ABSTRACT
The neurophysiological basis of social ranking perception underlying the execution of cooperative joint-actions was explored in the present study. Self-perception of social ranking, personality trait (Behavioral Activation System (BAS) and locus of control (LoC)) and alpha brain oscillations were considered. Subjects were required to match their cognitive performance in terms of accuracy (error rate) and response time. A positive feedback condition of a better joint-performance was provided and compared to absence of feedback. It was found that higher BAS participants and higher internal LoC responded in greater measure to post-feedback condition with better real performance probably based on their sensitivity to rewarding for high-BAS and the increased sense of self-efficacy. Moreover, higher-BAS showed an increased frontal left activity when they perceived increased cooperative efficacy. The present results confirmed the tendency to modulate both self-perceived social position and real performance based on the personal attitudes and the frontal.
Introduction
Only in the recent years, neuroscience begun to explore how the brain represents social hierarchies and social status (Freeman et al. Citation2009). Previous research suggested an important role for social interactions and social self-perception in achieving accurate self-knowledge and self-improvement, particularly in response to performance-related social comparisons and to social status in the context of performance-based feedback (Munafò et al. Citation2005). This direct comparison between subjects on a specific task may or may not improve our rank perception and social status representation in term of efficacy, taking into account the existing inter-personal condition. Indeed, in human beings, social hierarchies can be established along various dimensions: we can be socially ranked according to ability or skill, as well as economic, physical, and professional standing. Moreover, social status perception was shown to reciprocally affect performance on tasks that involve comparing our own performance with that of others (Munafò et al. Citation2005).
Recent social neuroscience studies showed that distinct neural systems are involved in the experience of social hierarchy and social status, and that activity within these brain regions is modulated by individual and personality factors. A neural circuit linking limbic, prefrontal cortex (PFC), and striatal structures was found to reflect the emotional, cognitive, and behavioral components of rank-related social interactions (Levitan et al. Citation2000). Recent investigations examining the structure and function of brain areas associated with social perception, social efficacy, and social ranking offer preliminary support for this neural mechanism of a human social system. Dorsal (DLPFC) and ventral (VLPFC) portions of lateral PFC – brain regions typically associated with regulating socioemotional responses and behavioral inhibition – are recruited during social status inference (Chiao et al. Citation2009; Balconi and Pagani Citation2014, Citation2015; Balconi and Vanutelli Citation2016). The engagement of DLPFC and VLPFC regions during the observation of social interactions and social status implications probably reflects recruitment of brain regions that can exert top-down control over specific processes, such as emotional responses to social hierarchy, to orchestrate a socially appropriate status response (Marsh et al. Citation2009).
Therefore, it is crucial to consider the implication of cortical areas, mainly the PFC, which was shown to be activated in response to social ranking perception in conjunction with some specific contextual conditions, i.e. subjective performance (self-perception of status due to cognitive skills for cooperation or competition) (Balconi and Pagani Citation2014, Citation2015; Balconi and Vanutelli Citation2016,Citation2017) and personality components, such as emotional and motivational behavior (Hall et al. Citation2005; Chiao et al. Citation2008). Indeed, it is relevant to distinguish the self-perception of our social efficacy and our position within a hierarchy in different conditions, that is in competitive or in cooperative conditions. Some previous studies explored the effect of competition on self-perception, efficacy in social interaction, and social ranking within the social hierarchy. It was found that competition may increase the effective subjective performance and perception of higher social ranking, but it contemporarily may induce a decreased sense of in-group and make the sense of social membership more weak (Goldman et al. Citation1977). That is the subject would pay for his/her better performance in terms of ‘being less socially part of ’.
Less studies applied similar paradigms in cooperative conditions (Funane et al. Citation2011; Cui et al. Citation2012; Chung et al. Citation2015). It was shown that cooperative strategies reinforce the sense of in-group, self-efficacy, and perception of higher social position as well as social well-being (Goldman et al. Citation1977). However in some cases, the real performance was worse than in competitive condition (Funane et al. Citation2011). In addition, the perceived improved efficacy during the cooperative task (with the sense of being able to produce a better performance) may induce also higher social ranking perception. For this reason, the ‘reinforce’ of self-perceived efficacy during a cooperation may be a usable tool to modulate the social perceived efficacy and the direct impact on the real performance (Balconi and Pagani Citation2015).
However, compared with previous research, two relevant aspects were underestimated and should be considered to evaluate the cooperative effect on social self-perception: the presence of a real cooperative/competitive dynamic interaction between co-partners, during the performance (Montague Citation2002); some personality effects related to emotional (such as approach/withdrawal attitude to emotions) (Gray Citation1990) and self-perception of internal/external control (such as locus of control (LoC)) (Rotter Citation1966).
About the first aspect, in the present research the subject’s scoring on a cognitive task, which dynamically modulated the subject’s perceived status in terms of performance, was artificially manipulated in a dyadic vis-à-vis cooperative condition which stressed the joint-effect of a coordinated strategy. Thus, we created a dyadic interactive task which strongly reinforced social relationship. In contrast with previous studies (Zink et al. Citation2008), we included a more ecologic task, where subjects were required to compare their performance constantly with that of the other subject. Specifically, a direct comparison with the second subject (interlocutor, I) was required so that the dynamic improved performances were constantly compared between partners. This comparison tested the effect of the subject’s own status modification related to I’s status. The real performance was also tested (better or worse attentional performance) in response to this fictitious increasing scoring.
Secondly, about the personality and motivational components, the way individuals judge their social ranking positions partially depends on some personality factors, such as the degree to which their own behavior is balanced between ‘approaching’ in response to rewards and non-punishments and ‘withdrawing’ from non-reward and punishments. These emotional and motivational components appear to be highly relevant with respect to social hierarchies. Indeed, recent research found that emotions are able to regulate the social hierarchies by inducing more positive versus negative predispositions in social relationships. Specifically, it was previously found that subjects with a higher the Behavioral Activation System (BAS; Gray Citation1994) were more likely to relate to the dominant character in a dyadic interaction, which was found to induce a positive effect, while those with a higher the Behavioral Inhibition System (BIS; Farrow et al. Citation2011) were more inclined to relate to the submissive character, inducing a negative affect (Demaree Citation2005). Moreover, in our previous research, a significant BAS effect was found in distinguishing social hierarchy (Balconi and Pagani Citation2014; Balconi, Crivelli, Vanutelli Citation2017). The BAS system is responsible for both approach and active behaviors, and emotions associated with these behaviors generally induce the subject to approach to situations that have generated the emotional response. The BAS is conceptualized as a motivational system that is sensitive to signals of reward, non-punishment, and that is important for engaging behavior toward a reward. In addition, BAS has been associated with feelings of optimism, sociality, and dominance (Gable et al. Citation2000; Gray and McNaughton Citation2000; Balconi and Mazza Citation2009, Citation2010). People with highly sensitive BAS may respond in great measure to approach-related emotional contexts that allow the subject to have a favorable and dominant behavior toward the environment (Davidson et al. Citation1990; Tomarken et al. Citation1992; Balconi et al. Citation2009a, Citation2009b, Citation2012). Conversely, highly sensitive BIS people inhibit behavior in response to stimuli that are novel, innately feared, and conditioned to be aversive. Thus, the BIS is conceptualized as a system that is sensitive to cues of punishment and non-reward, and that functions to interrupt ongoing behavior in order to facilitate the processing of these cues in preparation for a response (Fowles Citation2000; Yu and Dayan Citation2005). Gray also held that BIS functioning is responsible for the experience of negative feelings such as fear and anxiety in response to these cues (Gray Citation1987, Citation1994).
