ABSTRACT
Metastasis is an intricate process which involves the proliferation of a tumour to distant parts of the body from its original site. To successfully colonize a distant area in the body, a cancer cell must complete a series before it becomes clinically detectable. These steps involve a large number of proteins indulging in various pathways. Proteins such as matriptase require serine protease for activation, processing and degradation of any signal. Stim1/Orai1 controls the Ca2+ channel which is important for cell migration. Sox protein plays a vital role in various cellular activities and the disruption of its gene plays a role in instigating invasion. Unlike the above three, Metadherin has an inhibitory role to play. Protein inhibits the epithelial–mesenchymal transition (EMT); thus the loss of metadherin evokes metastasis. These proteins play a role in amalgamation with various pathways such as the AKT E-cadherin and EMT pathway, PI3K/AKT pathway, integrin-linked kinase (ILK) – integrin signalling pathway, PI3K/AKT and notch signalling pathway. In this article, we have combined various proteins and pathways that work in coordination to result in a metastatic colony. There are two major events that occur during metastasis, that is epithelial–mesenchymal transition and mesenchymal–epithelial transition. Both these events are an indispensable part for metastasis.
Cancer
The burden of cancer is projected to be more than twice of its current value globally. This situation has raised the prospect of an enormous public health. Change in distributions of risk factors as well as a rapidly changing pattern of ageing is contributing largely to the absolute burden of cancer (Siegel et al. Citation2014). According to the data of GLOBOCONN data3, an International Agency for Research on Cancer (IARC), 14.1 million cases were diagnosed and also 8.2 million deaths were reported worldwide in 2012 alone. These data did highlight the urgent need for expanding cancer-preventing efforts. In preventive measures, include population-based intervention like those that aim at controlling tobacco use, decreasing ultraviolet radiation exposures, reducing alcohol consumption, vaccinating against Human Papilloma Virus infection (Tarver Citation2012), etc. There are a number of treatments and medications available for cancer but the number of diseased people is still rising. One major issue with the treatment of cancer is its highly effective technique of transforming normal cells into metastatic ones. According to the study conducted by a group of researchers from Harvard-affiliated Brigham Women’s Hospital (BWH), cancer cells have the capability to extend their reach, transforming normal cells into metastatic and cancerous ones via ‘metastatic hijacking’. According to Shiladitya Sengupta, Bioengineering Division in the Department of Medicine and the corresponding author of the above study, metastasis will remain the final frontier in adopting and searching for a better cure for cancer. This research has been published in Nature Communications and gives an insight into the metastatic process. Thus, it is the need of the hour to search for loopholes in the metastatic pathway and protein functioning so that we can come up with a better cure.
Hallmarks of cancer
A review article authored by Douglas Hanahan ‘The hallmarks of cancer’ published in the Cell in 2000 categorized cancer on the basis of hallmarks. In total, six hallmarks were reported in the paper and later on new markers were also added to the list, making it 10. The main characteristics include the self-sufficiency of cancerous cells to initiate growth via growth signals (such cells are generally insensitive to the antigrowth signals), have the capability to evade apoptosis, have effective replicative potential, have sustained angiogenesis, which is a remarkable feature, invade new tissues and metastasis, have instability in the genome and being prone to mutation, cause inflammation in the affected tissue, reprogramme the energy metabolism and so on (Hanahan & Weinberg Citation2000).
EMT, metastasis and cancer
Despite the investments of huge resources, only incremental improvement has been observed in mortality. Most of the patients succumbed to either complicated treatment or metastatic disease. These huge data of reoccurrence suggest that the understanding of the metastatic machinery may lead to the development of effective anti-metastatic therapies that may provide an additional support in combating the morbidity and mortality of patients. The metastatic pathway involves a complex procedure of cell invasion from primary tumour, intravasation, extravasation of the circulatory system and all this followed by growth of new blood vessels, which is called angiogenesis (Gupta & Massagué Citation2006; Steeg Citation2006). The number and size of large lesions are indications of metastatic lesions, which can be detected by imaging. Known proteins are reported for the process of metastasis. Metastasis is a complex process that involves the spread of a tumour or cancer to distant parts of the body from its original site (Leber & Efferth Citation2009). However, this is a difficult process. To successfully colonize a distant area in the body, a cancer cell must complete a series of steps before it becomes a clinically detectable lesion. Metastasis comprises multiple complex pathway proteins and other microenvironmental factors that are interlinked. It encompasses various characteristics: intravasation proliferation in competent organs leading to colonization, have an ability to survive in the circulatory system, infiltration of tumour cells into the adjacent tissue (Eger & Mikulits Citation2005). The process of metastasis includes very efficient, well-coordinated multiple processes that are involved in making the whole phenomenon successful. If the process fails at any step, the complex machinery proceeds to the elimination of emigrating cancer cells at any of the multiple steps (Chambers et al. Citation2002; Mehlen & Puisieux Citation2006). There are large numbers of proteins involved in a cascade manner to lead to metastasis. In this article, we focus on the various RNAs, proteins, pathways and other molecules involved in the metastatic process, via elucidating the various cellular and molecular mechanisms, recognizing new targets to combat metastasis.
MET and its role in metastasis
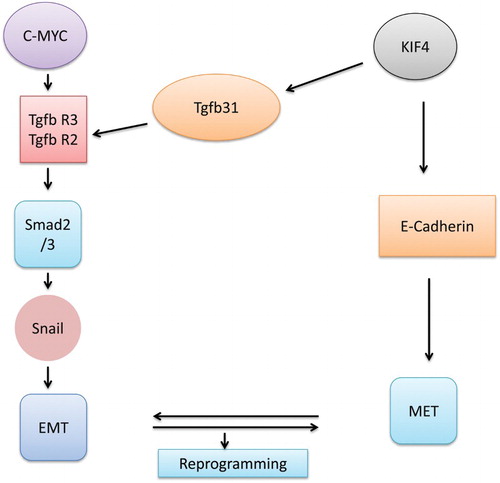
Embryonic development leads to the switching of various cellular processes from one form to another. Epithelial–mesenchymal transition (EMT) and mesenchymal–epithelial transition (MET) are two of them. Interswitching of both these phenomena results in the formation of a tumour at various cellular levels (Nakajima et al. Citation2000 ; Nakaya et al. Citation2004; Li et al. Citation2014). MET is a completely reversible phenomenon which involves various processes such as transiting cells from motile to planer arrays. There is a whole lot of differences in epithelial and mesenchymal cells in terms of interactions and biochemical nature. There are no mature cell–cell contacts. The cells have the ability to invade the extracellular matrix, and also possess the ability to express markers such as Snail, Twist, Fibronectin, Vimentin and N-cadherin (Thiery Citation2002; Baum et al. Citation2008). Determination of cell fate is a major issue that needs to be solved in developmental biology. Molecular mechanisms of reprogramming have led researchers to have an insight into decisions regarding the fate of cells. Reprogramming of the cells generally starts with MET, which suggests that the procedure is a complete reversal of EMT. This kind of switching generally occurs during the embryogenetic phase of the cell. Recent research has found two processes for the switching between these mechanisms. Since MET has been found to play a vital role in reprogramming it would be of greater use to study it in deciding the fate of the cell. It is a multistep procedure that involves an enormous number of proteins in the process of transforming cells from one type to another. Now the whole MET procedure is required for reprogramming but it also plays a significant role in the developmental event of carcinogenic modification. Now from various experimental studies it has been confirmed that MET is an important event along with EMT in order to complete the whole procedure of metastasis. The reprogramming procedure consists of various events such as initiation, maturation and the final step of stabilization (Samavarchi-Tehrani et al. Citation2010). Further analysis of gene expression has confirmed that MET is composed of downregulation of mesenchymal markers and transcriptional factors such as Zeb1, Snail and Cadherin (Li et al. Citation2010; Samavarchi-Tehrani et al. Citation2010). Thus MET is a definite first response of somatic reprogramming. shows the various pathways of conversion from MET to EMT.
Major proteins involved in interchange of MET and EMT
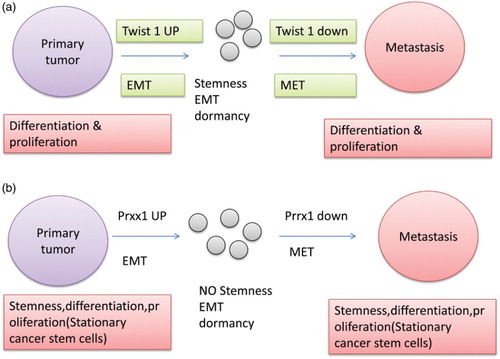
As already mentioned in various literature search and papers, it is evident that for the occurrence of metastasis, primary tumours must disassociate from the primary location and undergo de-differentiation through various activations and deactivations of the embryonic programme that involves EMT (Thiery et al., Citation2009). De-differentiated cancer cells in combination with EMT lead to the migration of the stem cells (Brabletz et al. Citation2005). Now TWIST1 is such a protein which is significant in co-inducing EMT and other stemness properties, thus leading to the linkage of EMT and the cancer stem cell property (Mani et al. Citation2008; Morel et al. Citation2008) (Shimono et al. Citation2007). Metastasis is not only governed by the EMT phenomenon but most of the metastatic cancers show re-differentiation in terms of MET. Here the one question that arises is why do the cells undergo re-differentiation? The probable answer to this is that in metastatic and invasive cells growth is arrested and the proliferation can only occur in re-differentiated cells (metastatic cells), leading to the conclusion that there should be a reverse phenomenon of EMT for the proliferation of cells and to make colonization possible. Many clinical experiments have reported evidence of the fact that cells undergo EMT–MET switching in the metastatic phenomenon but still the evidences provided are very less in number (e.g. Chaffer et al. Citation2006; Korpal et al., Citation2011). In studies it has been found that downregulation of TWIST1 is linked directly with the reversal of an EMT-related growth arrest. It has been considered that EMT plays a vital role in dissociation of the tumorous cells from the primary tumour site and that MET is required for proliferation and colonization of the disseminated cells; the sequence is shown in (a). Furthermore, it has been found that miR-200 plays an important role in re-differentiation. It also impacts SEC23A directly. SEC23A has been found to be involved in the production of protein, which leads to the suppression of metastatic progress (Korpal et al., Citation2011). The other protein that significantly affects the EMT phenomenon is prrx1 ‘paired-related homeobox transcription factor1’. In comparison to other factors affecting the metastasis, prrx1 leads to the suppression of stemness, thus negatively regulating the EMT phenomenon. Further, the protein works in combination with TWIST1, leading to the relevant changes of migration and invasion. In a study carried out by Tsai et al. (Citation2012), it was shown that loss of TWIST1 might lead to the disruption of the metastatic phenomenon, which was later disproved by conducting various experiments. Depletion of TWIST1 has only a marginal effect which indicates the indispensable role of prxx1 as a prime factor in regulating the whole event of metastasis. Further studies have shown that Prxx1 is responsible for negative regulation of stemness. Results are significant for a biologist in this field because they support the phenomenon of EMT/MET switching of metastasis and also they recognize a new mechanism for colonization of metastatic cells via incoupling of EMT with stemness, which leads to growth arrest in order to favour the maintenance of stemness, MET and proliferative phenotype ((b)). EMT and MET switching gets affected by many factors and proteins. The works of Tsai et al. (Citation2012) and Ocaña et al. (Citation2012) provide ample evidence that there is an upregulation of Twist1 which is an EMT activator in invasive cells of primary tumour. It is required to downregulate the EMT inducer in order to facilitate MET which is a redifferentiation process at various distant places. This helps in colonization of the tumours at various distant places. The peculiar characteristics of Twist1 include arrest of growth and induction of stemness. Twist leads to two occurrences: it provides the cell with stemness but leads to arrest of growth, which provides the cell with mobile but non-proliferating cancerous cells. Prxx1 is activated by EMT; this is a newly formed protein (Ocana et al. Citation2012). This protein leads to the suppression of stemness in EMT. It is required to downregulate the expression of prrx1 to activate stemness of the cells from the primary tumour and also to allow the colonization of the same. Thus now it is evident from all the literature search and experimental data that both EMT and MET are required for metastasis. EMT is required for dissociation of the cell and MET is required for colonization.
miRNA and its role in metastasis
The miRNA-200 family
It is a small noncoding RNA usually of 22 nucleotides. Generally, it is found in viruses, plants and animals and plays a role in silencing RNA and in regulation of gene expression (Ambros Citation2004; Bartel Citation2004). In plants and animals, it is encoded as eukaryotic nuclear DNA and in viruses b viral DNA. It functions by pairing with the complementary sequence of the mRNA molecules (Bartel Citation2009). This resembles siRNAs marginally except for the fact that miRNAs are derived from a longer region of RNA. The human genome might encode over 1000 miRNAs (Bentwich et al. Citation2005). These are abundant in almost all the cell types (Lagos-Quintana Citation2002; Lim et al. Citation2003). They target approximately 60% of the genes of humans (Lewis Citation2005; Friedman Citation2008). The family of miRNA-200 consists of at least five members and they are found to form two clusters which are located at two different genomic regions. Further studies have found that the first cluster, I miR-200 in humans, is constituted of miR-200b, -200a and -429(miR-200b/200a/429) which are located at chromosome number 1. Cluster II miR-200s contains miR-200c and 141(miR-200c/141) which are located at chromosome number 12 (Michael et al. Citation2003; Altuvia et al. Citation2005). Apart from their distinction on the basis of location, they can also be distinguished on the basis of their functional aspects. They only differ in seed sequence by a single nucleotide that is AAUACUG for the first group and AACACUG for the second one. Conserve sequences have been found in the miR-200 family in the vertebrate species. ZEB1 and ZEB2 have been found to be bound to the promoter regions of the miR-200 family members (Kim et al. Citation2011; Lesnikoff et al. Citation2014). Studies have shown that the miR-200 family is the most studied and researched miRNAs in cancer and metastasis. Its role has been found in all the stages of cancer that is initiation, invasion and movement of tumorous cells.
