of three lipase-encoding genes from the psychrotrophic Antarctic strain Moraxella TA144. Gene. 102:111–115. doi: 10.1016/0378-1119(91)90548-P
- Florczaka T, Daroch M, Wilkinson MC, Białkowska A, Bates AD, Turkiewicz M, Iwanejko LA. 2013. Purification, characterisation and expression in Saccharomyces cerevisiae of LipG7 an enantioselective, cold-adapted lipase from the Antarctic filamentous fungus Geomyces sp. P7 with unusual thermostability characteristics. Enzyme Microb Tech. 53:18–24. doi: 10.1016/j.enzmictec.2013.03.021
- Fujiwara S. 2002. Extremophiles: developments of their special functions and potential resources. J Biosci Bioeng. 94:518–525. doi: 10.1016/S1389-1723(02)80189-X
- Ganapati DY, Piyush SL. 2005. Lipase catalyzed transesterification of methyl acetoacetate with n-butanol. J Mol Cat B Enzy. 32:107–113. doi: 10.1016/j.molcatb.2004.10.003
- Georlette D, Blaise V, Collins T, D’Amico S, Gratia E, Hoyoux A, Marx JC, Sonan G, Feller G, Gerday C. 2004. Some like it cold: biocatalysis at low temperatures. FEMS Microbiol Rev. 28:25–42. doi: 10.1016/j.femsre.2003.07.003
- Gerday C, Aittaleb M, Arpigny JL, Baise E, Chessa JP, Garsoux G, Petrescu I, Feller G. 1997. Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta. 1342:119–131. doi: 10.1016/S0167-4838(97)00093-9
- Gomes J, Steiner W. 2004. The biocatalytic potential of extremophiles and extremozymes. Food Technol Biotechnol. 42:223–235.
- Gotor-Fernandez V, Brieva R, Gotor V. 2006a. Lipases: useful biocatalysts for the preparation of pharmaceuticals. J Mol Catal B Enzym. 40:111–120. doi: 10.1016/j.molcatb.2006.02.010
- Gotor-Fernandez V, Busto E, Gotor V. 2006b. Candida antarctica lipase B: an ideal biocatalyst for the preparation of nitrogenated organic compounds. Adv Synth Catal. 348:797–812. doi: 10.1002/adsc.200606057
- Grochulski P, Bouthillier F, Kazlauskas RJ, Serreqi AN, Schrag JD, Ziomek E, Cygler M. 1994. Analogs of reaction intermediates identify a unique substrate binding site in Candida rugosa lipase. Biochemistry. 33:3494–3500. doi: 10.1021/bi00178a005
- Gustavsson MT, Persson PV, Iversen T, Hult K, Martinelle M. 2004. Polyester coating of cellulose fiber surfaces catalyzed by a cellulose-binding module - Candida antarctica lipase B fusion protein. Biomacromolecules. 5:106–112. doi: 10.1021/bm034244y
- Hemachander C, Puvanakrishnan R. 2000. Lipase from Ralstonia pickettii as an additive in laundry detergent formulations. Process Biochem. 35:809–814. doi: 10.1016/S0032-9592(99)00140-5
- Imbert M, Gancel F. 2004. Effect of different temperature downshifts on protein synthesis by Aeromonas hydrophila. Curr Microbiol. 49:79–83. doi: 10.1007/s00284-004-4277-8
- Jadhav VV, Pote SS, Yadav A, Shouche YS, Bhadekar RK. 2013. Extracellular cold active lipase from the psychrotrophic Halomonas sp. BRI 8 isolated from the Antarctic sea water. Songklanakarin J Sci Technol. 35:623–630.
- Jaeger K-E, Eggert T. 2004. Enantioselective biocatalysis optimized by directed evolution. Curr Opin Biotechnol. 15:305–313. doi: 10.1016/j.copbio.2004.06.007
- Jaeger K-E, Reetz MT. 1998. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 16:396–403. doi: 10.1016/S0167-7799(98)01195-0
- Jinwei Z, Lin S, Zeng R. 2007. Cloning, expression and characterization of a cold-adapted lipase gene from an Antarctic deep-sea psychrotrophic bacterium, Psychrobacter sp. 7195. J Microbiol Biotechnol. 17:604–610.