About the cortical correlates of these systems, left PFC was shown to support the approach-related motivations and emotions, whereas the right PFC was found to be involved in withdrawal-related motivations and emotions (Bechara et al. Citation1999; Bechara and Martin Citation2004; Balconi and Mazza Citation2010; Balconi et al. Citation2012). These lateralized approach and withdrawal or punishment reward systems are viewed as mutually inhibitory. Therefore, the role of these two antithetic prefrontal systems, on the one hand, and that of the frontal ‘social’ brain circuit, on the other hand, was supposed to be able to elucidate the social self-perception hierarchy. Thus, we may suppose that, based on the lateralized approach/withdrawal model, there are different contributions of the left and right hemispheres on self-perception of social ranking.
A second personality component was the responsiveness to social situations. A relevant concept was identified in the ‘locus of control’ (LoC) which refers to the extent to which individuals believe they can control events affecting them. Indeed subjective ‘locus’ is conceptualized as either internal – the person believes they can control their life – or external – meaning subject believes his decisions and life are controlled by environmental factors which they cannot influence, or by chance. In general, about the perception of social ranking, it was shown that high level of internal LoC may be predictive of perceived and real higher self-efficacy, as shown in some recent research in which LoC predicted higher job-related self-efficacy (Strauser et al. Citation2002).
In relation to cooperation/competition, it was shown that in specific cooperative tasks internal LoC favors a cooperative behavior. It was suggested that individuals gradually learn to understand the subtle interplay between cooperation and self-interest and that internals are more astute in learning to cooperate because they are more endowed with the cognitive faculties necessary for quick learning than externals (Boone et al. Citation2002). It was also suggested the existence of a positive relationship between high LoC and compliant performance (Blau Citation1993). Moreover, as shown by previous research, there is support for a direct link and inter-relationship between individuals’ approach-avoidance tendencies and their LoC. It was observed that individuals with a predominant approach motivation tend to have an internal LoC, suggesting that these individuals are likely to see themselves in control of their own actions and outcomes (Kramer and Yoon Citation2007).
Therefore, the aim of the present study was to investigate the neurophysiological basis of social ranking perception underlying the execution of cooperative joint-actions. Based on our hypotheses, observed performance and external feedback (increased performance in cooperation), from one hand, and personality components, from the other hand, may affect the self-perception of social position and hierarchy, and they effectively may interact to impact our social success. That is, the perceived effectiveness of our performance during a cooperative task and some specific personality components related to reward mechanisms and LoC may positively guide self-perception of our position within the social ranking and consequently may impact on the social ability to stay with other people.
In addition, to explore respectively the BIS/BAS and the LoC contribution, we considered the cortical responsiveness (electroencephalographic activity, EEG) to cooperative situation during an attentional performance. Modulation of EEG alpha brain oscillations may be considered a valid measure of brain activation, and it has often been applied to describe distinct responsiveness by the two hemispheres to different social conditions (Sutton and Davidson Citation1997; Balconi and Mazza Citation2009; Balconi et al. Citation2012). In the frontal system, a reduction of alpha power in the left-frontal brain was found in response to approach attitude (Balconi and Mazza Citation2010; Balconi et al. Citation2011), whereas withdrawal conditions induced reduction in alpha power in the right frontal brain (Balconi et al. Citation2009a, Citation2009b). Resting EEG studies have shown that frontal hemispheric activation asymmetry in favor of the right PFC reflects an individual predisposition to respond in terms of withdrawal-related social behavior (Davidson Citation2004; Harmon-Jones Citation2004), whereas the left PFC reflects an individual predisposition to respond in terms of approach-related social behavior (Davidson Citation1992).
Specifically, the brain correlates of this dynamic cooperative exchange was examined and it should be plausible that the hemispheric ‘competition’ between the left and right sides would characterize social hierarchy behavior, showing a higher approach attitude in higher cooperative condition with an imbalance in favor of the left hemisphere. Specifically, we supposed that higher BAS participants (higher-BAS) may respond in greater measure to increased cooperative performance based on their sensitivity to rewarding and high dominant conditions. Therefore, decreased alpha activity should be found respectively for higher-BAS in the frontal left brain area in perceived increased cooperative efficacy. In addition, in the case of higher internal LoC component (higher-LoC) this effect should be enhanced since the increased performance effect should generate a more consistent response in internals (Strauser et al. Citation2002).
Concerning the real cognitive performance, consistent better performance should be found for higher-BAS and higher-LoC trait in the case of perceived higher ranking, as an effect of a more reinforcing condition. This ‘improving performance effect’ should be more significant in higher-BAS as a concomitant effect of perceived dominance and reward, which higher-BAS estimate in greater measure.
Finally, a significant relation should be found between these multiple measures, since we expected a correlated increased frontal left brain activity mainly in higher-BAS and higher-LoC, and that this activity should be related firstly with a better performance and secondly with the self-perception of an increased social ranking and social efficacy.
Materials and methods
Participants
Twenty-four undergraduate students (M = 22.73, SD = 2.11; male = 11) took part in the experiment. The participants were all right-handed and presented normal or corrected-to-normal visual acuity and they gave informed written consent to participate in the study. Exclusion criteria were history of psychopathology (Beck Depression Inventory, Beck et al. Citation1996) for the subjects and immediate family. In addition, State-Trait-Anxiety-Inventory (Spielberger et al. Citation1970) was submitted after the experimental session. No neurological or psychiatric pathologies were observed. No payment was provided for subjects’ performance. The research was approved by the local ethics committee of the Department of Psychology, Catholic University of Milan. The study was conducted in accordance with the Declaration of Helsinki.
Procedure
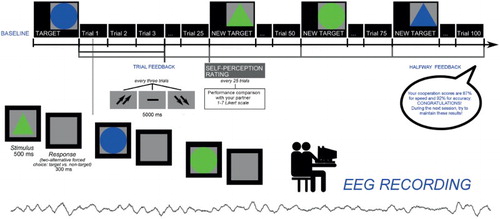
Subjects were seated comfortably in a moderately darkened room with a monitor screen positioned approximately 60 cm in front of their eyes. They performed a simple task for sustained selective attention (it was a modified version of Balconi and Pagani Citation2014). Subjects were told that some cognitive attentional measures were used to evaluate the subjective skills and, to reinforce their motivation, that these measures were usually applied as screening to test future professional career success and teamwork capabilities. In addition, the cooperative nature of the task was stressed. Indeed, subjects were told that the scoring was based on the capacity to synchronize their responses, in term of accuracy (number of correct responses: hits) and response times (RTs), with a second interlocutor (I). They were seated side-by-side, but separated by a black screen in a way that they could not see each other.
Subjects were required to select a target stimulus between non-targets, based on four different options of shape/color: the stimuli might interchangeably be a triangle or a circle, colored red or green. They were required to distinguish between target/non-target by focusing attention on each stimulus. The target was displayed on the video (indicated as the target for selection) and the successive stimuli were presented one after another. The target stimulus features changed every 25 trials. The subjects were instructed to make a two-alternative forced-choice response by pressing a left/right button. Each stimulus was presented for 500 ms, with a 300 ms inter-stimulus interval. After each trial, composed of three stimuli, subjects received a feedback signaled by two up-arrows (high cooperation score); a dash (mean performance); or two down-arrows (low cooperation score). This feedback remained for 5000 ms. After the feedback, an inter-trial interval occurred for other 5000 m. The task was composed of two sessions: the first which did not include a specific feedback to performance (4 blocks before the feedback, 100 trials); the second which included a specific positive feedback to performance (4 blocks with the feedback, 100 trials) (Figure ). Halfway, participants received a general evaluation of their cooperative performance: actually, both feedbacks and the evaluation were fixed, and subjects were told they had a good cooperation (synchronicity) score with 87% in terms of speed synchrony, and 92% in terms of accuracy synchrony. They were also encouraged to maintain their performance level during the second part of the experiment. Across the task, after the initial mean performance, subjects were constantly reinforced about their good cooperation by presenting the up-arrows in 70% of cases, while the dash or the down-arrows appeared in 30% of cases. In addition, after each block of 25 trials, subjects were required to evaluate their performance and efficacy in term of their ranking on a seven-point Likert scale (from one = most decreased ranking due to performance, to seven = most improved ranking due to performance). Participants were strongly engaged in the hierarchical context (92% told to be strongly engaged), as was evident by post-session questionnaire data. The subjects were also required to self-report their degree of trust of the exact feedback of the performance, which showed high trust (96%), a relevance of the task for social status (94%), the perceived improved ranking position during the task (93%).