Role of the miR-200 family in transformation of cells and in tumourigenesis
Initiation of tumour progression is a complicated procedure that leads to the transformation of the normal cells into malignant ones. During the whole mechanism of tumour formation, the molecular profile of the cell changes in order to make it tumorous.
The miR-200 family in cancer metastasis
The multistep signalling mechanism in a cascade manner helps in the occurrence of metastasis, where cells move from the primary site of the tumour to colonize at distant organs and form secondary metastatic tumours (Chaffer et al. Citation2013). Cells that attain metastatic movement generally undergo an extensive epigenetic and genetic modification in their molecular mechanism. Broadly, the whole event of metastasis has been divided into six steps (Weihua et al. Citation2008): (a) formation of tumour at the primary site, (b) detachment of the cancerous cells and their migration away from the primary site, (c) intravasation into the blood stream and lymph nodes of the tissues, (d) circulation in the blood stream, (e) extravasation, (f) colonization at some distant organs and formation of secondary tumours. Research has shown that molecules have the potential to affect every step of metastasis. There is an effect of miR-200 on various events of metastasis.
Effect of the miR-200 family on tumour growth, angiogenesis and nearby tissue invasion
After getting transformed into malignant cells, the checkpoints are bypassed, which results in uncontrolled growth. It has been found from studies that miR-200 has an inhibitory effect on tumour growth (Senol et al. Citation2014). On the contrary, many researches have shown that it has a very small role to play in tumour growth. Some researchers say that it plays a vital role in inhibiting tumours; others suggest that it has a small or no role to play in altering the metastatic pathways (Iliopoulos et al. Citation2010; Williams et al. Citation2013; Humphries et al. Citation2014; Li et al. Citation2014). Hence in light of these evidences, it is clear that more extensive work is needed in the study of miR-200.
Impact of miR-200 on EMT and migration of tumorous cells
Metastasis is one of the most lethal properties of tumour cells. They disseminate from the primary site and move to the site of secondary infection. One important process that has been explored extensively that results in this phenomenon is EMT. The EMT mechanism is a prime process during embryogenesis and now with all the findings it has been found to be a major contributor to the metastatic event. ZEB1 and ZEB2 are homeobox factors that play a vital role in maintaining the cell in its mesenchymal phenotype by keeping the transcription level of miR-200 and E-cadherin low. Thus, the interplay between the protein and various transcription factors, that is, ZEB1/ZEB2 and the miR-200 family, drives the cell into an EMT state. Furthermore, the study conducted by Gregory et al. (Citation2008) has shown that it helps in suppression of the cell movement (Gregory et al. Citation2008). This group has used the potent inhibitor of miR-200 and found an enormous increase in cell migration in the Madin-Darby canine kidney epithelial cells, which suggests that the miR-200 family does play an important role in suppressing metastasis (Park et al. Citation2008). There are various proteins and factors involved in certain pathways and mechanisms via which miR-200b works. It acts on moesin, thus regulating the migration and invasion. Upregulation of moesin facilitates the metastatic movement, whereas its downregulation leads the miR-200b to show its function and inhibit the migration. Further, it has also been found that miR-200b negatively regulates the level of PKCα; it alters its expression by targeting the 3′UTR of PKCα (Wang et al. Citation2014). Moreover, knockdown of PKCα reduces the migration significantly and also forced expression of the protein leads to the reversion of an inhibitory effect of miR-200b. These studies indicate that miR-200b inhibits migration of metastatic cells by targeting PKCα. Further, in the study it was found that miR-200 is involved in Rho-ROCK signalling, focal adhesion, MMP activity, invadopodia formation, etc.
Role of miR-200b in tumour intravasion
It is a complicated step that involves the entrance of cancerous cells into blood vessels/lymphatic system. Intravasion involves many signalling pathways in its completion. The two that are extensively involved are Notch and tumour-associated macrophages (Wyckoff et al. Citation2007; Sonoshita et al. Citation2011). Blood vessels of the primary tumour are somewhat leaky and facilitate the moving cell with the appropriate environment for intravasation (Jain Citation2005). In studies it has been found that miR-200 affects intravasion negatively but the mechanism of the same is still not known (Carmeliet & Jain Citation2011).
Role of miRNA34 in metastasis
The molecular determinant of metastasis is still very much under wraps or not known completely in spite of the fact that it causes the largest percentage of deaths due to cancer (Fidler Citation2003; Parkin et al. Citation2005; Thorgeirsson & Grisham Citation2002). Various researches have revealed the crucial role played by miRNAs in the process of metastasis. miRNAs regulate a large number of genes post-transcriptionally, which plays an important role in metastasis (Lim et al. Citation2005; Dalmay & Edwards Citation2006; Kim et al. Citation2008). In mammals, miRNAs are inculcated in RNA-inducing silencing complex and also they are associated with the 30 untranslated regions of a specific target, which suppresses the translation and also induces degradation (Pillai Citation2005; Zamore & Haley Citation2005). It has also been found that they play a significant role in differentiation of cells and proliferation (Chen et al. Citation2004; Croce & Calin Citation2005). They can behave both ways, as a suppressor of tumour or as an oncogene depending on their target (Cimmino et al. Citation2005; Johnson et al. Citation2005). It has been found that oncogenic miRNAs only express in tumorous growth such as miR-520c, miR-21, miR-373 and miR-10b (Zhu, et al. Citation2008). On the contrary, the suppressive ones are downregulated and these include miR-29c, miR-126, miR-335 and miR-146a, which lead to the invasion (Lin, Chinag, et al. Citation2008; Musiyenko et al. Citation2008; Sengupta et al. Citation2008; Tavazoie et al. Citation2008). Furthermore, in the study it has been found that miRNAs are overexpressed in all the tumours. There are peculiar miRNAs, for example, miR-529c, miR-373, miRNA-21, miRNA-10b, overexpressed in tumours (Asangani et al. Citation2008; Lin, Chiang, et al. Citation2008; Zhu et al. Citation2008). miR-34a works via targeting apoptosis, leads to arrest of G1 phase and also leads to the senescence of the cell by regulating cell cycle cyclin and cyclin dependent kinase such as cyclin D1 (CCND1), cyclin E2 (CCNE2) and Bcl-2. It also leads to the suppression of the tyrosine kinase receptor c-Met (oncogenic) (Kim et al. Citation2008). From these evidences, it can be deduced that miR-34a plays a role in metastatic moment and also helps in invasion. In one study, it was found that miRNA leads to the regulation of invasion and migration, and that it also helps in scattering of cells, in HCC tissues in HepG2 cell lines. C-Met is considered to be one of the prominent receptors for the hepatocyte growth factor and it is inhibited by miR-34a (Leelawat et al. Citation2006; Kim et al. Citation2008). This growth factor leads to the activation of the c-Met which triggers the phosphorylation of the signalling pathway such as ERK1/2, which influences the migration and invasion (Sipeki et al. Citation1999; Ma et al. Citation2007). c-Met also plays a role in scattering of the cells. These evidences indicate that miR-34a plays a role in metastasis. Constitutive instigation of the components of the downstream pathway of the MAPK pathway leads to the migration (Ma & Tretiakova et al. Citation2007). All the above studies along with data predict the role of the component, that is miR-34, could be investigated for therapeutic application of the metastatic movement.
Proteins involved in metastasis and invasion
Various proteins and pathways play a significant role in metastasis. These proteins interact with each other intensively to make cells metastatic. There are various proteins whose chemical or natural inhibitors have been reported while others may or may not have known inhibitors. These proteins have been classified and discussed under two categories, one category having chemical or natural inhibitors reported and the other without any known inhibitor, but in vitro experiments have proved their role in metastasis. A list of a few of the important proteins with their known inhibitors is presented in . Another list presented in consists of proteins and enzymes along with the area that they are affecting. These proteins may or may not have any effective known inhibitor.
Table 1. Important protein responsible for metastasis and their known inhibitors.
Table 2. Important protein and enzymes responsible for metastasis.
Matriptase
Matriptase is a member of the family of type II transmembrane serine proteases. It is also known by different names such as EPITHIN, ST14 and TADG (Bugge et al. Citation2009). It is a protein with a complex structure and regulatory mechanism (Leland & Wright Citation2006). Its weight is approximately 80–90 kDa. This protein is synthesized as an inactive zymogen and travels via the golgi apparatus to the plasma membrane. It has a short transmembrane part and a large C-terminal region containing a catalytic serine-protease domain and several non-catalytic domains. It also consists of an N-terminus to the cytoplasmic site of unknown function. Activation of matriptase is triggered by shedding of the extracellular part from the cell surface into the surrounding microenvironment (List et al. Citation2006). Studies have showed that the activation of protein matriptase can occur on the cell surface as well as inside the cells. Action of matriptase is important for activation, processing and degradation of cellular protein. There is considerable evidence indicating the presence of matriptase in tumour formation and metastasis. Even the slightest of overexpression of matriptase is enough to trigger the formation of tumours in mice (List Citation2009). There are several evidences that link matriptase to carcinogenesis in various cancer types, which include ovarian, prostate and cervical cancers (Lin, Tseng et al. Citation2008). Consequently, considerable efforts have been made in the development of matriptase inhibitors and methods to put a check over its activity in tumours (Shi et al. Citation1993; Galkin et al. Citation2004; Farady et al. Citation2007; Li et al. Citation2007; Napp et al. Citation2010). Matriptase was discovered originally as a matrix-degrading protease in breast cancer cells (Shi et al. Citation1993). Since matriptase plays a vital role in cell migration and invasion it could be explored as a target for inhibiting invasion.