- Joseph B, Ramteke PW. 2013. Extracellular solvent stable cold active lipase from psychrotrophic Bacillus sphaericus MTCC 7526: partial purification and characterization. Ann Microbiol. 63:363–370. doi: 10.1007/s13213-012-0483-y
- Joseph B, Ramteke PW, Kumar PA. 2006. Studies on the enhanced production of extracellular lipase by Staphylococcus epidermidis. J Gen Appl Microbiol. 52:315–320. doi: 10.2323/jgam.52.315
- Joseph B, Ramteke PW, Thomas G. 2008. Cold active microbial lipases: some hot issues and recent developments. Biotechnol Adv. 26:457–470. doi: 10.1016/j.biotechadv.2008.05.003
- Joseph B, Shrivastava N, Ramteke PW. 2012. Extracellular cold-active lipase of Microbacterium luteolum isolated from Gangotri glacier, Western Himalaya: isolation, partial purification and characterization. J Genet Eng Biotechnol. 10:137–144. doi: 10.1016/j.jgeb.2012.02.001
- Joseph B, Upadhyaya S, Ramteke P. 2011. Production of cold-active bacterial lipases through semisolid state fermentation using oil cakes. Enzyme Res. 2011:796407. doi:10.4061/2011/796407.
- Joshi GK, Kumar S, Tripathi BN, Sharma V. 2006. Production of alkaline lipase by Corynebacterium paurometabolum, MTCC 6841 isolated from Lake Naukuchiatal, Uttaranchal state, India. Curr Microbiol. 52:354–358. doi: 10.1007/s00284-005-0224-6
- Juhl PB, Doderer K, Hollmann F, Thum O, Pleiss J. 2010. Engineering of Candida antarctica lipase B for hydrolysis of bulky carboxylic acid esters. J Biotechnol. 150:474–480. doi: 10.1016/j.jbiotec.2010.09.951
- Jurado E, Bravo V, Luzon G, Fernandez-Serrano M, Garcia-Roman M, Altmajer-Vaz D, Vicaria JM. 2007. Hard-surface cleaning using lipases: enzyme–surfactant interactions and washing tests. J Surfactants Deterg. 10:61–70. doi: 10.1007/s11743-006-1009-z
- Kavitha M, Shanthi C. 2013. Isolation and characterization of cold active lipase producing Pseudomonas sp. 4 from marine samples of Tamilnadu coast. Res J Biotech. 8:57–62.
- Kim HR, Hou CT, Lee KT, Kim BH, Kim IH. 2010. Enzymatic synthesis of structured lipids using a novel cold-active lipase from Pichia lynferdii NRRL Y-7723. Food Chem. 122:846–849. doi: 10.1016/j.foodchem.2010.03.067
- Kim YO, Khosasih V, Nam BH, Lee SJ, Suwanto A, Kim HK. 2012. Gene cloning and catalytic characterization of cold-adapted lipase of Photobacterium sp. MA1-3 isolated from blood clam. J Biosci Bioeng. 114:589–595. doi: 10.1016/j.jbiosc.2012.06.013
- Kiran GS, Shanmughapriya S, Jayalakshmi J, Selvin J, Gandhimathi R, Sivaramakrishnan S, Arunkumar M, Thangavelu T, Natarajaseenivasan K. 2008. Optimization of extracellular psychrophilic alkaline lipase produced by marine Pseudomonas sp. (MSI057). Bioprocess Biosyst Eng. 31:483–492. doi: 10.1007/s00449-007-0186-0
- Krembs C, Eicken H, Junge K, Deming J. 2002. High concentrations of exopolymeric substances in Arctic winter sea ice: implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep Sea Res Part I: Oceanogr Res Pap. 49:2163–2181. doi: 10.1016/S0967-0637(02)00122-X
- Kulakovaa L, Galkina A, Nakayamab T, Nishinob T, Esakia N. 2004. Cold-active esterase from Psychrobacter sp. Ant300: gene cloning, characterization, and the effects of Gly Pro substitution near the active site on its catalytic activity and stability. Biochim Biophys Acta. 1696:59–65. doi: 10.1016/j.bbapap.2003.09.008
- Lafranconi PG, Caldarazzo SM, Villa A, Alberghina L, Lotti M. 2008. Unscrambling thermal stability and temperature adaptation in evolved variants of a cold active lipase. FEBS Lett. 582:2313–2318. doi: 10.1016/j.febslet.2008.05.037
- Lan DM, Yang N, Wang WK, Shen YF, Yang B, Wang YH. 2011. A novel cold-active lipase from Candida albicans: cloning, expression and characterization of the recombinant enzyme. Int J Mol Sci. 12:3950–3965. doi: 10.3390/ijms12063950
- Le QA, Joo JC, Yoo YJ, Kim YH. 2012. Development of thermostable Candida antarctica lipase B through novel in silico design of disulfide bridge. Biotechnol Bioeng. 109:867–876. doi: 10.1002/bit.24371
- Lee HK, Min JA, Sung HK, Won HS, Byeong CJ. 2003. Purification and characterization of cold active lipase from psychrotrophic Aeromonas sp. LPB4. J Microbiol. 41:22–27.