To exclude the learning or order effect and to confirm the feedback effect, a pre-experimental phase was included. In this first preliminary phase (pre-experimental control condition), subjects were not asked to activate a joined task, but they were only requested to execute the attention task individually without a specific cooperation feedback (t0). Therefore, including this task, the entire procedure was composed of three sessions: a first preliminary phase (control condition, pre-experimental phase) where subjects were asked only to execute the attention task individually (t0) (4 blocks, 100 trials). Then, for the successive experimental session, a second phase was included (t1) where subjects were required to synchronize their performance (4 blocks before the feedback, 100 trials), and a third phase (t2), which followed the positive social feedback described above (4 blocks after the feedback, 100 trials) (Figure ).
BAS scores
BAS scores were calculated for each subject by using the Italian version (Leone et al. Citation2002) of Carver and White Questionnaire (Citation1994). It included 24 items (20 score-items and 4 fillers, each measured on a 4-point Likert scale), and 2 total scores for BIS (range = 7–28; items 7) and BAS (range = 13–52; items 13). BAS also includes three subscales (Reward, 5 items; Drive, 4 items; and Fun Seeking, 4 items). The questionnaire was submitted to the subject after completing the experimental phase. Two total scores (BIS and BAS total) and three BAS subscale scores were calculated. The mean values and standard deviations for each scale were respectively: BAS: 48.35 (4.12); Reward: 23.33 (2.45); Drive: 13.41 (2.20); Fun Seeking: 13.58 (2.60). Finally, Cronbach’s alpha was calculated for BAS (0.90) and separately for each BAS subscale (Reward 0.89; Drive 0.86; and Fun Seeking 0.90). Based on these subscale ratings, we considered two sub-groups of subjects: higher-BAS and lower-BAS subjects. The first group includes subjects with high BAS scoring (more than 52, mean + 1 SD, N = 10); the second group includes subjects with low BAS scoring (less than 44, mean − 1 SD, N = 14). Two subjects were excluded from the final analysis since they showed a mixed-profile (both high-BAS and high-BIS).
LoC
The Locus of Control of Behavior Scale (LoC; Craig et al. Citation1984) was applied to measure the internal and external LoC. Here, the Italian version was used (Farma and Cortinovis Citation2000): it was composed of 17 items, each valuable on a 6-point Likert scale, ranging from 0 (‘strongly disagree’) to 5 intervals (‘strongly agree’): e.g. ‘I can anticipate difficulties and take action to avoid them’, ‘My mistakes and problems are my responsibility to deal with’. Seven questions (1, 5, 7, 8, 13, 15, 16) assess internal control, while the others evaluate external control. The score is obtained by summing the items for external control and the inverted scorings about internal control.
The mean values and standard deviations for the scale were 32.0 and 7.2. According to subjects’ ratings, we considered two sub-groups of subjects: higher-LoC (more internal component) and lower-LoC (less internal component) subjects. The first group includes subjects with high LoC scoring (more than 39, mean + 1 SD, N = 10); the second group includes subjects with low LoC scoring (less than 25, mean − 1 SD, N = 14). Two subjects were excluded from the final analysis since they did not show a clear high- vs. low-profile.
EEG
EEG recordings were performed with two 16-channel portable EEG-System (V-AMP: Brain Products, München. Truscan: Deymed Diagnostic, Hronov). An ElectroCap with Ag/AgCl electrodes was used to record EEGs from active scalp sites referred to the earlobes (10/5 system of electrode placement; Oostenveld and Praamstra Citation2001). Data were acquired using a sampling rate of 500 Hz, with a frequency band of 0.01–40 Hz. An off-line common average reference was successively computed to limit the problems associated with the signal-to-noise ratio (Ludwig et al. Citation2009). Additionally, one electroculogram (EOG) electrode was placed on the outer canthi to detect eye movements. The impedance of the recording electrodes was monitored for each subject prior to data collection and was always below 5 kΩ. After performing EOG correction and visual inspection, only artifact-free trials were considered (rejected epochs, 2%). The signal was visually scored, and a portion of the data that contained artifacts were removed to increase specificity. Blinks were also visually monitored. Ocular artifacts (eye movements and blinks) were corrected using an eye-movement correction algorithm that employs a regression analysis in combination with artifact averaging (Sapolsky Citation2004).
EEG activity was recorded on positions AFF1h, AFF2h, Fz, FFC3h, FFC4h, C3, C4, Cz, P3, P4, Pz, T7, T8, O1, O2. The digital EEG data were band-pass filtered in the frequency band 8–12 Hz (band-pass filtering 96 dB/octave rolloff, warm-up filter left and right to 100 ms). To obtain a signal proportion to the power of the EEG frequency band, the filtered signal samples (epoch 1000 ms) were squared (Pfurtscheller Citation1992). An average absolute power value for each experimental condition was calculated. An average of the pre-experimental absolute power (−200 ms before the beginning of the pre-experimental session) was used to determine the individual power during no task condition. For the statistical analysis, left and right frontal (FFC3h, FFC4h) alpha power activity was considered, to directly verify the role of DLPFC event-related alpha activity.
Results
A preliminary analysis was applied to t0 (pre-experimental task) compared to t1 (pre-feedback cooperative task) and t2 (post-feedback cooperative task). Systematic significant differences were found between t0 vs. t2, for both behavioral and neurophysiological measures, but not for the comparison between t0 vs. t1. These results support the specificity of feedback effect compared to absence of feedback for the cooperative task.
Four sets of analyses were performed with respect to behavioral (error rate, ER; RTs; ranking self-perception) and alpha band measures. Mixed-model ANOVAs were applied to these dependent measures. The independent factors were within-subjects factor condition (pre–post-feedback); between-subjects factors BAS (high-BAS vs. low-BAS) and LoC (high-LoC vs. low-LoC). They were applied to ER, RTs, and self-perception variables. For an alpha band dependent variable, the independent factors (mixed-model ANOVAs) were condition, BAS, LoC, and hemisphere side (Lat, left vs. right). The RTs were recorded from the stimulus onset, and ER was calculated as the total number of incorrect detections out of the total trial for each category. Higher values represented increased incorrect responses. About self-perception, the increased or decreased self-perceived ranking was considered. Alpha band modulation was calculated for each block. For all of the ANOVA tests, the degrees of freedom were corrected using Greenhouse–Geisser epsilon where appropriate. Post hoc comparisons (contrast analyses) were applied to the data. Post hoc analyses (simple effects for paired comparisons; contrast effects for mixed design ANOVA) were applied in the case of significant main or interactions effect.
Finally, correlational analyses were applied to BAS, LOC, alpha, and self-perception questionnaire.