STIM1/Orai1
Ca2+ is one of the critical regulators of cell migration and it is ubiquitous in nature (Pettit & Fay Citation1998). A store-operated Ca2+ entry mechanism is predominant in the mechanism of non-excitable cells (Lewis Citation2007; Parekh & Penner Citation1997). Ca2+ influx is an important and essential phenomenon for migration of normal as well as tumorous cells (Marks & Maxfield Citation1990; Komuro & Rakic Citation1993; Lee et al. Citation1999; Nishiyama et al. Citation2003; Li et al. Citation2005). Two genes have been identified from recent studies, STIM1 (stromal interaction molecule 1) and Orai1 (ORAI calcium release-activated calcium modulator 1), which are responsible for store-operated Ca2+ entry (Liou et al. Citation2005; Roos et al. Citation2005; Vig et al. Citation2006; Feske et al. Citation2006; Zhang et al. Citation2006). Orai1 is an essential pore-forming component of the store-operated Ca2+ entry channel (Yeromin et al. Citation2006), while STIM1 serves as a sensor of Ca2+ (Prakriya et al. Citation2006). Store-operated calcium channels are reconstituted by coexpression of Orai1 and STIM1 (Mercer et al. Citation2006; Peinelt et al. Citation2006; Soboloff et al. Citation2006). A variety of physiological and pathological processes are controlled by store-operated calcium influx (Fanger et al. Citation1995; Mogami et al. Citation1997; Dolmetsch et al. Citation1998; Lewis Citation2001; Feske et al. Citation2005; Yoo et al. Citation2000). Both the proteins STIM1 and Orai1 have been found to play a role in breast tumour cell migration, invasion and metastasis. The dynamic structure that is under spatial control at the subcellular level is termed as focal adhesion. Focal adhesion turnover consists of proteolysis of proteins and phosphorylation of tyrosine (Webb et al. Citation2004). An increase in cellular Ca2+ could increase the activity of the tyrosine kinase FAK (focal adhesion kinase) and the calcium-dependent protease calpain in focal adhesions (Siciliano et al. Citation1996; Huttenlocher et al. Citation2011; Achison et al. Citation2001; Dourdin et al. Citation2001). It has been found in studies that in FAK-deficient fibroblasts, migration is decreased with an increased number of focal adhesions (Ilic et al. Citation1995; Ren et al. Citation2000). Rac is activated by FAK through p130Cas, Crk and the DOCK180/ELMO complex (Brugnera et al. Citation2002). Calcineurin and myosin light-chain kinase could also mediate the Ca2+ effect on focal adhesion turnover (Lawson & Maxfield Citation1995; Eddy et al. Citation2000). Invadopodia has a role to play in cancer invasion, and podosome and invadopodia are also considered to be adhesive in structure (Linder Citation2009). Blocking store-operated Ca2+ influx slows down focal adhesion turnover, resulting in larger focal adhesions and consequently stronger adherence. Strong adherence could lead to the fast migration of cells, including metastatic cancer cells. And hence there are agents like SKF96365, siRNAs for Orai1 and STIM1 that can block the store-operated Ca2+ channel’s activity of store-operated calcium, and can be considered as potential therapeutics for tumour metastasis.
SOX
A family of transcription factors containing the DNA-binding domain of SRY is encoded by SOX genes (Dong et al. Citation2004). In total, 20 different SOX proteins are identified in mammals and these proteins are further categorized into 8 subgroups (A, B1, B2, C, D, E, F, G, H). This categorization is done on the basis of gene structure, presence of specific functional domains HMG box domains, transrepression and transactivation (Castillo Citation2012). Expression of a targeted protein is either repressed or activated on the basis of the presence of domains and their specific binding partners (Wegner et al. Citation2010; Kamachi and Kondoh Citation2013). SOX proteins are involved in various functions during embryonic development such as development of the central nervous, haematopoietic system, sex determination and cell differentiation (Kamachi and Kondoh Citation2013). Disruption of these genes plays a crucial role in invasion, as it is involved in many of the cellular activities, cellular programming, regulation and adhesion of cell–cell interaction (Sarkar & Hochedlinger Citation2013).
Metadherin
Invasion involves many steps before its final execution; these steps are intravasation, survival in circulation, extravasation, micrometastasis and final colonization (Thompson et al. Citation2005; Chaffer & Weinberg Citation2011). EMT is considered to be the primary and most important step in cell invasion and metastasis in situ (Yang & Weinberg Citation2008; Thiery et al. Citation2009; Gonzalez de Castro et al. Citation2013). It can be understood as adhesion between two cell “cell-cell” adhesion is interrupted which is followed by transformation of the cytoskeleton of epithelial cells adhesion is interrupted which is followed by transformation of cytoskeleton of epithelial cells into mesenchymal-like cells (Acloque et al. Citation2009; Chao et al. Citation2009). These studies suggest that a protein that plays an important role in cell adhesion and inhibits cytoskeletal remodelling can be considered to be an important target for inhibiting EMT and tumour metastasis. Overexpression of MTDH leads to inhibition of EMT by reversing the pro-metastatic actin and remodelling cytoskeleton. And loss of or inhibitory expression of MTDH leads to reversal of action and facilitates the invasion and metastasis of proteins. It was demonstrated that MTDJ co-localized with tight junction protein ZO-1 and occludins in polarized epithelial cells (Thompson et al. Citation1994; Kleinerman et al. Citation1995; Britt et al. Citation2004). The occurrence leads to disruption at junction complexes, MTDH dissociates from ZO-1 and it is again reloaded during maturation of the tight junction complex (Britt et al. Citation2004).
Cathepsin
Cathepsin B induction is required for the oesophageal cancer invasion into ECM (Andl et al. Citation2010). Overexpression of Cathepsin D results in increased fibroblast motility and invasion (Pruitt et al. Citation2013). Activity of integrin gets affected by Cathepsin H, which further influences the process of migration and invasion (Jevnikar et al. Citation2013). Regulation of expression of MMP9 and Cathepsin K occurs by coronin 3, which further leads to the expression of invasional characteristics (Ren et al. Citation2012). It has been revealed by immune-staining that there is a strong expression of Cathepsin K in most of the primary melanomas’ metastasis. Therefore, it could be concluded that Cathepsin K has an important role in invasion and metastasis (Quintanilla-Dieck et al. Citation2008). Progression of prostate tumour is facilitated in bone by Cathepsin K (Keller et al. Citation2013). In case of colorectal carcinomas it is said that Cathepsin S plays novel roles in migration and invasion (Burden et al. Citation2012). And also Cathepsin S has a role in invasion and migration in case of gastric and hepatocellular carcinoma (HCC, Yang et al. Citation2010; Fan et al. Citation2015). Upregulation of Cathepsin X was also directly associated with higher invasiveness (Krueger et al. Citation2005). Inactivation of the tumour-suppressive proteins like profiling 1 by Cathepsin also leads to the migration or invasive feature of cells (Pečar Fonović et al. Citation2013).
Involvement of factors
Metastasis is a complex process that involves multiple cellular process such as angiogenesis (generation of new blood cells), migration (motility), invasion (movement) and growth. These processes are complicated and involve various factors that either suppress or enhance the activity of specific proteins. All these factors are released in normal as well as cancerous conditions but there are imbalances of certain factors leading to characters such as angiogenesis and extra growth. Change in adhesion is also one of the major factors required for invasion, but these processes are complex and require disruption of local cell–cell interactions on one hand and formation of cell–cell interactions on the other hand. Therefore, here we are proceeding to the individual gene and protein involved in it.
Growth factors and their receptors
Although metastasis is a late event in the natural history of carcinogenesis, early gene changes can influence later events. Growth factors and their receptors are often perceived to be early changes due to their ability to act as oncogenes in some systems, and their ability to induce transition through the cell cycle and so induce proliferation (Jakowlew Citation2006). The event of metastasis gets affected significantly by signalling through growth factor receptors
However, it has become apparent that these impacts include regulation of the cytoskeleton, effects on cell shape, adhesion of cell–cell and expression of a variety of genes related to cell growth such as proteins that modulate invasion.
PRRX
The PRRX1 gene encodes a DNA-associated protein called prrx. The protein encoded by the gene comes under the family of Homeobox proteins that is localized to the nucleus of a cell. It acts as a coactivator of transcription, thus enhancing the binding affinity of DNA to the serum response factor. Muscle creatin kinase is also regulated by this protein, thus indicating the vital role it plays in the development of various mesodermal muscle types. Splicing leads to the formation of two isoforms of the same protein that variedly differ in their expression and pattern. It has been found during experimental work that the mice that lack both prrx 1 and prrx2 (Suzuki et al. Citation1997; Wissmüller et al. Citation2006). PRXX1 regulates various proteins which includes differentiation of mesenchymal precursor. One example of this kind of regulation is inhibition of adipogenesis which occurs by activating the transforming growth factor-beta (TGF-beta) signalling (Gregory et al. Citation2006). It has also been found in recent studies that it downregulates the tumour necrosis factor-alpha in order to inhibit osteoblast differentiation (Kim et al. Citation2007). These recent findings have clearly instigated researchers to find more about the role and significance of protein. Furthermore, it has been discovered in in vivo studies that for metastasis to occur efficiently it is required to lose the Prrx1 and this procedure leads to the acquisition of stem cell properties. Unlike traditional EMT transcription factors, Prrx 1 leads to the uncoupling of EMT and stemness and is also considered as a biomarker for patients who have survived the lethal effect of metastasis. Apart from the entire regulatory role that it plays there is a paradox related to the protein functioning in that its downregulation is needed for induction of stem cell property but its expression to a certain level is required for invasion.
E-cadherin and b-catenin
E-cadherin is supposed to help in adhesion of molecules with catenin; when the level of adhesive molecules depletes due to any reason it leads to a disruption of intercellular contacts. And this disruption of cellular contacts is considered to be the prime step towards early metastasis. Only destruction could not guarantee a successful metastatic procedure. The depletion of the E-cadherin protein does via inducing the epithelial to mesenchymal transition and invasiveness. It has been found in studies that loss of E-cadherin induces series of transcriptional changes, thus leading to the activation and deactivation of various proteins of metastasis. Thus, the overall loss of E-cadherin provokes the phenomenon of EMT, which is followed by elevated motility of cells, increased invasiveness and also resistance to apoptosis (Cancer Res 2008). There are two methods employed by tumours cell to deactivate E-cadherin: in one method only the level of E-cadherin gets affected and in the other truncated or inactive protein production occurs. It was found that mutation in the extracellular domain of the protein does not lead to initiation of any metastatic event instead a complete elimination is required for instigation of the event (Berx et al. Citation1998) are not likely to result in an EMT or to afford functional traits that allow completion of the later steps of metastasis. Complete loss of the protein leads to multiple events in a cascade manner to finally complete the process of metastasis.
Vimentin
The VIM gene is accountable for the production of the vim protein. It is expressed in mesenchymal cells and is considered to be the type III intermediate filament. Protein vimentin play vital role in humans and also it has shown its findings in metazoan and bacteria (Eriksson et al. Citation2009; Cabeen & Jacobs-Wagner Citation2010). Since vimentin is one of the major structural constituents of the mesenchymal cell it is often used as a biomarker for cells which are derived mesenchymally or which undergo (EMT) transition. It also plays a significant role in positioning and anchoring of the organelles in the cytosol. Its attachment to the various organelles such as mitochondria, endoplasmic reticulum and nucleus is laterally or terminally (Katsumoto et al. Citation1990). It provides flexibility to the cell, and integrity (Goldman et al. Citation1996). Through the various experimental setups it has been found that it is used as a marker for sarcoma tumour to identify the mesenchyme (Leader et al. Citation1987). Further investigation reveals that it regulates the interaction of various cytoskeletal proteins with that of cell adhesion molecules and thereby plays a pivotal role in migration, invasion, signalling and adhesion. Phosphorylation, polymerization and depolymerization act as regulatory mechanisms for the protein and thus affect cell–cell interaction. These new findings about the protein put it in the category of a potent target for anti-metastatic therapies and research.
SLUG
Expression of SLUG has been found to be elevated in tumour metastasis in various types of tumours (Zhang et al. Citation2010). In an experiment conducted on a breast cancer mouse model, it was found that SLUG promotes metastasis by partially inhibiting E-cadherin (Casas et al. 2011). SLUG acts on the metastatic pathway in many ways; apart from inhibiting E-cadherin it regulates CXCL12. Overexpression of this protein leads to the upregulation of CXCL12 and promotes migration and invasion prostate cancer and also reduces the adhesion. In contrast to the above study, knockdown of the same CXCL12 leads to the impairment of SLUG-mediated MMP9 expression and also affects the migration and invasion in PC3 cells. From the above studies, it can be seen that CXCL12 leads to the promotion of invasion by upregulating MMP9 (Shen et al. Citation2009), which leads to the degradation of extracellular matrix components. SLUG upregulates the expression of both CXCL12 and MMP9. It still has to be determined whether MMP9 is indispensable for SLUG-mediated invasion of prostate cancer cells (Arya et al. Citation2004). Research has shown that SLUG regulates the expression of the CXCL12/CXCR4 axis positively in human prostate cancer cell lines. Furthermore, in the study it was found that activated SLUG expression leads to the invasion and migration by activating the CXCR4/CXCL12 axis. Thus CXCL12, SLUG and MMP9 can be targeted for the prostate cancer metastasis (Patrussi et al. Citation2011).