- Leonov SL. 2010. Screening for novel cold-active lipases from wild type bacteria isolates. Innov Rom Food Biotechnol. 6:12–17.
- Li M, Yang LR, Xu G, Wu JP. 2013. Screening, purification and characterization of a novel cold-active and organic solvent-tolerant lipase from Stenotrophomonas maltophilia CGMCC 4254. Bioresour Technol. 148:114–120. doi: 10.1016/j.biortech.2013.08.101
- Lo Giudice A, Michaud L, de Pascale D, De Domenico M, di Prisco G, Fani R, Bruni V. 2006. Lipolytic activity of Antarctic cold adapted marine bacteria. J Appl Microbiol. 101:1039–1048. doi: 10.1111/j.1365-2672.2006.03006.x
- Maraite A, Hoyos P, Carballeira JD, Cabrera ÁC, Ansorge-Schumacher MB, Alcántara AR. 2013. Lipase from Pseudomonas stutzeri: purification, homology modelling and rational explanation of the substrate binding mode. J Mol Catal B Enzym. 87:88–98. doi: 10.1016/j.molcatb.2012.11.005
- Marchi P, Longhi V, Zangrossi S, Gaetani E, Briani F, Deho G. 2007. Autogenous regulation of Escherichia coli polynucleotide phosphorylase during cold acclimation by transcription termination and antitermination. Mol Genet Genom. 278:75–84. doi: 10.1007/s00438-007-0231-3
- Marsal A, Cot J, Boza EG, Celma PJ, Manich AM. 1999. Oxidizing unhairing process with hair recovery. Part I. Experiments on the prior hair immunization. J Soc Leather Technol Chem. 83:310–315.
- Martinelle M, Hult K. 1995. Kinetics of acyl transfer reactions in organic media catalyzed by Candida antarctica lipase B. Biochim Biophys Acta. 1251:191–197. doi: 10.1016/0167-4838(95)00096-D
- Mayordomo I, Randez-Gil F, Prieto JA. 2000. Isolation, purification, and characterization of a cold active lipase from Aspergillus nidulans. J Agric Food Chem. 48:105–109. doi: 10.1021/jf9903354
- Mohamad Ali MS, Mohd FSF, Ganasen M, Rahman RNZRA, Basri M, Salleh AB. 2013. Structural adaptation of cold-active RTX lipase from Pseudomonas sp. strain AMS8 revealed via homology and molecular dynamics simulation approaches. BioMed Res Int. 2013:925373. doi: 10.1155/2013/925373.
- Muryoi N, Sato M, Kaneko S, Kawahara H, Obata H, Yaish MWF, Griffith M, Glick BR. 2004. Cloning and expression of afpA, a gene encoding an antifreeze protein from the arctic plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. J Bacteriol. 186:5661–5671. doi: 10.1128/JB.186.17.5661-5671.2004
- Muthukumaran N, Dhar SC. 1982. Comparative studies on the degreasing of skins using acid lipase and solvent with reference to the quality of finished leathers. Leather Sci. 29:417–424.