ANOVA
ER
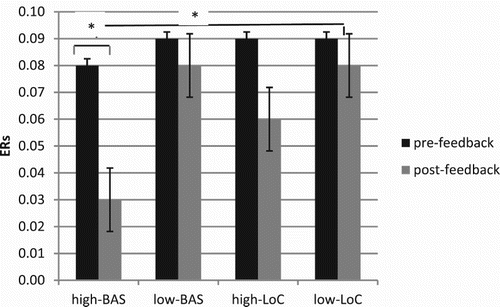
ANOVA indicated significant main effects for Cond (F[1, 23] = 7.18, p ≤ .001, η2 = .36), with decreased ER for post-feedback; BAS (F[1, 23] = 7.90, p ≤ .001, η2 = .38), with decreased ER for high-BAS; and interaction effects BAS × Cond (F[1, 23] = 8.11, p ≤ .001, η2 = .41); LoC × Cond (F[1, 23] = 8.09, p ≤ .001, η2 = .39); BAS × LoC × Cond (F[1, 23] = 7.70, p ≤ .001, η2 = .37). About the first interaction effect, high-BAS showed a decreased ER in post-feedback compared to pre-feedback condition (F[1, 23] = 7.51, p ≤ .001, η2 = .36). Secondly, ER decreased for high-LoC in post-feedback compared to pre-feedback (F[1, 23] = 8.54, p ≤ .001, η2 = .40). Thirdly, about the BAS × LoC × Cond, high-BAS compared to all the other level showed decreased ER in post-feedback (respectively high-LoC F[1, 23] = 7.30, p ≤ .001, η2 = .36; low-LoC F[1, 23] = 7.15, p ≤ .001, η2 = .36; low-BAS F[1, 23] = 7.09, p ≤ .001, η2 = .36) (Figure ).
RTs
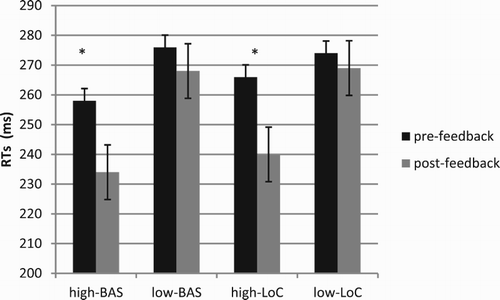
ANOVA indicated significant main effects for Cond (F[1, 23] = 8.16, p ≤ .001, η2 = .40), with decreased RTs for post-feedback; BAS (F[1, 23] = 9.12, p ≤ .001, η2 = .43), with decreased RTs for high-BAS; and interaction effects BAS × Cond (F[1, 23] = 8.50, p ≤ .001, η2 = .41); and LoC × Cond (F[1, 23] = 8.12, p ≤.001, η2 = .39). About the first interaction effect, high-BAS showed decreased RTs in post-feedback compared to pre-feedback condition (F[1, 23] = 8.50, p ≤ .001, η2 = .40). Similarly, high-LoC showed decreased RTs in post-feedback compared to pre-feedback condition (F[1, 23] = 9.23, p ≤ .001, η2 = .45) (Figure ).
Self-ranking
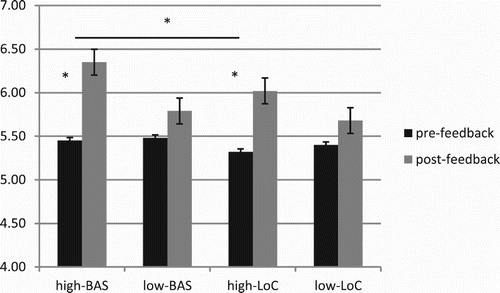
About the evaluation of their ranking position in term of performance, ANOVA indicated significant interaction effects for BAS × Cond (F[1, 23] = 8.80, p ≤ .001, η2 = .41); LoC × Cond (F[1, 23] = 9.73, p ≤ .001, η2 = .44); and BAS × LoC × Cond (F[1, 23] = 8.40, p ≤ .001, η2 = .42). About the first interaction effect, high-BAS showed higher ranking perception than low-BAS in post-feedback (F[1, 23] =7.14, p ≤ .001, η2 = .37). In addition, high-BAS revealed higher ranking in post- than pre-feedback (F[1, 23] = 7.89, p ≤ .001, η2 = .38). Similarly, high-LoC showed increased perception of ranking in post-feedback compared to pre-feedback condition (F[1, 23] = 9.09, p ≤ .001, η2 = .45). Finally, about the threefold interaction effect, high-BAS revealed increased self-perception of high ranking than high-LoC in post-feedback (F[1, 23] = 8.33, p ≤ .001, η2 = .40), as well as for low-LoC and low-BAS (Figure ).
Alpha band
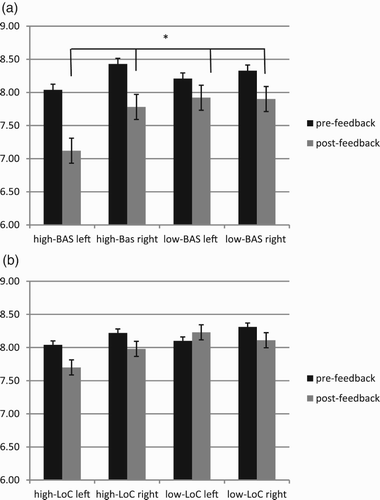
ANOVA indicated significant main effects for Lat ×Cond (F[1, 23] = 7.09, p ≤ .001, η2 = .35), with decreased left alpha activity for post-feedback compared to pre-feedback condition; LoC × Cond (F[1, 23] = 9.78, p ≤ .001, η2 = .45) with decreased left alpha activity for high-LoC than low-LoC in post-feedback condition (F[1, 23] = 8.32, p ≤ .001, η2 = .40); BAS × Lat (F[1, 23] = 9.78, p ≤ .001, η2 = .45), with decreased left alpha activity for high-BAS than low-BAS F[1, 23] = 9.78, p ≤ .001, η2 = .45); BAS × Lat × Cond (F[1, 23] = 9.16, p ≤ .001, η2 = .44), with decreased left alpha for high-BAS in post-feedback than pre-feedback condition (F[1, 23] = 8.90, p ≤ .001, η2 = .43); and with decreased left alpha response for high-BAS than low-BAS in post-feedback condition (F[1, 23] = 9.08, p ≤ .001, η2 = .44).
Finally BAS × LoC × Lat × Cond was significant (F[1, 23] = 10.08, p ≤ .001, η2 = .48). Post hoc comparisons (contrast paired comparisons) revealed a significant difference between high-BAS and high-LoC, since high-BAS showed decreased left alpha activity in post-feedback than high-LoC (F[1, 23] = 9.08, p ≤ .001, η2 = .44) (Figure (a,b)).
Correlational analysis
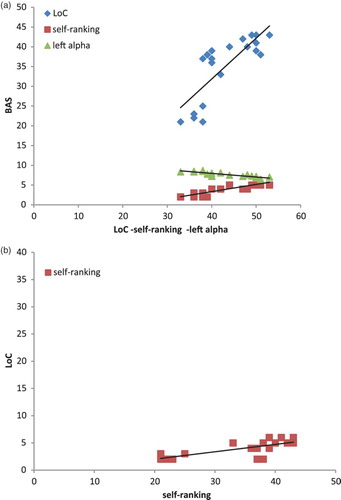
A series of correlational analysis was applied to BAS, LoC, self-ranking, and alpha measures. Pearson correlation coefficients were calculated between them. BAS revealed a significant positive correlation with self-ranking (r2 = .455, p ≤ .001) and LoC (r2 = .525, p ≤ .001). Similarly, LoC was positively correlated with self-ranking (r2 = .478, p ≤ .001). In addition, alpha was inversely correlated with BAS within the left hemisphere (r2 = −.385, p ≤ .001) (increased left brain activity in concomitance with higher BAS), as well with self-ranking (r2 = −.409, p ≤ .001) (increased left activity in concomitance with higher self-ranking). No other effect was statistically significant (Figure (a,b)).