Syntenin
Acquisition of invasiveness is an important feature of progression of a tumour. Mechanism of metastasis involves, primarly a tissue destined for invasion is required to get recognized and interact with the microenvironment that surrounds it. Secondly, it requires undergoing remodelling of the extracellular matrix in such a way so as to achieve the next step. Studies have shown that the migration step is the most important and it is this step that acts as a limiting factor for invasion (Hanahan & Weinberg Citation2000; Kassis et al. Citation2001).
It has been found that it is the specific PDZ domain of protein that interacts with other proteins. These proteins may include Syndecans and B ephrin. Eph B-ephrin plays a vital role in regulation of cell migration (Klein Citation2001) and the PDZ domain of protein colocalize with both Eph receptors and their ephrins. Furthermore, investigation has revealed that the expression of syntenin is elevated in metastatic human breast and gastric cell lines and also that it invokes the migration of the same.
TWIST
TWIST proteins have oncogenic properties which intimate crosstalk between failsafe program escape and cancer cell dissemination. TWIST itself does not lead to metastasis; instead it promotes it by affecting other micro-environmental factors. In further experimental research conducted through oncogenic cooperation assays on epithelial cells, researchers have demonstrated that TWIST works by overriding ERBB2 or RAS-induced senescence, which can promote EMT (Ansieau et al. Citation2008). It has been found that activation of RAS has a strong impact on various components of the cytoskeleton and that it can synchronize the TWIST proteins. This protein is more likely to put an impact on TWIST’s stabilization, thus effecting its activation significantly. It has generally been assumed that the process of metastatic initiation has been restricted to the later part of the cancer but in recent studies it has been found that some cancer dissemination might get initiated at an early stage, which gives rise to evolving of a single cell independently from a primary tumour. TWIST 1 reactivation leads to early tumour progression and its dissociation (Hüsemann et al. Citation2008).
P2Y2
Studies have shown that the P2Y receptor helps in stimulation of metastasis by acting on extracellular adenosine 5′-triphosphate (ATP). Various studies have shown the role of P2Y2 in metastasis of tumorous cells. One such receptor is P2Y which has been in focus in the current scenario to investigate its role in metastasis. In an experiment conducted to find out the role of P2Y2, the expression of the protein was silenced via RNAi. Later on in vitro and in vivo experiments were conducted to investigate the role of P2Y2 receptors in cellular metastasis and invasion. Apart from this, a cDNA microarray was also performed to identify the genes which are expressed differentially. The experiment has shown a significant expression of the protein in prostate cancer. Knocking down of the p2y2 receptor leads to suppression of invasion and metastasis in vitro as well as in vivo. Further studies have led to the fact that ATP has an ability to promote IL-8 and snail expression and also regulates Claudin-1 and E-cadherin expression negatively. Further the experiment has shown that knocking down of the protein has affected the expression of EMT/invasion-related genes in vitro and in vivo. In light of these findings, the P2Y2 receptor could be considered to be a potent therapeutic target for treating prostate cancer.
Livin
Livin comes under the category of IAPs (Identical ancestors proteins), which are inhibitors of apoptosis proteins. Livin is the most recently discovered IAP. Previously, it has already been established that IAPs play a significant role in tumour invasion and metastasis. Further research has shown that Livin might play a significant role in cell proliferation by regulating the G1-S cell cycle transition. In one study it was found that the expression of LIVIN is higher in PCa tissues in comparison to non-metastatic tissues. And also its expression is regulated by siRNA, which could inhibit or promote PC-3/LNCaP cell invasion. However, a lot is needed to be discovered regarding regulation of metastasis and invasion. Previous findings indicate that silencing the expression of Livin could reduce the invasive capacity in an SMMc-7721 cell line (Liu et al. Citation2010), which is an indication that expression of the Livin gene might be associated with tumour cell invasion and metastasis.
MMP9 and MMP2
MMPs are generally concerned with remodelling of a matrix, especially in terms of invasion and angiogenesis. Apart from this, its role has been expanded exclusively to other functions also, which include processing of various substrates related to cancer and its various stages.
These findings clearly make MMPS to the category of potential target against cancer. Despite such an apparent finding for the protein the field of MMP research in cancer is still not as efficient as required because of drastic failure of molecules in multiple phases of clinical trials against MMP (Coussens et al. Citation2002; Pavlaki & Zucker Citation2003). There could be various reasons for the failure of these inhibitors, the prime reason being the lack of specificity of the inhibitors and another reason being the side effects caused by dose limitations; the mechanism of dose-limiting effect has still not been understood completely (Pavlaki et al. Citation2010; Fingleton Citation2008; Sela-Passwell et al. Citation2011; Dufour & Overall Citation2013). Despite the setback one has got in finding potential molecules against MMP inhibitors knowledge and research in field of MMPs have increased tremendously. For inhibiting these MMPs, drugs with novel binding sites are required. In spite of all theextravagant research and findings, it is still challenging to find out ways to find a potent inhibitor molecule and to deliver it perfectly by integrating it with various available techniques such as MRI, PET/SPECT. So it is still a question for many whether to investigate it further or not.
Egfl7
Epidermal growth factor-like domain 7 (Egfl7) is considered to be expressed mainly in endothelial cells. It is one of the secretory proteins. In various studies, elevated levels of protein have been found but the mechanism by which it acts or instigates metastasis and invasion is still unknown. Expression of this factor is predominant in lung cancer, especially HCC cells. For functional characterization of Egfl7 in HCC, its expression in HCCLM3 cells has been depleted by using siRNA. Reduction of the expression of Egfl7 leads to the suppression of migration of HCCLM3 cells. This study reveals that Egfl7 could be taken as a unique marker for metastasis of HCC and also it could be considered as a therapeutic target (Wu et al. Citation2009)
LOX
Metastasis is an efficient mechanism which involves several steps and which leads to more than 90% death of cancer patients. For development of an effective treatment of cancer, it is required to understand the mechanism and events of the metastasis. The pathway and interaction study will help us to identify the potential drug candidate. One such protein is LOX (lysyl oxidase). Studies have indicated the LOX- dependent cross-linking of collagen which involves in creating a microenvironment which facilitates the metastatic growth by evoking persistence and survival of tumour. Therapeutic targeting of LOX has shown the prevention of metastatic colonization. LOX works in unison with collagen and its cross-linking with the same drastically increases the proliferation of cells and also enhances colonization of metastatic cells. These researches have shown the significance of cross-linking of both the proteins. Thus these proteins could be targeted as a potential target against metastatic tumour.
Periostin
Periostin is found in extracellular matrix which has dual function attachment and it acts as an autocrine, which also helps in adhesion of molecules like integrins αvβ3 and αvβ5. It also helps in cardiac development, formation of bone, tumour development and wound repair. It is of significant importance to the biologist dealing with metastasis as its overexpression has been ground in almost all the tumorous cells. This involves the maintaining of the naive nature of the stem cells, formation of niche, survival of tumorous cells and angiogenesis. All the above-mentioned mechanisms are indispensable for the EMT and invasion step of tumorous cells. Apart from playing a role in the above-mentioned mechanisms it was also found to activate PI-3K/AKT, Wnt and FAK-mediated signalling pathways to promote metastasis. Since it has a role in multiple pathways and signals it appears to be a potentially good candidate for targeting inhibition.
Survivin
Overexpression of Survivin has been found in various melanomas; it is a mitotic regulator by function. It also acts as a marker for patients with metastatic disease. One study has shown that overexpression of the protein in YUSAC2 human melanoma cells has led to the increased formation of colonies on soft agar. It can further be explored to put it in the category of potent candidate for metastasis (McKenzie et al. Citation2013)
Pathways involved
Cancer is a complex amalgam of interactions where various factors, proteins and other signalling pathways work together in unison to produce cancer with its peculiar characteristics of metastasis, invasion, extravasion, angiogenesis and so on. Thus, it makes the study of involved pathways very important, so that the overall mechanism of cancer could be understood.
AKT, E-cadherin and EMT
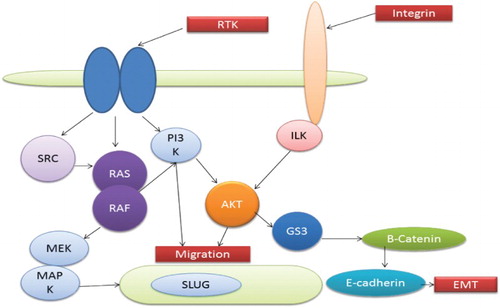
Initially, AKT was classified under the category of oncogene (Staal Citation1987; Bellacosa et al. Citation1991). Broadly, three AKTs have been classified and all these have serine-threonine kinase activity (Testa & Bellacosa Citation2001; Bellacosa et al. Citation1991). AKT2 is supposed to be upregulated and activated in certain cancers such as ovarian, breast and pancreatic tumours. AKT has many roles to play in cellular processes, which include progression of cell cycle, proliferation of cell, cell survival, EMT and metabolism. AKT induces EMT and it leads to various cellular changes like reduction in cell–matrix adhesion, alteration in production and distribution of specific proteins, decreases cell adhesion, change in morphology, cell motility induction, apicobasolateral loss, there are various examples of alteration of functional proteins for an example desmoplankin is a protein that involves in maintenance of desmosomes is internalized, vimetin is induced in many mesenchymal cells (). Akt has also a role to play in synthesis of metalloproteinases and invasion of cells (Kim et al. Citation2001; Park et al. Citation2002). In epithelial cells, Akt has two major consequences for E-cadherin; the first is that transcription of the gene responsible for E-cadherin is repressed strongly and the second is that a small amount of protein gets concentrated in the perinuclear region (Grille et al. Citation2003). These regulations maintain the cell to confine it in a mesenchymal state during exponential growth. The transcriptional repression of E-cadherin is affected by the activation of the Snail gene while its sequestration is related to the activation of Rab5 which is mediated by AKT (Barbieri et al. Citation1998; Batlle et al. Citation2000; Cano et al. Citation2000).
Interactions between the PI3K/AKT and other pathways of EMT
Interaction between RAS and ILK, PI3K/AKT plays vital role in instigating EMT. EMT occurs only due to perfectly defined connections between these signal transducers (Boyer et al. Citation2000; Thiery Citation2002; Bellacosa et al. Citation1991). Other EMT pathways are less established and need to be investigated. However, studies have shown some peculiar connections between Wnt/b-catenin and Notch signalling.
Integrin-linked kinase (ILK) and integrin signalling
Focal adhesion requires integrin, which depends on the integrin-linked kinase (ILK) (Wu Citation1999). Targets of ILK involve AKT by activating phosphorylation on glycogen synthase 3 and S473 (Delcommenne et al. Citation1998). Overexpression of ILK leads to the nuclear translocation of protein b-catenin, which further increases the invasion and repression of E-cadherin (Novak et al. Citation1998; Wu et al. Citation1998) via upregulating Snail transcription (Tan et al. Citation2001; Barbera et al. Citation2004). Involvement of ILK was also seen in ILK TGF-b-mediated EMT of human keratinocytes (Lee et al. Citation2007). shows the various pathways interacting to lead to the EMT phenomenon.
E-cadherin pathways
EMT is a major phenomenon that invokes the event of invasion of EMT and vice versa. Both of these functions are dependent on the E-cadherin molecule. Cadherins are regulated at various levels as at the level of mRNA and at the level of proteins by varying the translational, transcriptional and degradation machinery. It is considered to be a tumour suppressor for the reason that its expression is silenced in various carcinomas (Vleminckx et al. Citation1991). Production of a defective protein or transcriptional silencing because of hypermethylation may result in the loss of functionality of the E-Cadherin. In addition to its mutation, abnormal post-translational modifications or protein degradation may lead to defective production of E-cadherin. Upregulation of E-cadherin has been reported in tumour progressions, particularly at the time of intravasation (Kang & Massagué Citation2004; Thiery & Morgan Citation2004). E-boxes have a peculiar repeating sequence called consensus sequence; in this case it is CANNTG Zeb1; Slug, Sip1 and Sail are the E-boxes that repress the E-cadherin transcription. Transcription of E-cadherin is inversely correlated with the expression of snail. Snail production in large amount is generally associated with the loss of E-cadherin expression (Birchmeier & Behrens Citation1994; De Craene et al. Citation2005). The overproduction of Snail or Slug induces EMT in vitro (Batlle et al. Citation2000). E-cadherin upregulation is associated with the repression of Snail RNA. There is a system of positive and negative regulation of E-cadherin (Palmer et al. Citation2004). Regulation of E-cadherin occurs via 1, 25(OH) 2D3 along the help of vitamin D receptor and its repression can occur by Snail regulate E-cadherin levels (Palmer et al. Citation2004). Repressor and nuclear retention activity of Snail are promoted by PAK1 which is activated by phosphorylated p21 (Yang et al. Citation2005). These examples have shown the complex process of regulation of E-cadherin during EMT. Sip1 helps in modulating the TGF-b.