- Narinx E, Baise E, Gerday C. 1997. Subtilisin from psychrophilic Antarctic bacteria: characterization and site directed mutagenesis of residues possible involved in the adaptation to cold. Protein Eng. 10:1271–1279. doi: 10.1093/protein/10.11.1271
- Nawani N, Kaur J. 2007. Studies on lipolytic isoenzymes from a thermophilic Bacillus sp: Production, purification and biochemical characterization. Enzyme Microb Tech. 40:881–887. doi: 10.1016/j.enzmictec.2006.07.006
- Nielsen MH, Jepsen SJ, Outtrup H. 1981. Enzymes for low temperature washing. J Am Oil Chem Soc. 58:644–649. doi: 10.1007/BF02672384
- Novototskaya-Vlasova KA, Petrovskaya LE, Rivkina EM, Dolgikh DA, Kirpichnikov MP. 2013. Characterization of a Cold active lipase from Psychrobacter cryohalolentis K5T and its deletion mutants. Biochemistry (Moscow). 78:385–394. doi: 10.1134/S000629791304007X
- Ohgiya S, Hoshino T, Okuyama H, Tanaka S, Ishizaki K. 1999. Biotechnological applications of cold-adapted organisms. Berlin: Springer Verlag; p. 17–34.
- Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al. 1992. The α/β hydrolase fold. Protein Eng. 5:197–211. doi: 10.1093/protein/5.3.197
- Ong AL, Kamaruddin AH, Bhatia SW, Long WS, Lim ST, Kumari R. 2006. Performance of free Candida antarctica lipase B in the enantioselective esterification of (R)-ketoprofen. Enzyme Microb Technol. 39:924–929. doi: 10.1016/j.enzmictec.2006.01.029
- Otto Y, Sawamoto T, Hasuo M. 2000. Tributyrin specifically induces a lipase with a preference for the sn-2 position of triglyceride in Geotrichum sp. F0401B. Biosci Biotechnol Biochem. 64:2497–2499. doi: 10.1271/bbb.64.2497
- Pandey A, Benjamin S, Soccol CR, Nigam P, Krieger N, Soccol V. 1999. The realm of microbial lipases in biotechnology. Biotechnol Appl Biochem. 29:119–131.
- Park IH, Kim SH, Lee YS, Lee SC, Zhou Y, Kim CM, Ahn SC, Choi YL. 2009. Gene cloning, purification, and characterization of a cold-adapted lipase produced by Acinetobacter baumannii BD5. J Microbiol Biotechnol. 19:128–135. doi: 10.4014/jmb.0802.130.
- Park Y-D, Baik KS, Seong CN, Bae KS, Kim S, Chun J. 2006. Photobacterium ganghwense sp. nov., a halophilic bacterium isolated from sea water. Int J Syst Evol Microbiol. 56:745–749. doi: 10.1099/ijs.0.63811-0
- Parra LP, Reyes F, Acevedo JP, Salaza O, Andrews BA, Asenjo JA. 2008. Cloning and fusion expression of a cold-active lipase from marine Antarctic origin. Enzyme Microb Technol. 42:371–377. doi: 10.1016/j.enzmictec.2007.11.003
- Patkar SA, Bjorking F, Zundai M, Schulein M, Svendsen A, Heldt-Hansen HP, Gormsen E. 1993. Purification of two lipases from Candida antarctica and their inhibition by various inhibitors. Ind J Chem. 32B:76–80.
- Pemberton JM, Kidd SP, Schmidt R. 1997. Secreted enzymes of Aeromonas. FEMS Microbiol Lett. 152:1–10. doi: 10.1111/j.1574-6968.1997.tb10401.x
- Pirozzi D, Greco GJ. 2004. Activity and stability of lipases in the synthesis of butyl lactate. Enzym Microb Technol. 34:94–100. doi: 10.1016/j.enzmictec.2003.01.002
- Preuss J, Kaiser I, Gehring U. 2001. Molecular characterization of a phosphatidylcholine –hydrolyzing phospholipase C. Eur J Biochem. 268:5081–5091. doi: 10.1046/j.0014-2956.2001.02440.x
- Rabus R, Ruepp A, Frickey T, Rattei T, Fartmann B, Stark M, Bauer M, Zibat A, Lombardot T, Becker I, et al. 2004. The genome of Desulfotalea psychrophila, a sulfate-reducing bacterium from permanently cold Arctic sediments. Environ Microbiol. 6:887–902. doi: 10.1111/j.1462-2920.2004.00665.x
- Ramasami T, Rao JR, Chandrababu NK, Parthasarathi K, Rao PG, Saravanan P, Gayatri R, Sreeram KJ. 1999. Beamhouse and tanning operations. J Soc Leather Technol. 83:39–45.