Discussion
The present research intended to explore ranking perception during a social cooperative task which included a joint-action where a performance-based feedback was provided. Specifically, we considered the contribution of cortical correlates (alpha band modulation) and some personality traits components (such as BAS and LoC) in achieving accurate self-representation and self-improvement in social contexts. Based on our results, three main effects were elucidated. A first main effect was related to the systematic frontal brain (PFC) responses to social status perception in cooperation. This PFC activation was found mainly when a positive reinforce (post-feedback) was furnished to the subjects for their joint-action. Therefore, this brain activity was reinforced by self-representation of a better joint-performance in cooperation. Secondly, a significant lateralization effect was revealed, with a more engaged left hemisphere. Mainly after the positive reinforce, the role of left PFC was preponderant as activated by the perception of efficacy in cooperative behavior. These results were also confirmed by self-representation of rating, with increased perception of higher status and social ranking, and by real subjective performance, with decreased ERs and RTs, in concomitance with higher left PFC responsiveness and mainly in response to positive feedback. Thirdly, both BAS and LoC were found to be effective in modulating alpha activity, social ranking perception, and real performance, with higher left responsiveness, self-perception of ranking, as well as improved performance post-feedback for high-BAS and high-LoC.
About the first effect, a consistent PFC contribution was observed in response to cooperative condition. Specifically, as shown by EEG data, the DLPFC was mainly implicated when subjects were informed on their efficient joint-action. This effect was supported in the present study by modulation of alpha frequency band that is increased brain activity (reduced alpha power) in response to social interaction which asks for cooperation between subjects. Prior work has suggested a main role for the ventromedial prefrontalcortex (VMPFC) in responding to status (Karafin et al. Citation2004). Specifically, it was found that patients with VMPFC lesions made less use of information in their dominance judgments (Karafin et al. Citation2004). Recent studies investigating the effect of partner strategies found differential activation in the DLPFC when playing with cooperative, neutral, and non-cooperative human partners (Suzuki et al. Citation2011) and activation in the superior temporal sulcus during successful adaption to the strategies of computer agents (Haruno and Kawato Citation2009). Additionally, De Vico Fallani et al. (Citation2010) using the EEG hyper-scanning technique has reported activation in this region during reciprocal interaction in iterated Prisoner’s Dilemma games.
Given the evolutionary prevalence and importance of social ranking and social perception in cooperative contexts, where hierarchy across species and across human social groups is crucial, it is plausible that the ‘social’ brain has specialized mechanisms for perceiving social status and joint-actions in integration with specific personality factors.
More interestingly, the post-feedback condition revealed an increased DLPFC responsiveness. As shown by correlational analyses, this brain modulation (alpha decreasing) was accompanied by the significant increased ranking perception as well as by the increased performance (decreased ERs and RTs). Indeed, it was found that subjects highly improved their real performance in response to the external feedback, that is the perception of their outcomes in relationship with those of joint-I. Therefore, the self-evidence of a good cooperation (positive feedback condition) produced also a more consistent impact, with higher effect for the ranking position and the cognitive performance compared to the absence of external feedback.
We may suppose that the relevant effect, when this rating was compared with that of a cooperative I, was related to the impact of the perceived performance on the cognitive real performance. Therefore, firstly, the manipulation of the feedback has an impact on social rank representation, with a possible direct effect on self-representation. Secondly, this intrinsic relation also highlights the possibility of considering the reciprocal influence of cognitive behavior and self-perception as two sides of the same coin. That is, a sort of ‘self-fulfilling prophecy’ may be adduced: the social significance of the performance for the social hierarchy appears to be highly relevant in modulating the subjects’ performance across the task. This effect was observed for the entire duration of the experiment with a consistent and parallel increasing of ranking perception and subjective performance.
At this regard, Festinger’s long-standing, prominent theory of social comparison processes (Festinger Citation1954) suggested an important role for hierarchical rank in achieving accurate self-knowledge, self-representation, and self-improvement for subjects. Therefore, these three components (social ranking perception; social reinforce; brain activity) may be considered as main factors able to affect the subjective behavior, and the PFC activation was the underlying correlate of this efficacious mechanism. However, it should be noted that, in comparison with some previous research (Dötsch and Schubö Citation2015), in the present study both the performance and self-representation modification were not generated when a generic positive feedback provided, but the induced reinforce was specifically related to their cooperative joint-efficacy: they ‘perceived’ themselves to be more efficacious in cooperation with other I. We may suggest that in the present condition subjects represent their social cooperative efficacy as the key point of their performance, and, in this regard not only PFC modulates its activity when performance is perceived as increasing, but it is also associated to the perception of increased social efficacy in joint-behavior.
The second main result of the present research was related to a clear hemispheric lateralization effect. As elucidated by the present data, lateralized left cortical network within the PFC (left DLPFC) supported self-perception of ranking and improved performance derived by cooperative tasks. The fact that this cortical ‘unbalance’ in favor of the left hemisphere in response to positive reinforcing conditions was also accompanied by a better performance and an increased social efficacy in term of ranking attribution, as shown by the correlational analysis, which underlined the direct link between the left cortical activity, the external social ranking representation, and the effective behavior. Moreover, previous research demonstrated that high social power perception is indeed associated with greater left-frontal brain activity compared to low social power (Boksem et al. Citation2012). The specific cortical localization may suggest the consistent over-activation of the cortical left system and a concomitant predominance of this brain area in managing the cognitive behavior of the subjects when they perceive to be higher in ranking.
More generally, in this study, the left hemisphere effect was demonstrated to be prominent to explain our results. As pointed out by Koslov et al. (Citation2013), correlational research suggests that frontal cortical asymmetry in favor of the left hemisphere is associated with approach motivation, with the ability to regulate negative emotions, and with general well-being (Davidson Citation1993; Jackson et al. Citation2003; Urry et al. Citation2004; Balconi and Mazza Citation2010; Harmon-Jones et al. Citation2010). Starting from this evidence, they explored resting intracortical activity during social threat and found that participants with higher resting activity in the left vs. right DLPFC cortex exhibited more adaptive, approach-oriented cardiovascular stress responses.
However, these main results had to be discussed at light of two other core components, that are the approach attitude construct (BAS) and the internal LoC. Indeed, it was observed that personality approach attitude (high-BAS) was able to modulate brain activity, social ranking perception, and cognitive performance. In addition, the lateralization effect was also confirmed in the case of high-BAS with higher left PFC mainly post-feedback reinforce. These results suggest that social status may not be a ‘universally valid’ and immutable phenomenon; rather, perception of our own ranking, particularly during conditions of cooperation with others, may be directly and strongly related with personality approach-related component. This is in line with previous studies (Demaree Citation2005), which reported that those individuals with a higher BAS strength were more likely to relate to the dominant and ‘proactive’ character in situations which were shown to induce a positive effect, while those with a higher BIS sensitivity were more inclined to relate to the submissive and passive character, inducing a negative effect. This raises the possibility that our personalities and our subjective comprehension of social hierarchies may interact to impact our social success and sense of well-being. Furthermore, it is possible that high-BAS more than low-BAS implicitly assessed their own (self-referential) social hierarchical status in relation to the task they performed, with particular respect to increased social efficacy perception. It is also possible that the improved self-perception of ranking (induced by the external feedback) may have introduced a reinforcing cue able to significantly modify the behavioral performance (Chiao Citation2010).
In addition, it should also be noted that, about the significance of the BAS component, higher BAS subjects may be more attentive to conditions that produce a significant positive reinforce, and that reinforce the behaviors which are active in nature, ingenerating positive emotions, and positive self-perception of approaching attitude (Balconi et al. Citation2009b), as shown in previous research which has used a non-social condition (Balconi and Pagani Citation2014, Citation2015). As observed in the present research, this effect could be valid and consistent also when a social task was provided. Thus, in line with our previous hypotheses, we observed in higher BAS subjects a prevalence in responding to approach condition that includes a positive joint-action. This result is consistent with prior research showing that social status is associated with greater BAS during the processing of cooperative situations. More generally we have to consider the extent to which individuals of higher-BAS are more proactive in achieving their outcomes when a cooperative goal is to be obtained (Magee and Galinsky Citation2008; Pothos and Busemeyer Citation2009). By virtue of having relatively a greater proactive attitude, they must rely more on their resources to meet their needs (Kraus et al. Citation2009).