PI3K/AKT and notch signalling
It has been found in recent studies that Notch ligand expression in human keratinocytes and cancerous cell lines of cervical tissue leads to AKT phosphorylation and also it induces PI3K-dependent EMT that can be characterized by upregulation of vimentin and fibronectin, downregulation of E-cadherin, increased motility and morphological changes (Veeraraghavalu et al. Citation2005).
RAS pathway
Regulation of activated downstream receptor protein tyrosine kinases of EMT is affected by RAS production. Jun and Fos are involved downstream of RAS Epistatic analysis (Boyer et al. Citation1996). There are many signalling factors involved in EMT and signalling from RAS to jun, fos, snail, slug and others involves MEK and RAF. Both these are inducers of EMT and MAPK (Edme et al. Citation2002). Another effector that alters the functioning of RAS is PI3. mRAS also affects the Rho, Rac and GTPases via PI3K. These two molecules play a role in EMT via regulating the adherent junctions, myosin phosphorylation, focal adhesion scattering and motility (Edme et al. Citation2002; Thiery Citation2002).
PI3K/AKT and Wnt/b-catenin signalling
Wnt and PI3/AKT signalling can converge at the level of inhibition of GSK3. It is evident from the studies that two pathways affect the different pools of GSK3 (Weston & Davis Citation2001; Grille et al. Citation2003). Other possible pathways may include the stimulation of AKT activity via Wnt/Disheveled signalling (Fukumoto et al. Citation1997) and b-catenin transcription stimulation (and possibly phosphorylation) by AKT and 14-3-3z (Tian et al. Citation2004). In spite of establishment of the indirect role of these interactions between PI3K/AKT and Wnt/b-catenin signalling, their direct role in EMT is still needed to be investigated.
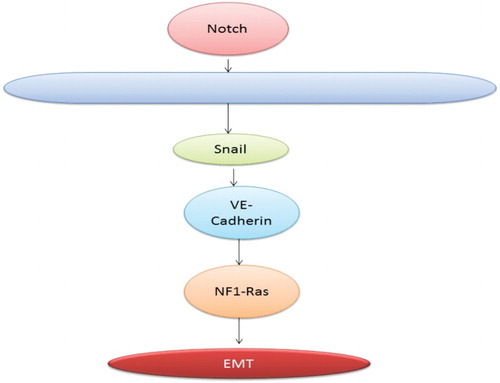
Notch pathway leading to EMT
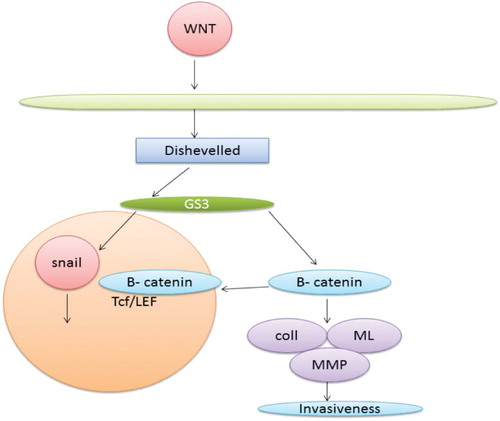
Notch is involved in determination of fate of a cell during development. It has been found from recent studies that notch pathway also plays a major role in inducing EMT. It performs this function by repressing VE-cadherin transcription or by inducing snail. In a same manner if Notch is overexpressed it results in EMT because it leads to the activation of Snail and repressing VE-cadherin (Timmerman et al. Citation2004). shows the interaction of various proteins with Wnt and B-catenin resulting in invasiveness.
Rac1b and reactive oxygen species (ROS)
ROS has been found to have a role in EMT (Radisky et al. Citation2005). Production of ROS is stimulated by Rac1b which in turn leads to stimulation of Snail expression and initiation of EMT. When treated with an antioxidant N-acetyl cysteine snail induction and EMT get prevented (Radisky et al. Citation2005). These findings connect between oxidative damage, extracellular matrix and EMT (Thiery Citation2002). shows the progression of invasion via the Notch pathway.
TGF-b pathway
TGF-b transforming growth factor b imparts its effect on EMT by getting regulated via different factors such as SIP1, Snail and Slug. These factors work in a manner which leads to the suppression of the E-cadherin which is a primary adhesion molecule between two cells.
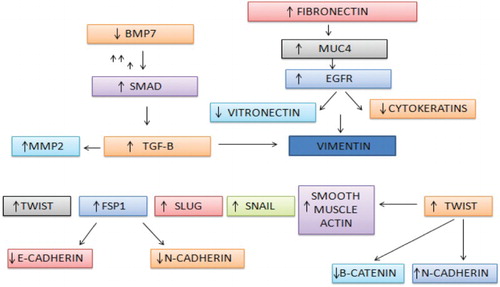
Its activity is regulated by BMP7 and upregulation of the same leads to production of altered physiological EMT. It does this by inhibiting the Smad-mediated TGF-b signalling and also by reducing the expression of vimentin. It has been shown that the expression of BMP7 is inversely proportional to the tumourigenicity (Buijs et al. Citation2007; Yang et al. Citation2011; Buijs et al. Citation2012; Liu et al. Citation2012).
TWIST and HIF pathway
TWIST is considered to be the master regulator of the EMT (Van Dusen & Firulli Citation2012; Xue & Hemmings Citation2012; Saunders & McClay Citation2014). It has an evident role to play in type I and type III metastases (reviewed in Khan et al. Citation2003; Mallini et al. Citation2004). When the expression of this protein exceeds the normal level, it leads to the metastasis (Croset et al. Citation2014). It is widely expressed in a variety of metastatic breast cancer cells. It has been found in an experiment that knocking down the TWIST with its siRNA drastically lowers the metastatic movement. Further experiment was carried out on human mammary epithelial cells. Further clinical samples have provided evidences of induction of metastasis by TWIST activity. It has been evident from the research that the epigenetic pathway regulates the mechanism which stimulates the function of migration and invasion by TWIST. It has been evident from the various cell lines that TWIST leads to the expression of the miRNA, miR-10b (Ma et al. Citation2007). Expression of the same in primary carcinomas is related to the clinical progression (Ma et al. Citation2007). These findings indicate that both the genetic alteration and epigenetic factors are responsible for the metastatic movement of the cells. Many studies have pointed out that stress conditions like the hypoxic one create an environment that favours metastatic growth and movement (Zhang et al. Citation2013). Evident proof has been retrieved from the in vitro experiments conducted on MCF7 cells which show that HIF-1A is a driving factor for EMT and metastasis. It has been found to directly regulate the expression of TWIST (Yang et al. Citation2008). HIF-1a works under a hypoxic condition and it directly interacts with the TWIST, thus activating it efficiently. It consecutively upregulates the endothelial growth factor and also increases the formation of new blood vessels which is followed by an enormous increase of E-cadherin and by loss of vimentin and N-cadherin. The process can totally be reversed via inhibiting HIF-1a with that of siRNA qualifies HIF-1a as a key regulator of EMT (Yang et al. Citation2008). It can therefore be concluded that the hypoxic condition can lead to induction of HIF-1a which subsequently leads to the activation of TWIST signalling, enabling the molecule to escape into circulation.
E-cadherin pathway
E-cadherin is considered to be one of the most prominent hallmarks of EMT assays and it has been found through various sources that loss of the molecule is very high for initiating the first step of metastasis. Loss of the protein leads to the disruption of the cell–cell adhesion which leads to the cellular polarity, allowing various growth factors which normally would have been removed from the system (Wells et al. Citation2008). b-Catenin along with cadherin alters many pathways, thus leading to playing a vital role in metastasis. shows the interaction of various pathways, thus leading to the up- or downregulation of various proteins involved in the invasion phenomenon.
PI3K AKT fibronectin pathway
Fibronectin is one more ECM protein which plays a pivotal role in migration, adhesion and transformation by instigating the PI3/Akt pathway. It completes the pathway after getting bound to the avb1 integrin molecule (Bae et al. Citation2013). In vitro experiments on human mammary breast cancer cells have shown that fibronectin plays a vital role in initiating EMT. When in an experiment MCF-10A cells were exposed to fibronectin it has led to the stimulation of the migration and induction of an EMT event which includes the upregulation of the markers of EMT, for example MMP2, Vimentin, Snail, Smad2, N-cadherin, etc. (Park et al. Citation2011) These studies are a clear indication of the major role of fibronectin in EMT through stimulating the activity of TGF-b. In addition, exogenous fibronectin is able to induce EMT under serum-free conditions; this process could be reversed following addition of a TGF-b-neutralizing antibody (Park et al. Citation2011). These data suggest that fibronectin can induce EMT in breast cancers by enhancing the activity of endogenous TGF-b.
Conclusion
Tumour metastasis could be explained as a multistep procedure that leads to dissemination from the primary site of infection and forming secondary tumours at a distant site. There is a complete series of events involved in transforming a solid tumour to the metastatic one: invasion, intravasion, transport, extravasations and formation of colony at some distant site. EMT has shown to play an indispensable part in promoting and spreading metastasis. Proteins E-cadherin, b-catenin, Integrin, Syntenin, Survivin, Periostin are some that have shown to be involved in the interplay of metastasis and EMT. Apart from EMT, various studies have shown the role of MET as one of the important factors in re-differentiation of the tumorous cells at distant places. Thus, it is evident from already established data and experiments that all the events are interlinked and inter-conversion of EMT and MET is required for secondary explosion of tumours at a distant place. miRNA 200 and miRNA 34 have also been found to manifest their impact on the process of metastasis. Thus it is evident from the literature and experimental proofs that metastasis is not controlled by a singular factor but by an amalgam of a complex network involving the entire cellular component starting from RNAs, protein and the webbed network of pathways. Thus the process can be explored further as a target against metastatic movement of cancerous tissues. And also therapeutics could be developed targeting the mechanism via utilizing its various components.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Achison M, Elton CM, Hargreaves PG, Knight CG, Barnes MJ, Farndale RW. 2001. Integrin-independent tyrosine phosphorylation of p125(fak) in human platelets stimulated by collagen. J Biol Chem. 276:3167–3174. doi: 10.1074/jbc.M007186200
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. 2009. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J clin invest. 119(6):1438–1449. doi: 10.1172/JCI38019
- Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T, Margalit H. 2005. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 33:2697–2706. doi: 10.1093/nar/gki567
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R. 2008. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 14(1):79–89. doi: 10.1016/j.ccr.2008.06.005
- Ambros V. 2004. The functions of animal microRNA. Nature. 431:350–355. doi: 10.1038/nature02871
- Andl CD, McCowan KM, Allison GL, Rustgi AK. 2010. Cathepsin B is the driving force of esophageal cell invasion in a fibroblast-dependent manner. Neoplasia. 12:485–498. doi: 10.1593/neo.10216
- Arya M, Patel H, McGURK C, Tatoud R, Klocker H, Masters J, Williamson M. 2004. The importance of the CXCL12-CXCR4 chemokine ligand-receptor interaction in prostate cancer metastasis. J Exp Therap Oncol. 4:291–303.
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. 2008. MicroRNA-21 (miR-21)post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2:72128–72136.
- Bae YK, Kim A, Kim MK, Choi JE, Kang SH, Lee SJ. 2013. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum Pathol. 44:2028–2037. doi: 10.1016/j.humpath.2013.03.006
- Barbera MJ, Puig I, Domínguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, Baulida J, Francí C, Dedhar S, Larue L, de Herreros AG. 2004. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 23(44):7345–7354. doi: 10.1038/sj.onc.1207990
- Barbieri MA, Kohn AD, Roth RA, Stahl PD. 1998. Protein kinase B/akt and Rab5 mediate Ras activation of endocytosis. J Biol Chem. 273:19367–19370. doi: 10.1074/jbc.273.31.19367
- Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297. doi: 10.1016/S0092-8674(04)00045-5
- Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell. 136:215–233. doi: 10.1016/j.cell.2009.01.002
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. 2000. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2:84–89. doi: 10.1038/35000034
- Baum B, Settleman J, Quinlan MP. 2008. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 19:294–308. doi: 10.1016/j.semcdb.2008.02.001
- Bellacosa A, Franke TF, Gonzalez-Portal ME, Datta K, Taguchi T, Gardner J, Cheng JQ, Testa JR, Tsichlis PN. 1993. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene. 8(3):745–754.