- Rao JR, Nair BU, Ramasami T. 1997. Isolation and characterization of low affinity chromium(III) complex in chrome tanning solutions. J Soc Leather Technol Chem. 81:234–238.
- Rashid N, Shimada Y, Ezaki S, Atomi H, Imanaka T. 2001. Low-temperature lipase from psychrotrophic Pseudomonas sp. strain KB700A. Appl Environ Microbiol. 67:4064–4069. doi: 10.1128/AEM.67.9.4064-4069.2001
- Reddy GSN, Matsumoto GI, Schumann P, Stackebrandt E, Shivaji S. 2004. Psychrophilic pseudomonads from Antarctica: pseudomonas antarctica sp. nov., Pseudomonas meridiana sp. nov. and Pseudomonas proteolytica sp. nov. Int J Syst Evol Microbiol. 54:713–719. doi: 10.1099/ijs.0.02827-0
- Reetz MT, Carballeira JD. 2007. Iterative saturation mutagenesis (ISM) for rapid directed evolution of functional enzymes. Nat Protoc. 2:891–903. doi: 10.1038/nprot.2007.72
- Reetz MT, Carballeira JD, Vogel A. 2006. Iterative saturation mutagenesis on the basis of B factors as a strategy for increasing protein thermostability. Angew Chem Int Ed Engl. 45:7745–7751. doi: 10.1002/anie.200602795
- Reyes-Duarte D, Polaina J, Lopez-Corte’s N, Alcade M, Plou FJ, Elborough K, Ballesteros A, Timmis KN, Golyshin PN, Ferrer M. 2005. Conversion of a carboxylesterase into a triacylglycerol lipase by a random mutation. Angew Chem Int Ed Engl. 44:7553–7557. doi: 10.1002/anie.200502461
- Rotticci D, Ottosson J, Norin T, Hult K. 2001. Candida antarctica lipase B: a tool for the preparation of optically active alcohols. Methods in Biotechnol. 15:261–276.
- Ruslan R, Abd Rahman RN, Leow TC, Ali MSM, Basri M, Salleh AB. 2012. Improvement of thermal stability via outer-loop ion pair interaction of mutated T1 lipase from Geobacillus zalihae strain T1. Int J Mol Sci. 13:943–960. doi: 10.3390/ijms13010943
- Ryu HS, Kim HK, Choi WC, Kim MH, Park SY, Han NS, Oh TK, Lee JK. 2006. New cold-adapted lipase from Photobacterium lipolyticum sp. nov. that is closely related to filamentous fungal lipases. Appl Microbiol Biotechnol. 70:321–326. doi: 10.1007/s00253-005-0058-y
- Santarossa G, Lafranconi PG, Alquati C, DeGioia L, Alberghina L, Fantucci P, Lotti M. 2005. Mutations in the ‘lid’ region affect chain length specificity and thermostability of a Pseudomonas fragi lipase. FEBS Lett. 579:2383–2386. doi: 10.1016/j.febslet.2005.03.037
- Sarrouh B, Santos TM, Miyoshi A, Dias R, Azevedo V. 2012. Up-to-date insight on industrial enzymes applications and global market. J Bioprocess Biotech. doi:10.4172/2155-9821.S4-002.