In addition, a specific lateralization effect was found for high-BAS, mainly in response to positive post-feedback condition. These results are in line with some previous studies which demonstrated that high cortical left unbalance is related to approach-related conditions, with higher prevalence of high-frequency oscillations in the left PFC more than right PFC (Balconi and Mazza Citation2010). It is possible to explain this lateralization effect pointing out that approach attitude, generally associated to increased left PFC responsiveness, is able per se to affect both the self-perception of efficacy and cooperativeness and, consequently, the subjects’ real performance. Indeed, we may state that a more consistent approach-attitude and positive motivation may support a concomitant left-side hyperactivation which supports the self-representation of an increased social ranking in cooperative contexts, with an improved real cognitive performance. Nevertheless, the role of BAS was not able in absolute to explain the present results, since we had a significant generalized higher left-hemisphere activity also independently from the BAS contribution. In other terms, the ‘basic’ left-lateralized BAS effect due to approach attitude might not exhaustively explain the increased effect found in post-feedback condition, with higher left DLPFC activity as a consequence of positive reinforcing for cooperative actions. Future research should better consider the specific role of BAS and left DLPFC area in describing the cooperative behavior.
Also, LoC affects DLPFC activity, as well as both the real increased performance and the self-perception as pointed out by correlational analysis. That is, the BAS significance was paralleled by LoC effect, with increased self-efficacy perception in the case of high-LoC. Whereas this result was not unattended based on the main conceptual components of internal LoC (which is normally related to increased sense of efficacy), the innovative effect of the present research was linked to the strength association between high level of internal LoC and social ranking perception during a cooperative interaction. Indeed, previously it was argued the concepts of LoC, generalized self-efficacy and self-esteem measured the same, single factor and demonstrated them to be related concepts (Judge et al. Citation2002). However, here we more directly considered the social variant of self-efficacy tested in cooperatively joint condition. Indeed, not only the sense of social self-efficacy was modulated by LoC, but it was also influent to determine the specific perception of an increased social status and social ranking in term of high level joint-performance. Therefore, we can state that both the sense of self-efficacy and social position were determined by internal LoC with significant impact on the effective cognitive outcomes. In addition, in previous experiments, it was found that internal LoC subjects played significantly more cooperatively than external LoC (Boone et al. Citation1999a, Citation1999b). However, it was observed that this difference could not be the result of ‘internals’ being more altruistic, but rather of their tendency to use behavior strategically in order to control their environment to obtain valued outcomes. In other words, internals play more cooperatively, on average, because it furthers their self-interest. They have to understand that, in the long run, cooperation is in their self-interest. In fact, they readily switch to a competitive strategy when this is more appropriate to obtain a higher payoff. It can be suggested that internal LoC subjects, who believe in their own potency to control and modify their environment, are much more likely than external LoC subjects to use all their faculties to understand and influence their surrounding world as this heightens the probability of successfully regulating behavior.
It is also hypothesizable that internal LoC subjects tend to verify their assumptions more and they might be more attentive to cues and feedback relevant to their decisional processes because they believe this may improve their performance. All these provide strong support for the validity of LoC construct as it is indicative of a basic striving of internal individuals to actively engage in the seeking for relevant cues in their environment to determine their social position and social perception, and to guide or adapt their behavior accordingly.
Nevertheless, it should be noted that, contrarily to BAS construct, no lateralization effect was revealed in concomitance to high-LoC. Indeed, a more generalized DLPFC activity was observed post-feedback condition compared with pre-feedback, without a specific left-lateralization. Therefore, we may state that the specific hemispheric effect we found may be more directly supported by approach attitude and by BAS component, as elucidated by the present results. Secondly, these evidences more strongly suggest a not complete overlapping between the two constructs of LoC and BAS and their partially different relationship with the brain correlates during cooperative actions. It should be underlined that these differences may be also related to the different impact that positive feedback has in relation to BAS and LoC and to how this feedback impact on these constructs and, consequently, on both the performance and the ranking perception. Indeed, the direct comparison between high-BAS and high-LoC highlighted the more consistent increasing of social ranking and a better performance for high-BAS in post-feedback condition. This result may suggest that a more proactive and positively motivated attitude, as represented by high-BAS, produces the maximal effect on the cooperative context, maximizing both the subjective performance and the self-representation of social position. Nevertheless, future research should better elucidate this important point.
In addition, a tentative explanation of the main role exercised by both BAS and LoC may be summarized taking into consideration the underlying concept of core self-evaluation (CSE, Judge et al. Citation1997). CSE refers to fundamental assessments that people make about their worthiness, competence, and capabilities. Recently, it was explored how CSE influences outcomes (Judge et al. Citation2004). In this respect, CSE has been conceptualized as an indicator of high approach temperament (Judge et al. Citation1998), orienting individuals toward seeking positive outcomes, which subsequently influence performance and well-being. In addition, CSE may be conceptualized as a hinge between approach attitude and LoC as representable in term of fundamental evaluations that people hold about themselves and their ability to control and manage the external forming the basis of their self-appraisals and self-efficacy.
To summarize the results, this study appears to confirm the tendency to modulate both self-perceived social position and real performance based on the personal attitudes (BAS and LoC) and the frontal activity related to alpha frequency band during an inter-personal cooperative performance which is considered relevant for social hierarchy. Higher level hierarchy related to cognitive performance is linked to a clear increased activity in the DLPFC for high-BAS and high-LoC subjects, when subjects perceived themselves as skillful. Positive reinforce increases this effect in relation to both the personality measures. Relevant effects were observed about the cortical prefrontal activity. Indeed for both BAS and LoC, we may state that social status relates to neural activity in the frontal network with an increased effect when subjects were higher in BAS and LoC. These effects were supported in the present study by modulation of the DLPFC alpha frequency band, that is, increased brain activity (reduced alpha power) in response to high-ranking perception and increased cognitive performance. In addition, we found that the left-side system – more related to BAS polarity – accounts for the increased performance and improved self-perception: BAS subjects showed a more intense response within the left hemisphere in the case of high reinforced cooperation. The current research provides initial evidence for this hypothesis and lays a foundation for future research examining the extent to which the human brain selectively processes social dominance cues. Finally, a significant and systematic correlation was observed between these multiple personality and brain measures. However, LoC and BAS were two interrelated, but not overlapping, constructs, since more directly and consistently BAS showed its specificity in term of a lateralized left network for cooperative joint-action.
Future research should more exhaustively consider a direct comparison of cooperative task with competitive task, to elucidate the main impact of DLPFC contribution as a function of social contexts in the case of cooperative vs. non-cooperative joint-actions. In addition, for future explorations, a more specific cortical side effect and hemispheric contribution in response to joint-action should be analyzed with some adjunctive measures, such as neuroimaging. At this regard, also the specific central of posterior sites response to cooperation could better explain the full cortical activity. In addition, a more exhaustive analysis based on the full brain spectral oscillations could be considered to better elucidate the functional role of other frequency bands. Finally, the intrinsic commonalities and some basic differences between BAS and LoC concepts should be more deeply evaluated in cooperative tasks, taking into account the significant differences we observed in relationship with the left-lateralization effect.