- Bellacosa A, Testa JR, Staal SP, Tsichlis PN. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 254(5029):274–277. doi: 10.1126/science.1833819
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al. 2005. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 37:766–770. doi: 10.1038/ng1590
- Berx G, Becker KF, Hofler H, van Roy F. 1998. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 12:226–237. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D
- Birchmeier W, Behrens J. 1994. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1198:11–26.
- Boyer B, Valles AM, Edme N. 2000. Induction and regulation of epithelial–mesenchymal transitions. Biochem Pharmacol. 60:1091–1099. doi: 10.1016/S0006-2952(00)00427-5
- Boyer B, Valles AM, Thiery JP. 1996. Model systems of Epithelium Mesenchyme transitions. Cells Tissues Organs. 156(3):227–239. doi: 10.1159/000147849
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. 2005. Migrating cancer stem cells–an integrated concept of malignant tumour progression. Nat Rev Cancer. 5(9):744–749. doi: 10.1038/nrc1694
- Britt DE, Yang DF, Yang DQ, Flanagan D, Callanan H, Lim YP, Lin SH, Hixson DC. 2004. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Exp cell Res. 300(1):134–148. doi: 10.1016/j.yexcr.2004.06.026
- Brugnera E, Haney C, Grimsley M, Lu SF, Walk AC, Tosello-Trampont IG, Macara HM, Fink GR, Ravichandra KS. 2002. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 4, 574–582.
- Bugge TH, Antalis TM, Wu Q. 2009. Type II transmembrane serine proteases. J Biol Chem. 284:23177–23181. doi: 10.1074/jbc.R109.021006
- Buijs JT, Henriquez NV, van Overveld PGM, van der Horst G, Que I, Schwaninger R, Rentsch C, ten Dijke P, Cleton-Jansen A-M, Driouch K, et al. 2007. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 67:8742–8751. doi: 10.1158/0008-5472.CAN-06-2490
- Buijs JT, van der Horst G, van den Hoogen C, Cheung H, de Rooij B, Kroon J, Petersen M, van Overveld PGM, Pelger RCM, van der Pluijm G. 2012. The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene. 31:2164–2174. doi: 10.1038/onc.2011.400
- Burden RE, Gormley JA, Kuehn D, Ward C, Kwok HF, Gazdoiu M, McClurg A, Jaquin TJ, Johnston JA, Scott CJ, Olwill SA. 2012. Inhibition of cathepsin S by Fsn0503 enhances the efficacy of chemotherapy in colorectal carcinomas. Biochimie. 94:487–493. doi: 10.1016/j.biochi.2011.08.017
- Cabeen MT, Jacobs-Wagner C. 2010. The bacterial cytoskeleton. Annu Rev Genet. 44:365–392. doi: 10.1146/annurev-genet-102108-134845
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. 2000. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2:76–83. doi: 10.1038/35000025
- Carmeliet P, Jain RK. 2011. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 10:417–427. doi: 10.1038/nrd3455
- Castillo SDM. 2012. The SOX family of genes in cancer development: biological relevance and opportunities for therapy. Expert Opin Therap Target. 16:903–919. doi: 10.1517/14728222.2012.709239
- Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. 2006. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 66(23):11271–11278. doi: 10.1158/0008-5472.CAN-06-2044
- Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D'Alessio AC, Young RA, Weinberg RA. 2013. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 154(1):61–74. doi: 10.1016/j.cell.2013.06.005
- Chaffer CL, Weinberg RA. 2011. A perspective on cancer cell metastasis. Science. 331:1559–1564. doi: 10.1126/science.1203543
- Chambers AF, Groom AC, MacDonald IC. 2002. Macdonald dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2:563–572. doi: 10.1038/nrc865
- Chao YC, Pan SH, Yang SC, Yu SL, Che TF, Lin CW, Tsai MS, Chang GC, Wu CH, Wu YY, Lee YC. 2009. Claudin-1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 179(2):123–133. doi: 10.1164/rccm.200803-456OC
- Chen CZ, Li L, Lodish HF, Bartel DP. 2004. MicroRNAs modulate hematopoietic lineage differentiation. Science. 303:83–86. doi: 10.1126/science.1091903
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M. 2005. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 102:13944–13949. doi: 10.1073/pnas.0506654102
- Coussens LM, Fingleton B, Matrisian LM. 2002. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 295:2387–2392. doi: 10.1126/science.1067100
- Croce CM, Calin GA. 2005. miRNAs, cancer, and stem cell division. Cell. 122:6–7. doi: 10.1016/j.cell.2005.06.036
- Croset M, Goehrig D, Frackowiak A, Bonnelye E, Ansieau S, Puisieux A, Clézardin P. 2014. TWIST1 expression in breast cancer cells facilitates bone metastasis formation. J BoneMiner Res. 29:1886–1899. doi: 10.1002/jbmr.2215
- Dalmay T, Edwards DR. 2006. MicroRNAs and the hallmarks of cancer. Oncogene. 25:6170–6175. doi: 10.1038/sj.onc.1209911
- De Craene B, Van Roy F, Berx G. 2005. Unraveling signalling cascades for the Snail family of transcription factors. Cell Signal. 17(5):535–547. doi: 10.1016/j.cellsig.2004.10.011
- Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. 1998. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci. 95(19):11211–11216. doi: 10.1073/pnas.95.19.11211
- Dolmetsch RE, Xu K, Lewis RS. 1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 392:933–936. doi: 10.1038/31960
- Dong C, Wilhelm D, Koopman P. 2004. Sox genes and cancer. Cytogenetic Genome Res. 105:442–447. doi: 10.1159/000078217
- Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, Huttenlocher A. 2001. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 276:48382–48388.
- Dufour A, Overall CM. 2013. Missing the target: matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol Sci. 34:233–242. doi: 10.1016/j.tips.2013.02.004
- Eddy RJ, Pierini LM, Matsumura F, Maxfield FR. 2000. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J Cell Sci. 113:1287–1298.
- Edme N, Downward J, Thiery JP, Boyer B. 2002. Ras induces NBT-II epithelial cell scattering through the coordinate activities of Rac and MAPK pathways. J Cell Sci. 115:2591–2601.
- Eger A, Mikulits W. 2005. Models of epithelial–mesenchymal transition. Drug Discov Today Dis Models. 2:57–63. doi: 10.1016/j.ddmod.2005.04.001
- Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. 2009. Introducing intermediate filaments: from discovery to disease. J Clin Invest, 119:1763–1771. doi: 10.1172/JCI38339
- Fan K, Li D, Zhang Y, Han C, Liang J, Hou C, Xiao H, Ikenaka K, Ma J. 2015. The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroinflammation. J Neuroinflammation. 12(1):1. doi: 10.1186/s12974-015-0268-x
- Fanger CM, Hoth M, Crabtree GR, Lewis RS. 1995. Characterization of T cell mutants with defects in capacitative calcium entry: genetic evidence for the physiological roles of CRAC channels. J Cell Biol. 131:655–667. doi: 10.1083/jcb.131.3.655
- Farady CJ, Sun J, Darragh MR, Miller SM, Craik CS. 2007. The mechanism of inhibition of antibody-based inhibitors of membrane-type serine protease 1 (MT-SP1). J Mol Biol. 369:1041–1051. doi: 10.1016/j.jmb.2007.03.078
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 441:179–185. doi: 10.1038/nature04702
- Feske S, Prakriya M, Rao A, Lewis RS. 2005. A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med. 202:651–662. doi: 10.1084/jem.20050687
- Fidler IJ. 2003. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 3, 453–458. doi: 10.1038/nrc1098
- Fingleton B. 2008. MMPs as therapeutic targets—still a viable option? Semin Cell Dev Biol. 19:61–68. doi: 10.1016/j.semcdb.2007.06.006
- Friedman RC, Farh KK, Burge CB, Bartel DP. 2008. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19:92–105. doi: 10.1101/gr.082701.108
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Huttenlocher A, Palecek SP, et al. 1997. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 272:32719–32722. doi: 10.1074/jbc.272.21.13816
- Galkin AV, Mullen L, Fox WD, Brown J, Duncan D, Moreno O, Madison EL, Agus DB. 2004. CVS-3983, a selective matriptase inhibitor, suppresses the growth of androgen independent prostate tumour xenografts. Prostate. 61:228–235. doi: 10.1002/pros.20094
- Goldman RD, Khuon S, Chou YH, Opal P, Steinert PM. 1996. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 134:971–983. doi: 10.1083/jcb.134.4.971
- Gonzalez de Castro D, Clarke PA, Al-Lazikani B, Workman P. 2013. Personalized cancer medicine: molecular diagnostics, predictive biomarkers, and drug resistance. Clin Pharmacol Ther. 93(3):252–259. doi: 10.1038/clpt.2012.237
- Gregory SG, Barlow KF, McLay KE, Kaul R, Swarbreck D, Dunham A, Scott CE, Howe KL, Woodfine K, Spencer CC, et al. 2006. The DNA sequence and biological annotation of human chromosome 1. Nature. 441:315–321. doi: 10.1038/nature04727
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. 2008. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601. doi: 10.1038/ncb1722
- Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. 2003. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
- Gupta GP, Massagué J. 2006. Cancer metastasis: building a framework. Cell. 127(4):679–695. doi: 10.1016/j.cell.2006.11.001
- Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell. 100:57–70. doi: 10.1016/S0092-8674(00)81683-9
- Humphries B, Wang Z, Oom AL, Fisher T, Tan D, Cui Y, Jiang Y, Yang C. 2014. MicroRNA-200b targets protein kinase C and suppresses triple-negative breast cancer metastasis. Carcinogenesis. 35:2254–2263. doi: 10.1093/carcin/bgu133
- Hüsemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G, Klein CA. 2008. Systemic spread is an early step in breast cancer. Cancer Cell. 13(1):58–68. doi: 10.1016/j.ccr.2007.12.003
- Huttenlocher A, Horwitz AR. 2011. Integrins in cell migration. CSH Perspect Biol. 3(9):a005074.
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 377:539–544. doi: 10.1038/377539a0
- Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. 2010. Loss of miR-200 inhibition of SUZ12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 39:761–772. doi: 10.1016/j.molcel.2010.08.013
- Jain RK. 2005. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 307:58–62. doi: 10.1126/science.1104819
- Jakowlew SB. 2006. Transforming growth factor-beta in cancer and metastasis. Caner Metastasis Rev. 25:435–457. doi: 10.1007/s10555-006-9006-2
- Jevnikar Z, Rojnik M, Jamnik P, Doljak B, Fonovic UP, Kos J. 2013. Cathepsin H mediates the processing of talin and regulates migration of prostate cancer cells. J Biol Chem. 288:2201–2209. doi: 10.1074/jbc.M112.436394
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. 2005. RAS is regulated by the let-7 microRNA family. Cell. 120:635–647. doi: 10.1016/j.cell.2005.01.014
- Kamachi Y, Kondoh H. 2013. Sox proteins: regulators of cell fate specification and differentiation. Development. 140:4129–4144. doi: 10.1242/dev.091793
- Kang Y, Massagué J. 2004. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 118(3):277–279. doi: 10.1016/j.cell.2004.07.011
- Kassis J, Lauffenburger DA, Turner T, Wells A. 2001. Semin. Cancer Biol. 11:105–117. doi: 10.1006/scbi.2000.0362
- Katsumoto T, Mitsushima A, Kurimura T. 1990. The role of the vimentin intermediate filaments in rat 3Y1 cells elucidated by immunoelectron microscopy and computer-graphic reconstruction. Biol Cell. 68:139–146. doi: 10.1016/0248-4900(90)90299-I
- Keller JM, Schade GR, Ives K, Cheng X, Rosol TJ, Piert M, Siddiqui J, Roberts WW, Keller ET. 2013. A novel canine model for prostate cancer. Prostate. 73:952–959. doi: 10.1002/pros.22642
- Khan MN, Bhattacharyya T, Andrikopoulos P, Esteban MA, Barod R, Connor T, Ashcroft M, Maxwell PH, Kiriakidis S. 2011. Factor inhibiting HIF (FIH-1) promotes renal cancer cell survival by protecting cells from HIF-1α-mediated apoptosis. Brit J Cancer. 104(7):1151–1159. doi: 10.1038/bjc.2011.73
- Kim S, Lee UJ, Kim MN, Lee EJ, Kim JY, Lee MY, Choung S, Kim YJ, Choi Y-C. 2008. MicroRNA miR-199a* regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2). J Biol Chem. 283:18158–18166. doi: 10.1074/jbc.M800186200
- Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. 2007. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 67:546–554. doi: 10.1158/0008-5472.CAN-06-2401
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. 2001. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 21(3):893–901. doi: 10.1128/MCB.21.3.893-901.2001
- Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, et al. 2011. P53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 208:875–883. doi: 10.1084/jem.20110235
- Klein R. 2001. Excitatory Eph receptors and adhesive ephrin ligands. Curr Opin Cell Biol. 13(2):196–203. doi: 10.1016/S0955-0674(00)00197-6
- Kleinerman DI, Troncoso P, Lin SH, Pisters LL, Sherwood ER, Brooks T, von Eschenbach AC, Hsieh JT. 1995. Consistent expression of an epithelial cell adhesion molecule (C-CAM) during human prostate development and loss of expression in prostate cancer: implication as a tumor suppressor. Cancer Res. 55(6):1215–1220.