- Seo HJ, Bae SS, Yang SH, Lee JH, Kim SJ. 2005. Photobacterium aplysiae sp. nov., a lipolytic marine bacterium isolated from eggs of the sea hare Aplysia kurodai. Int J Syst Evol Microbiol. 55:2293–2296. doi: 10.1099/ijs.0.63765-0
- Seo JB, Kim HS, Jung GY, Nam MH, Chung JH, Kim JY, Yoo JS, Kim CW, Kwon O. 2004. Psychrophilicity of Bacillus psychrosaccharolyticus: a proteomic study. Proteomics. 4:3654–3659. doi: 10.1002/pmic.200401025
- Sharma PK, Kumar R, Kumar R, Mohammed O, Singh R, Kaur J. 2012. Engineering of a metagenome derived lipase toward thermal tolerance: effect of asparagine to lysine mutation on the protein surface. Gene. 491:264–271. doi: 10.1016/j.gene.2011.09.028
- Shieh WY, Chen YW, Chaw SM, Chiu HH. 2003. Vibrio ruber sp. nov., a red, facultatively anaerobic, marine bacterium isolated from sea water. Int J Syst Evol Microbiol. 53:479–484. doi: 10.1099/ijs.0.02307-0
- Shimada Y, Watanabe Y, Sugihara A, Baba T, Ooguri T, Moriyama S, Terai T, Tominaga Y. 2001. Ethyl esterification of docosahexaenoic acid in an organic solvent-free system with immobilized Candida antarctica lipase. J Biosci Bioeng. 92:19–23. doi: 10.1016/S1389-1723(01)80192-4
- Siadat OR, Lougarre A, Lamouroux L, Ladurantie C, Fournier D. 2006. The effect of engineered disulfide bonds on the stability of Drosophila melanogaster acetylcholinesterase. BMC Biochem. 7:12–18. doi: 10.1186/1471-2091-7-12
- Siddiqui KS, Cavicchioli R. 2005. Improved thermal stability and activity in the cold-adapted lipase B from Candida Antarctica following chemical modification with oxidized polysaccharides. Extremophiles. 9:471–476. doi: 10.1007/s00792-005-0464-1
- Siddiqui KS, Cavicchioli R. 2006. Cold-adapted enzymes. Ann Rev Biochem. 75:403–433. doi: 10.1146/annurev.biochem.75.103004.142723
- Sih CJ, Wu SH. 1989. Resolution of enantiomers via biocatalysis. Topics Stereochem. 19:63–125. doi: 10.1002/9780470147283.ch2
- Smidt H, Fischer A, Fischer P, Schmid RD. 1996. Preparation of optically pure chiral amines by lipase catalyzed enantioselective hydrolysis of N-acyl-amines. Biotechnol Tech. 10:335–338. doi: 10.1007/BF00173249
- Srinivas TNR, Vijaya Bhaskar Y, Bhumika V, Anil Kumar P. 2013. Photobacterium marinum sp. nov., a marine bacterium isolated from a sediment sample from Palk Bay, India. Syst Appl Microbiol. 36:160–165. doi: 10.1016/j.syapm.2012.12.002
- Suhre K, Claverie JM. 2003. Genomic correlates of hyperthermostability, an update. J Biol Chem. 278:17198–17202. doi: 10.1074/jbc.M301327200
- Suzuki T, Nakayama T, Kurihara T, Nishino T, Esaki N. 2001. Cold-active lipolytic activity of psychrotrophic Acinetobacter sp. strain no. 6. J Biosci Bioeng. 92:144–148. doi: 10.1016/S1389-1723(01)80215-2
- Suzuki Y, Haruki M, Takano K, Morikawa M, Kanaya S. 2004. Possible involvement of an FKBP family member protein from a psychrotrophic bacterium Shewanella sp. SIB1 in cold-adaptation. Eur J Biochem. 271:1372–1381. doi: 10.1111/j.1432-1033.2004.04049.x
- Tan S, Owusu ARK, Knapp J. 1996. Low temperature organic phase biocatalysis using cold adapted lipase from psychrotrophic Pseudomonas P38. Food Chem. 57:415–418. doi: 10.1016/0308-8146(95)00243-X
- Thanikaivelan P, Rao JR, Nair BU, Ramasami T. 2004. Progress and recent trends in biotechnological methods for leather processing. Trends Biotechnol. 22:181–188. doi: 10.1016/j.tibtech.2004.02.008
- Tutino ML, di Prisco G, Marino G, de Pascale D. 2009. Cold-adapted esterases and lipases: from fundamentals to application. Protein Pept Lett. 16:1172–1180. doi: 10.2174/092986609789071270
- Uppenberg J, Hansen MT, Patkar S, Jones TA. 1994a. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure. 2:293–308. doi: 10.1016/S0969-2126(00)00031-9
- Uppenberg J, Ohrner N, Norin M, Hult K, Kleywegt GJ, Patkar S, Waagen V, Anthonsen T, Jones TA. 1995. Crystallographic and molecular-modeling studies of lipase B from Candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry. 34:16838–16851. doi: 10.1021/bi00051a035
- Uppenberg J, Patkar S, Bergfors T, Jones TA. 1994b. Crystallization and preliminary X-ray studies of lipase B from Candida antarctica. J Mol Biol. 235:790–792. doi: 10.1006/jmbi.1994.1035
- Vielle C, Zeikus GJ. 2001. Hyperthermophilic enzymes: sources, uses and molecular mechanisms for thermostability. Microbiol Mol Biol Rev. 65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001
- Vulfson EN. 1994. Industrial applications of lipases. Cambridge: Cambridge University Press.