Acknowledgements
Michela Balconi projected the experiment and supervised the experiment and the data analysis. Maria Elide Vanutelli realized the experiment and the data analysis. All data will be accessible via web www.psychoneuronet.com.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Michela Balconi http://orcid.org/0000-0002-8634-1951
References
- Balconi M, Bortolotti A, Gonzaga L. 2011. Emotional face recognition, EMG response, and medial prefrontal activity in empathic behaviour. Neurosci Res. 71:251–259. doi: 10.1016/j.neures.2011.07.1833
- Balconi M, Brambilla E, Falbo L. 2009a. Appetitive vs. defensive responses to emotional cues. autonomic measures and brain oscillation modulation. Brain Res. 1296:72–84. doi: 10.1016/j.brainres.2009.08.056
- Balconi M, Brambilla E, Falbo L. 2009b. Bis/BAS, cortical oscillations and coherence in response to emotional cues. Brain Res Bull. 80:151–157. doi: 10.1016/j.brainresbull.2009.07.001
- Balconi M, Crivelli D, Vanutelli ME. 2017. Why to cooperate is better than to compete: brain and personality components. BMC Neurosci. 18:68. doi: 10.1186/s12868-017-0386-8
- Balconi M, Falbo L, Conte VA. 2012. Bis and BAS correlates with psychophysiological and cortical response systems during aversive and appetitive emotional stimuli processing. Motiv Emot. 36:218–231. doi: 10.1007/s11031-011-9244-7
- Balconi M, Mazza G. 2009. Brain oscillations and BIS/BAS (behavioral inhibition/activation system) effects on processing masked emotional cues. ERS/ERD and coherence measures of alpha band. Int J Psychophysiol. 74:158–165. doi: 10.1016/j.ijpsycho.2009.08.006
- Balconi M, Mazza G. 2010. Lateralisation effect in comprehension of emotional facial expression: a comparison between EEG alpha band power and behavioural inhibition (BIS) and activation (BAS) systems. Laterality. 15:361–384. doi: 10.1080/13576500902886056
- Balconi M, Pagani S. 2014. Personality correlates (BAS-BIS), self-perception of social ranking, and cortical (alpha frequency band) modulation in peer-group comparison. Physiol Behav. 133:207–215. doi: 10.1016/j.physbeh.2014.05.043
- Balconi M, Pagani S. 2015. Social hierarchies and emotions: cortical prefrontal activity, facial feedback (EMG), and cognitive performance in a dynamic interaction. Soc Neurosci. 10:166–178. doi: 10.1080/17470919.2014.977403
- Balconi M, Vanutelli ME. 2016. Competition in the brain. The contribution of EEG and fNIRS modulation and personality effects in social ranking. Front Psychol. 7:1587. doi: 10.3389/fpsyg.2016.01587
- Balconi M, Vanutelli ME. 2017. Interbrains cooperation: hyperscanning and self-perception in joint actions. J Clin Exp Neuropsychol. 39:607–620. doi: 10.1080/13803395.2016.1253666
- Bechara A, Damasio H, Damasio AR, Lee GP. 1999. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999
- Bechara A, Martin EM. 2004. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 18:152–162. doi: 10.1037/0894-4105.18.1.152
- Beck AT, Steer RA, Brown GK. 1996. Manual for the Beck Depression Inventory – II. San Antonio: Psychological Corporation.
- Blau G. 1993. Testing the relationship of locus of control to different performance dimensions. J Occup Organ Psychol. 66:125–138. doi: 10.1111/j.2044-8325.1993.tb00522.x
- Boksem MAS, Smolders R, Cremer DD. 2012. Social power and approach-related neural activity. Soc Cogn Affect Neurosci. 7:516–520. doi: 10.1093/scan/nsp006
- Boone C, De Brabander B, Carree M, De Jong G, van Olffen W, van Witteloostuijn A. 2002. Locus of control and learning to cooperate in a prisoner’s dilemma game. Personal Individ Differ. 32:929–946. doi: 10.1016/S0191-8869(01)00100-3
- Boone C, De Brabander B, van Witteloostuijn A. 1999a. Locus of control and strategic behaviour in a prisoner’s dilemma game. Personal Individ Differ. 27:695–706. doi: 10.1016/S0191-8869(98)00269-4
- Boone C, De Brabander B, van Witteloostuijn A. 1999b. The impact of personality on behavior in five Prisoner’s Dilemma games. J Econ Psychol. 20:343–377. doi: 10.1016/S0167-4870(99)00012-4
- Carver CS, White TL. 1994. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 67:319–333. doi: 10.1037/0022-3514.67.2.319
- Chiao JY. 2010. Neural basis of social status hierarchy across species. Curr Opin Neurobiol. 20:803–809. doi: 10.1016/j.conb.2010.08.006
- Chiao JY, Adams RBJ, Tse PU, Lowenthal L, Richeson JA, Ambady N. 2008. Knowing who’s boss: fMRI and ERP investigations of social dominance perception. Gr Process Intergr Relations. 11:201–214. doi: 10.1177/1368430207088038
- Chiao JY, Harada T, Oby ER, Li Z, Parrish T, Bridge DJ. 2009. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 47:354–363. doi: 10.1016/j.neuropsychologia.2008.09.023
- Chung D, Yun K, Jeong J. 2015. Decoding covert motivations of free riding and cooperation from multi-feature pattern analysis of EEG signals. Soc Cogn Affect Neurosci. 10:1210–1218. doi: 10.1093/scan/nsv006
- Craig AR, Franklin JA, Andrews G. 1984. A scale to measure locus of control of behaviour. Psychol Psychother. 57:173–180.
- Cui X, Bryant DM, Reiss AL. 2012. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. Neuroimage. 59:2430–2437. doi: 10.1016/j.neuroimage.2011.09.003
- Davidson RJ. 1992. Emotion and affective style: hemispheric substrates. Psychol Sci. 3:39–43. doi: 10.1111/j.1467-9280.1992.tb00254.x
- Davidson RJ. 1993. Cerebral asymmetry and emotion: conceptual and methodological conundrums. Cogn Emot. 7:115–138. doi: 10.1080/02699939308409180
- Davidson RJ. 2004. What does the prefrontal cortex ‘do’ in affect: perspectives on frontal EEG asymmetry research. Biol Psychol. 67:219–234. doi: 10.1016/j.biopsycho.2004.03.008
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. 1990. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology. I. J Pers Soc Psychol. 58:330–341. doi: 10.1037/0022-3514.58.2.330
- Demaree HA. 2005. Brain lateralization of emotional processing: historical roots and a future incorporating ‘dominance’. Behav Cogn Neurosci Rev. 4:3–20. doi: 10.1177/1534582305276837
- De Vico Fallani F, Nicosia V, Sinatra R, Astolfi L, Cincotti F, Mattia D, Wilke C, Doud A, Latora V, He B, Babiloni F. 2010. Defecting or not defecting: how to ‘read’ human behavior during cooperative games by EEG measurements. PLoS One. 5:e14187. doi: 10.1371/journal.pone.0014187
- Dötsch D, Schubö A. 2015. Social categorization and cooperation in motor joint action: evidence for a joint end-state comfort. Exp Brain Res. 233:2323–2334. doi: 10.1007/s00221-015-4301-1
- Farma T, Cortinovis I. 2000. Un questionario sul ‘locus of control’: suo utilizzo nel contesto italiano. Ric Psicoter. 3:147–155.
- Farrow TFD, Jones SC, Kaylor-Hughes CJ, Wilkinson ID, Woodruff PWR, Hunter MD, Spence SA. 2011. Higher or lower? The functional anatomy of perceived allocentric social hierarchies. Neuroimage. 57:1552–1560. doi: 10.1016/j.neuroimage.2011.05.069
- Festinger L. 1954. A theory of social comparison processes. Hum Relations. 7:117–140. doi: 10.1177/001872675400700202
- Fowles DC. 2000. Electrodermal hyporeactivity and antisocial behavior: does anxiety mediate the relationship? J Affect Disord. 61:177–189. doi: 10.1016/S0165-0327(00)00336-0
- Freeman JB, Rule NO, Adams RB, Ambady N. 2009. Culture shapes a mesolimbic response to signals of dominance and subordination that associates with behavior. Neuroimage. 47:353–359. doi: 10.1016/j.neuroimage.2009.04.038
- Funane T, Kiguchi M, Atsumori H, Sato H, Kubota K, Koizumi H. 2011. Synchronous activity of two people’s prefrontal cortices during a cooperative task measured by simultaneous near-infrared spectroscopy. J Biomed Opt. 16:077011. doi: 10.1117/1.3602853
- Gable SL, Reis HT, Elliot AJ. 2000. Behavioral activation and inhibition in everyday life. J Pers Soc Psychol. 78:1135–1149. doi: 10.1037/0022-3514.78.6.1135
- Goldman M, Stockbauer JW, McAuliffe TG. 1977. Intergroup and intragroup competition and cooperation. J Exp Soc Psychol. 13:81–88. doi: 10.1016/0022-1031(77)90015-4
- Gray JA. 1987. The neuropsychology of emotion and personality. In: Stahl S.M., Iversen S.D., Goodman E.C., editor. Cognitive neurochemistry. Oxford: Oxford University Press; p. 171–190.