- Korpal M, Ell BJ, Buffa FM, Ibrahum T, Blanco MA, Celia-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, et al. 2011. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 17:1101–1108. doi: 10.1038/nm.2401
- Komuro H, Rakic P. 1993. Modulation of neuronal migration by NMDA receptors. Science. 260:95–95. doi: 10.1126/science.8096653
- Krueger S, Kalinski T, Hundertmark T, Wex T, Küster D, Peitz U, Ebert M, Nägler DK, Kellner U, Malfertheiner P, et al. 2005. Up-regulation of cathepsin X in helicobacter pylori gastritis and gastric cancer. J Pathol. 207:32–42. doi: 10.1002/path.1820
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. 2002. Identification of tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. doi: 10.1016/S0960-9822(02)00809-6
- Lawson MA, Maxfield FR. 1995. Ca2+ and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 377:75–79. doi: 10.1038/377075a0
- Leader M, Collins M, Patel J, Henry K. 1987. Vimentin: an evaluation of its role as a tumour marker. Histopathology. 11:63–72. doi: 10.1111/j.1365-2559.1987.tb02609.x
- Leber MF, Efferth T. 2009. Molecular principles of cancer invasion and metastasis. Int J Oncol. 34:881–895.
- Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. 1999. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 400:382–386. doi: 10.1038/22578
- Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. 2007. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 26:3957–3967. doi: 10.1038/sj.emboj.7601818
- Leland ES, Wright PK. 2006. Resonance tuning of piezoelectric vibration energy scavenging generators using compressive axial preload. Smart Mater Struct. 15(5):1413–1420. doi: 10.1088/0964-1726/15/5/030
- Leelawat K, Leelawat S, Tepaksorn P, Rattanasinganchan P, Leungchaweng A, Tohtong R, Sobhon P. 2006. Involvement of c-Met/hepatocyte growth factor pathway in cholangiocarcinoma cell invasion and its therapeutic inhibition with small interfering RNA specific for c-Met. J Surg Res. 136:78–84. doi: 10.1016/j.jss.2006.05.031
- Lesnikoff N, Attema JL, Roslan S, Bert AG, Schwarz QP, Gregory PA, Goodall GJ. 2014. Specificity protein 1 (Sp1) maintains basal epithelial expression of the miR-200 family: implications for epithelial–mesenchymal transition. J Biol Chem. 289:11194–11205. doi: 10.1074/jbc.M113.529172
- Lewis RS. 2007. The molecular choreography of a store-operated calcium channel. Nature. 446:284–287. doi: 10.1038/nature05637
- Lewis RS. 2001. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 19:497–521. doi: 10.1146/annurev.immunol.19.1.497
- Lewis BP, Burge CB, Bartel DP. 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 120:15–20. doi: 10.1016/j.cell.2004.12.035
- Li P, Jiang S, Lee SL, Lin CY, Johnson MD, Dickson RB, Michejda CJ, Roller PP. 2007. Design and synthesis of novel and potent inhibitors of the type II transmembrane serine protease, matriptase, based upon the sunflower trypsin inhibitor-1. J Med Chem. 50:5976–5983. doi: 10.1021/jm0704898
- Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B. 2010. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 7(1):51–63. doi: 10.1016/j.stem.2010.04.014
- Li X, Pei D, Zheng H. 2014. Transitions between epithelial and mesenchymal states during cell fate conversions. Protein Cell. 5(8):580–591. doi: 10.1007/s13238-014-0064-x
- Li X, Roslan S, Johnstone CN, Wright JA, Bracken CP, Anderson M, Bert AG, Selth LA, Anderson RL, Goodall GJ, et al. 2014. MiR-200 can repress breast cancer metastasis through ZEB1-independent but moesin-dependent pathways. Oncogene. 33:4077–4088. doi: 10.1038/onc.2013.370
- Li G, Shrotriya V, Huang J, Yao Y, Moriarty T, Emery K, Yang Y. 2005. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat Mater. 4(11):864–868. doi: 10.1038/nmat1500
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. 2005. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 433:769–773. doi: 10.1038/nature03315
- Lim LP, Lau NC, Weinstein EG, Abdelhakim A, Yekta S, Rhoades MW, Burge CB, Bartel DP. 2003. The microRNAs of Caenorhabditis elegans. Genes Dev. 17:991–1008. doi: 10.1101/gad.1074403
- Lin SL, Chiang A, Chang D, Ying SY. 2008. Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 14:417–424. doi: 10.1261/rna.874808
- Lin CY, Tseng IC, Chou FP, Su SF, Chen YW, Johnson MD, Dickson RB. 2008. Zymogen activation, inhibition, and ectodomain shedding of matriptase. Front Biosci. 13:621–635. doi: 10.2741/2707
- Linder S. 2009. Invadosomes at a glance. J Cell Sci. 122(17):3009–3013. doi: 10.1242/jcs.032631
- Liou J, Kim ML, Do Heo W, Jones JT, Myers JW, Ferrell JE, Meyer T. 2005. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055
- List K. 2009. Matriptase: a culprit in cancer? Future Oncol. 5:97–104. doi: 10.2217/14796694.5.1.97
- List K, Bugge TH, Szabo R. 2006. Matriptase: potent proteolysis on the cell surface. Mol Med. 12:1–7. doi: 10.2119/2006-00022.List
- Liu H, Wang S, Sun H, Pan Z, Zhou W, Wu M. 2010. Inhibition of tumorigenesis and invasion of hepatocellular carcinoma by siRNA mediated silencing of the livin gene. Mol Med Report. 3:903–907.
- Liu Z, Zhang B, Liu K, Ding Z, Hu X. 2012. Schisandrin B attenuates cancer invasion and metastasis via inhibiting epithelial-mesenchymal transition. PLoS ONE. 7(7):e40480. doi: 10.1371/journal.pone.0040480
- Ma L, Teruya-Feldstein J, Weinberg RA. 2007. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 449(7163):682–688. doi: 10.1038/nature06174
- Ma PC, Tretiakova MS, Nallasura V, Jagadeeswaran R, Husain AN, Salgia R. 2007. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 97:368–377. doi: 10.1038/sj.bjc.6603884
- Mallini P, Lennard T, Kirby J, Meeson A. 2014. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev. 40(3):341–348. doi: 10.1016/j.ctrv.2013.09.008
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL. 2008. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 133(4):704–715. doi: 10.1016/j.cell.2008.03.027
- Marks PW, Maxfield FR. 1990. Transient increases in cytosolic free calcium appear to be required for the migration of adherent human neutrophils. J. Cell Biol. 110:43–52. doi: 10.1083/jcb.110.1.43
- McKenzie JA, Liu T, Jung JY, Jones BB, Ekiz HA, Welm AL, Grossman D. 2013. Survivin promotion of melanoma metastasis requires upregulation of α5 integrin. Carcinogenesis. 34(9):2137–2144. doi: 10.1093/carcin/bgt155
- Mehlen P, Puisieux A. 2006. Metastasis: a question of life or death. Nat Rev Cancer. 6:449–458. doi: 10.1038/nrc1886
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney Jr, JW. 2006. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem, 281:24979–24990. doi: 10.1074/jbc.M604589200
- Michael MZ, O’Connor SM, van Holst Pellekaan NG, Young GP, James RJ. 2003. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 1:882–891.
- Mogami H, Nakano K, Tepikin AV, Petersen OH. 1997. Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell. 88:49–55. doi: 10.1016/S0092-8674(00)81857-7
- Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. 2008. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PloS one. 3(8):e2888. doi: 10.1371/journal.pone.0002888
- Musiyenko A, Bitko V, Barik S. 2008. Ectopic expression regulates protein translation and invasiveness of prostate cancer LNCaP cells. J Mol Med. 86:313–322. doi: 10.1007/s00109-007-0296-9
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. 2000. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat Rec, 258:119–145. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U
- Nakaya Y, Kuroda S, Katagiri YT, Kaibuchi K, Takahashi Y. 2004. Mesenchymal–epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev Cell. 7:425–438. doi: 10.1016/j.devcel.2004.08.003
- Napp J, Dullin C, Muller F, Uhland K, Petri JB, van de Locht A, Steinmetzer T, Alves F. 2010. Time-domain in vivo near infrared fluorescence imaging for evaluation of matriptase as a potential target for the development of novel, inhibitor-based tumour therapies. Int J Cancer. 127:1958–1974. doi: 10.1002/ijc.25405
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. 2003. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 423, 990–995. doi: 10.1038/nature01751
- Novak A, Hsu SC, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R, Dedhar S. 1998. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc Natl Acad Sci USA. 95:4374–4379. doi: 10.1073/pnas.95.8.4374
- Ocaña OH, Córcoles R, Fabra Á, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. 2012. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 22(6):709–724. doi: 10.1016/j.ccr.2012.10.012
- Palmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, Puig I, Rodriguez R, de la Fuente R, Bernad A, et al. 2004. Nat Med. 10:917–919. doi: 10.1038/nm1095
- Parekh AB, Penner R. 1997. Store depletion and calcium influx. Physiol Rev. 7:901–930.
- Park SM, Gaur AB, Lengyel E, Peter ME. 2008. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 22:894–907. doi: 10.1101/gad.1640608
- Park HS, Kim MS, Huh SH, Park J, Chung J, Kang SS, Choi EJ. 2002. Akt (protein kinase B) negatively regulates SEK1 by means of protein phosphorylation. J Biol Chem. 277(4):2573–2578. doi: 10.1074/jbc.M110299200
- Park CY, Kim DK, Sheen YY. 2011. EW-7203, a novel small molecule inhibitor of transforming growth factor-ß (TGF-ß) type I receptor/activin receptor/like kinase-5, blocks TGF-ß1- mediated epithelial-to-mesenchymal transition in mammary epithelial cells. Cancer Sci. 102:1889–1896. doi: 10.1111/j.1349-7006.2011.02014.x
- Parkin DM, Bray F, Ferlay J, Pisani P. 2005. Global cancer statistics. CA Cancer J Clin. 55:74–108. doi: 10.3322/canjclin.55.2.74
- Patrussi L, Baldari CT. 2011. The CXCL12/CXCR4 axis as a therapeutic target in cancer and HIV-1 infection. Curr Med Chem. 18(4):497–512. doi: 10.2174/092986711794480159
- Pavlaki M, Zucker S. 2003. Matrix metalloproteinase inhibitors (MMPIs): the beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev. 22:177–203. doi: 10.1023/A:1023047431869
- Pavlaki M, Zucker S, Dufour A, Calabrese N, Bahou W, Cao J. 2010. Furin functions as a nonproteolytic chaperone for matrix metalloproteinase-28: MMP-28 propeptide sequence requirement. Biochem res int. 2011. doi:10.1155/2011/630319.
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. 2006. Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat Cell Biol. 8:771–773. doi: 10.1038/ncb1435
- Pettit EJ, Fay FS. 1998. Cytosolic free calcium and the cytoskeleton in the control of leukocyte chemotaxis. Physiol Rev. 78:949–967.