- Wang Q, Zhang C, Hou Y, Lin X, Shen J, Guan X. 2013. Optimization of cold-active lipase production from psychrophilic bacterium Moritella sp. 2-5-10-1 by statistical experimental methods. Biosci Biotechnol Biochem. 77:17–21. doi: 10.1271/bbb.120104
- Wang YX, Srivastava KC, Shen GJ, Wang HY. 1995. Thermostable alkaline lipase from a newly isolated thermophilic Bacillus, strain A30-1 (ATCC53841). J Ferment Bioeng. 79:433–438. doi: 10.1016/0922-338X(95)91257-6
- Watanabe Y, Shimada Y, Sugihara A, Tominaga Y. 2002. Conversion of degummed soybean oil to biodiesel fuel with immobilized Candida antarctica lipase. J Mol Catal B Enzy. 17:151–155. doi: 10.1016/S1381-1177(02)00022-X
- Wei X, Jiang X, Ye L, Yuan S, Chen Z, Wu M, Yu H. 2013. Cloning, expression and characterization of a new enantioselective esterase from a marine bacterium Pelagibacterium halotolerans B2T. J Mol Catal B Enzym. 97:270–277. doi: 10.1016/j.molcatb.2013.09.002
- Wintrode PL, Miyazaki K, Arnold FH. 2000. Cold adaptation of a mesophilic subtilisin-like protease by laboratory evolution. J Biol Chem. 275:31635–31640. doi: 10.1074/jbc.M004503200
- Xiang Z, Xiao X, Wang P, Wang F. 2004. Screening and characterization of psychrotrophic lipolytic bacteria from deep-sea sediments. J Microbiol Biotechnol. 14:952–958.
- Xuezheng L, Shuoshuo C, Guoying X, Shuai W, Ning D, Jihong S. 2010. Cloning and heterologous expression of two cold-active lipases from the Antarctic bacterium Psychrobacter sp. G. Polar Res. 29:421–429. doi: 10.1111/j.1751-8369.2010.00189.x
- Yeshodha K, Dhar SC, Santappa M. 1978. Studies on degreasing of skins using a microbial lipase. Leather Sci. 25:77–86.
- Yuan D, Lan D, Xin R, Yang B, Wang Y. 2014. Biochemical Properties of a new cold-active mono- and diacylglycerol lipase from marine member Janibacter sp. strain HTCC2649. Int J Mol Sci. 15:10554–10566. doi: 10.3390/ijms150610554
- Yumoto I, Hirota K, Sogabe Y, Nodasaka Y, Yokota Y, Hoshino T. 2003. Psychrobacter okhotskensis sp., nov., a lipase-producing facultative psychrophile isolated from the coast of the Okhotsk Sea. Int J Syst Evol Microbiol. 53:1985–1989. doi: 10.1099/ijs.0.02686-0
- Zeng X, Xiao X, Wang P, Wang F. 2004. Screening and characterization of psychrotrophic lipolytic bacteria from deep-sea sediments. J Microbiol Biotechnol. 14:952–958.
- Zhang JH, Lin Y, Sun YF, Ye YR, Zheng SP, Han SY. 2012. High throughput screening of B factor saturation mutated Rhizomucor miehei lipase thermostability based on synthetic reaction. Enzyme Microb Technol. 50:325–330. doi: 10.1016/j.enzmictec.2012.03.002
- Zhang N, Suen WC, Windsor W, Xiao L, Madison V, Zaks A. 2003. Improving tolerance of Candida antarctica lipase B towards irreversible thermal inactivation through directed evolution. Prot Eng. 16:599–605. doi: 10.1093/protein/gzg074