- Gray JA. 1990. Brain systems that mediate both emotion and cognition. Cogn Emot. 4:269–288. doi: 10.1080/02699939008410799
- Gray JA. 1994. Framework for a taxonomy of psychiatric disorder. In: Van Goozen S.H.M., Van de Poll N.E., Sergeant J.A., editor. Emotions: essays on emotion theory. Mahwah: Lawrence Erlbaum Associates; p. 29–59.
- Gray JA, McNaughton N. 2000. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system, 2nd ed. Oxford: Oxford University Press.
- Hall JA, Coats EJ, Lebeau LS. 2005. Nonverbal behavior and the vertical dimension of social relations: a meta-analysis. Psychol Bull. 131:898–924. doi: 10.1037/0033-2909.131.6.898
- Harmon-Jones E. 2004. On the relationship of frontal brain activity and anger: examining the role of attitude toward anger. Cogn Emot. 18:337–361. doi: 10.1080/02699930341000059
- Harmon-Jones E, Gable PA, Peterson CK. 2010. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol Psychol. 84:451–462. doi: 10.1016/j.biopsycho.2009.08.010
- Haruno M, Kawato M. 2009. Activity in the superior temporal sulcus highlights learning competence in an interaction game. J Neurosci. 29:4542–4547. doi: 10.1523/JNEUROSCI.2707-08.2009
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, Rosenkranz Ma, Ryff CD, Singer BH, Davidson RJ. 2003. Now you feel it, now you don’t: frontal brain electrical assymetry and individual differences in emotion regulation. Psychol Sci. 14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x
- Judge TA, Erez A, Bono JE, Thoresen CJ. 2002. Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? J Pers Soc Psychol. 83:693–710. doi: 10.1037/0022-3514.83.3.693
- Judge TA, Locke EA, Durham CC. 1997. The dispositional causes of job satisfaction: a core evaluations approach. Res Organ Behav. 19:151–188.
- Judge TA, Locke EA, Durham CC, Kluger AN. 1998. Dispositional effects on job and life satisfaction: the role of core evaluations. J Appl Psychol. 83:17–34. doi: 10.1037/0021-9010.83.1.17
- Judge TA, Van Vianen AEM, De Pater IE. 2004. Emotional stability, core self-evaluations, and job outcomes: a review of the evidence and an agenda for future research. Hum Perform. 17:325–346. doi: 10.1207/s15327043hup1703_4
- Karafin MS, Tranel D, Adolphs R. 2004. Dominance attributions following damage to the ventromedial prefrontal cortex. J Cogn Neurosci. 16:1796–1804. doi: 10.1162/0898929042947856
- Koslov K, Mendes WB, Pajtas PE, Pizzagalli DA. 2013. Greater left resting intracortical activity as a buffer to social threat. Psychol Sci. 22:641–649. doi: 10.1177/0956797611403156
- Kramer T, Yoon S. 2007. Approach-avoidance motivation and the use of affect as information. J Consum Psychol. 17:128–138. doi: 10.1016/S1057-7408(07)70019-0
- Kraus MW, Piff PK, Keltner D. 2009. Social class, sense of control, and social explanation. J Pers Soc Psychol. 97:992–1004. doi: 10.1037/a0016357
- Leone L, Pierro A, Mannetti L. 2002. Validità della versione italiana delle scale bis/bas di Carver e White (1994): generalizzabilità della struttura e relazioni con costrutti affini. G Ital Psicol. 29:413–434.
- Levitan R, Hasey G, Sloman L. 2000. Major depression and the involuntary defeat strategy: biological correlates. In: Gilbert P., Sloman L., editor. Subordination and defeat: An evolutionary approach to mood disorders and their therapy. Manhaw: Lawrence Erlbaum Associates; p. 95–120.
- Ludwig KA, Miriani RM, Langhals NB, Joseph MD, Anderson DJ, Kipke DR. 2009. Using a common average reference to improve cortical neuron recordings from microelectrode arrays. J Neurophysiol. 101:1679–1689. doi: 10.1152/jn.90989.2008
- Magee JC, Galinsky AD. 2008. Social hierarchy: the self-reinforcing nature of power and status. Acad Manag Ann. 2:351–398. doi: 10.1080/19416520802211628
- Marsh AA, Blair KS, Jones MM, Soliman N, Blair RJR. 2009. Dominance and submission: the ventrolateral prefrontal cortex and responses to status cues. J Cogn Neurosci. 21:713–724. doi: 10.1162/jocn.2009.21052
- Montague P. 2002. Hyperscanning: simultaneous fMRI during linked social interactions. Neuroimage. 16:1159–1164. doi: 10.1006/nimg.2002.1150
- Munafò MR, Clark T, Flint J. 2005. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Mol Psychiatry. 10:415–419. doi: 10.1038/sj.mp.4001627
- Oostenveld R, Praamstra P. 2001. The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol. 112:713–719. doi: 10.1016/S1388-2457(00)00527-7
- Pfurtscheller G. 1992. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr Clin Neurophysiol. 83:62–69. doi: 10.1016/0013-4694(92)90133-3
- Pothos EM, Busemeyer JR. 2009. A quantum probability explanation for violations of 'rational' decision theory. Proc R Soc B Biol Sci. 276:2171–2178. doi: 10.1098/rspb.2009.0121
- Rotter JB. 1966. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr Gen Appl. 80:1–28. doi: 10.1037/h0092976
- Sapolsky RM. 2004. Social status and health in humans and other animals. Annu Rev Anthropol. 33:393–418. doi: 10.1146/annurev.anthro.33.070203.144000
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR, Jacobs GA. 1970. STAI manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press.
- Strauser DR, Ketz K, Keim J. 2002. The relationship between self-efficacy, locus of control and work personality. J Rehabil. 68:20–26.
- Sutton SK, Davidson RJ. 1997. Prefrontal brain asymmetry: a biological substrate of the behavioral approach and inhibition systems. Psychol Sci. 8:204–210. doi: 10.1111/j.1467-9280.1997.tb00413.x
- Suzuki S, Niki K, Fujisaki S, Akiyama E. 2011. Neural basis of conditional cooperation. Soc Cogn Affect Neurosci. 6:338–347. doi: 10.1093/scan/nsq042
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. 1992. Psychometric properties of resting anterior EEG asymmetry: temporal stability and internal consistency. Psychophysiology 29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x
- Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, Rosenkranz Ma, Ryff CD, Singer BH, Davidson RJ. 2004. Making a life worth living. Neural correlates of well-being. Psychol Sci. 15:367–372. doi: 10.1111/j.0956-7976.2004.00686.x
- Yu AJ, Dayan P. 2005. Uncertainty, neuromodulation, and attention. Neuron. 46:681–692. doi: 10.1016/j.neuron.2005.04.026
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-lindenberg A. 2008. Know your place: neural processing of social hierarchy in humans. Neuron. 58:273–283. doi: 10.1016/j.neuron.2008.01.025