- Pillai RS. 2005. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 11:1753–1761. doi: 10.1261/rna.2248605
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature. 443:230–233. doi: 10.1038/nature05122
- Pruitt FL, He Y, Franco OE, Jiang M, Cates JM, Hayward SW. 2013. Cathepsin D acts as an essential mediator to promote malignancy of benign prostatic epithelium. Am J Pathol. 73:476–488.
- Quintanilla-Dieck MJ, Codriansky K, Keady M, Bhawan J, Rünger TM. 2008. Cathepsin K in melanoma invasion. J Invest Dermatol. 128:2281–2288. doi: 10.1038/jid.2008.63
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. 2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 436:123–127. doi: 10.1038/nature03688
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. 2000. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 113:3673–3678.
- Ren G, Tian Q, An Y, Feng B, Lu Y, Liang J, Li K, Shang Y, Nie Y, Wang X, Fan D. 2012. Coronin 3 promotes gastric cancer metastasis via the up-regulation of MMP-9 and cathepsin K. Mol Cancer. 11:67. doi: 10.1186/1476-4598-11-67.
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G. 2005. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 169(3):435–445. doi: 10.1083/jcb.200502019
- Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL. 2010. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 7(1):64–77. doi: 10.1016/j.stem.2010.04.015
- Sarkar A, Hochedlinger K. 2013. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 12:15–30. doi: 10.1016/j.stem.2012.12.007
- Saunders LR, McClay DR. 2014. Sub-circuits of a gene regulatory network control a developmental epithelial-mesenchymal transition. Development. 141:1503–1513. doi: 10.1242/dev.101436
- Sela-Passwell N, Trahtenherts A, Kruger A, Sagi I. 2011. New opportunities in drug design of metalloproteinase inhibitors: combination between structure–function experimental approaches and systems biology. Expert Opin Drug Discov. 6:527–542. doi: 10.1517/17460441.2011.560936
- Sengupta S, den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, Chen C-J, Hildesheim A, Sugden B, Ahlquist P. 2008. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA. 105:5874–5878. doi: 10.1073/pnas.0801130105
- Senol O, Schaaij-Visser TBM, Erkan EP, Dorfer C, Lewandrowski G, Pham TV, Piersma SR, Peerdeman SM, Ströbel T, Tannous B, et al. 2014. miR-200a-mediated suppression of non-muscle heavy chain IIb inhibits meningioma cell migration and tumor growth in vivo. Oncogene. 120(14):1790–1798.
- Shen X, Wang S, Wang H, Liang M, Xiao L, Wang Z. 2009. The role of SDF-1/ CXCR4 axis in ovarian cancer metastasis. J Huazhong Univ Sci Technol Med Sci. 29:363–367. doi: 10.1007/s11596-009-0320-0
- Shi YE, Torri J, Yieh L, Wellstein A, Lippman ME, Dickson RB. 1993. Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res. 53:1409–1415.
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, Dirbas FM. 2009. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 138(3):592–603. doi: 10.1016/j.cell.2009.07.011
- Siciliano JC, Toutant M, Derkinderen P, Sasaki T, Girault JA. 1996. Differential regulation of proline-rich tyrosine kinase 2/cell adhesion kinase beta (PYK2/CAKbeta) and pp125(FAK) by glutamate and depolarization in rat hippocampus. J Biol Chem. 271:28942–28946. doi: 10.1074/jbc.271.46.28942
- Siegel R, Ma J, Zou Z, Jemal A. 2014. Cancer statistics. CA Cancer J Clin. 64: 9–29 doi: 10.3322/caac.21208
- Sipeki S, Bander E, Buday L, Farkas G, Bácsy E, Ways DK, Faragó A. 1999. Phosphatidylinositol 3-kinase contributes to Erk1/Erk2 MAP kinase activation associated with hepatocyte growth factor-induced cell scattering. Cell Signal. 11:885–890. doi: 10.1016/S0898-6568(99)00060-1
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. 2006. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 281:20661–20665. doi: 10.1074/jbc.C600126200
- Sonoshita M, Aoki M, Fuwa H, Aoki K, Hosogi H, Sakai Y, Hasida H, Takabayashi A, Sasaki M, Robine S, et al. 2011. Suppression of colon cancer metastasis by Aes through inhibition of notch signaling. Cancer Cell. 19:125–137. doi: 10.1016/j.ccr.2010.11.008
- Staal SP. 1987. Proc Natl Acad Sci USA. 84:5034–5037. doi: 10.1073/pnas.84.14.5034
- Steeg PS. 2006. Tumour metastasis: mechanistic insights and clinical challenges. Nat Med. 12:895–904. doi: 10.1038/nm1469
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S. 1997. Construction and characterization of a full length-enriched and a 5′-end-enriched cDNA library. Gene. 200:149–156. doi: 10.1016/S0378-1119(97)00411-3
- Tan C, Costello P, Sanghera J, Dominguez D, Baulida J, de Herreros AG, Dedhar S. 2001. Inhibition of integrin linked kinase (ILK) suppresses b-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene. 20:133–140. doi: 10.1038/sj.onc.1204052
- Tarver T. 2012. Cancer Facts & Figures 2012. American Cancer Society (ACS) Atlanta, GA: American Cancer Society, 2012. J Consum Health Internet. 16(3):366–367. doi: 10.1080/15398285.2012.701177
- Tavazoie SF, Alarcón C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massagué J. 2008. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 451:147–152. doi: 10.1038/nature06487
- Testa JR, Bellacosa A. 2001. Proc Natl Acad Sci USA. 98:10983–10985. doi: 10.1073/pnas.211430998
- Thiery JP. 2002. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2:442–454. doi: 10.1038/nrc822. PMID 12189386.
- Thiery JP, Acloque H, Huang RYJ, Nieto MA. 2009. Epithelial–mesenchymal transitions in development and disease. Cell. 139(5):871–890. doi: 10.1016/j.cell.2009.11.007
- Thiery JP, Morgan M. 2004. Breast cancer progression with a Twist. Nat med. 10(8):777–778. doi: 10.1038/nm0804-777
- Thompson NL, Lin SH, Panzica MA, Hixson DC. 1994. Cell CAM 105 isoform RNA expression is differentially regulated during rat liver regeneration and carcinogenesis. Pathobiology. 62(4):209–220. doi: 10.1159/000163912
- Thompson EW, Newgreen DF. 2005. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 65(14):5991–5995. doi: 10.1158/0008-5472.CAN-05-0616
- Thorgeirsson SS, Grisham JW. 2002. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 31:339–346. doi: 10.1038/ng0802-339
- Tian Q, Feetham MC, Tao WA, He XC, Li L, Aebersold R, Hood L. 2004. Proteomic analysis identifies that 14-3-3zeta interacts with beta-catenin and facilitates its activation by Akt. Proc Natl Acad Sci USA. 101:15370–15375. doi: 10.1073/pnas.0406499101
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. 2004. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 18:99–115. doi: 10.1101/gad.276304
- Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. 2012. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 22(6):725–736. doi: 10.1016/j.ccr.2012.09.022
- Van Dusen NJ, Firulli AB. 2012. Twist factor regulation of non-cardiomyocyte cell lineages in the developing heart. Differentiation. 84:79–88. doi: 10.1016/j.diff.2012.03.002
- Veeraraghavalu K, Subbaiah VK, Srivastava S, Chakrabarti O, Syal R, Krishna S. 2005. Complementation of human papillomavirus type 16 E6 and E7 by Jagged1-specific Notch1-phosphatidylinositol 3-kinase signaling involves pleiotropic oncogenic functions independent of CBF1;Su(H);Lag-1 activation. J Virol. 79:7889–7898. doi: 10.1128/JVI.79.12.7889-7898.2005
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. 2006. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 312(5777):1220–1223. doi: 10.1126/science.1127883
- Vleminckx K, Vakaet Jr, L, Mareel M, Fiers W, van Roy F. 1991. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 66:107–119. doi: 10.1016/0092-8674(91)90143-M
- Wang Z, Humphries B, Xiao H, Jiang Y, Yang C. 2014. MicroRNA-200b suppresses arsenic-transformed cell migration by targeting protein kinase Cα and Wnt5b protein kinase Cα-positive feedback loop and inhibiting Rac1 activation. J Biol Chem. 289:18373–18386. doi: 10.1074/jbc.M114.554246
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 6:154–161. doi: 10.1038/ncb1094
- Wegner SV, Arslan H, Sunbul M, Yin J, He C. 2010. Dynamic copper (I) imaging in mammalian cells with a genetically encoded fluorescent copper (I) sensor. J Am Chem Soc. 132(8):2567–2569. doi: 10.1021/ja9097324
- Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH, Fidler IJ, Hung MC. 2008. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 13(5):385–393. doi: 10.1016/j.ccr.2008.03.015
- Wells A, Yates C, Shepard CR. 2008. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metast. 25:621–628. doi: 10.1007/s10585-008-9167-1
- Weston CR, Davis RJ. 2001. Signal transduction – signaling specificity – a complex affair. Science. 292:2439–2440. doi: 10.1126/science.1063279
- Williams LV, Veliceasa D, Vinokour E, Volpert OV. 2013. miR-200b inhibits prostate cancer, EMT, growth and metastasis. PLoS ONE. 34(6):1735–1744.
- Wissmüller S, Kosian T, Wolf M, Finzsch M, Wegner M. 2006. The high-mobility-group domain of Sox proteins interacts with DNA-binding domains of many transcription factors. Nucleic Acids Res. 34:1735–1744. doi: 10.1093/nar/gkl105
- Wu C. 1999. Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J Cell Sci. 112:4485–4489.
- Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S. 1998. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem. 273(1):528–536. doi: 10.1074/jbc.273.1.528
- Wu F, Yang LY, Li YF, Ou DP, Chen DP, Fan C. 2009. Novel role for epidermal growth factor-like domain 7 in metastasis of human hepatocellular carcinoma. Hepatology. 50:1839–1850. doi: 10.1002/hep.23197
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. 2007. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823
- Xue G, Hemmings BA. 2012. Phosphorylation of basic helix-loop-helix transcription factor twist in development and disease. Biochem Soc Trans. 40:90–93. doi: 10.1042/BST20110678
- Yang YL, Ju HZ, Liu SF, Lee TC, Shih YW, Chuang LY, Guh J-Y, Yang Y-Y, Liao T-N, Hung T-J, Hung M-Y. 2011. BMP-2 suppresses renal interstitial fibrosis by regulating epithelial–mesenchymal transition. J Cell Biochem. 112:2558–2565. doi: 10.1002/jcb.23180
- Yang Y, Lim SK, Choong LY, Lee H, Chen Y, Chong PK, Ashktorab H, Wang TT, Salto-Tellez M, Yeoh KG, et al. 2010. Cathepsin S mediates gastric cancer cell migration and invasion via a putative network of metastasis-associated proteins. J Proteome Res. 9:4767–4778. doi: 10.1021/pr100492x
- Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. 2005. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail’s subcellular localization and functions. Cancer Res. 65:3179–3184.
- Yang J, Weinberg RA. 2008. Epithelial-mesenchymal transition: at the crossroads of development and tumour metastasis. Dev Cell. 14:818–829. doi: 10.1016/j.devcel.2008.05.009
- Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng S-C, Wu K-J. 2008. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 10:295–305. doi: 10.1038/ncb1691
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. 2006. Cahalan molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 443:226–229. doi: 10.1038/nature05108
- Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, et al. 2000. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 27:561–572. doi: 10.1016/S0896-6273(00)00066-0
- Zamore PD, Haley B. 2005. Ribo-gnome: the big world of small RNAs. Science. 309:1519–1524. doi: 10.1126/science.1111444
- Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, et al. 2013. Hypoxia induces epithelial–mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1a in hepatocellular carcinoma. BMC Cancer. 13:444. doi: 10.1186/1471-2407-13-108. doi: 10.1186/1471-2407-13-444
- Zhang K, Zhang S, Jiao X, Wang H, Zhang D, Niu Z, Shen Y, Lv L, Zhou Y. 2010. Slug regulates proliferation and invasiveness of esophageal adenocarcinoma cells in vitro and in vivo. Med Oncol. 28:1089–1100. doi: 10.1007/s12032-010-9652-7
- Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. 2008. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 18:350–359. doi: 10.1038/cr.2008.24
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. 2006. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci USA. 103(24):9357–9362. ( Export to RIS). doi: 10.1073/pnas.0603161103