ABSTRACT
Information accumulated over the past decades on the physiological functions and metabolic pathways of biosynthesis and degradation of D-amino acids has led to a renewed interest in their study. These isomers are known to form both in nature and during the chemical synthesis of L-amino acids for feeding and pharmacological purposes, as well as in the industrial processing of some raw materials. This article discusses the positive and negative effects of D-amino acids on the human body, animals and the environment. In addition, the scientific data concerning the mechanisms of cytotoxic action of D-amino acids and their industrial and biomedical potential are summarized.
Introduction
Amino acids, as components of peptides, peptide hormones, structural and immune proteins, are the most important bioregulators involved in all life processes along with nucleic acids, carbohydrates and lipids. Due to their high biological activity and widespread usage, their production is constantly growing. The main consumers of amino acids are food, biotech, pharmaceutical industries, medicine and animal husbandry (Martínez-Rodríguez et al. Citation2010; Sorokina et al. Citation2010).
An important biochemical feature of amino acids is their optical activity. All of them except glycine have one or two asymmetric atoms and can therefore exist in two (D- and L-) optically active (enantiomeric) forms that have different physiological and biological activities. Whereas L-amino acids in most cases have a positive effect on the body, D-amino acids may have adverse effects. Thus, it is very important to control their optical purity (Chernobrovkin et al. Citation2004).
In recent years, numerous new studies of biochemical properties of D-amino acids have been published, opening up potentially undeveloped application fields for these isomers in various industries and biomedicine.
Stereoisomerism of D-amino acids
When the chemistry of D-amino acids is mentioned, their stereochemistry and the associated reactions of chemical transformations are usually implied.
As already noted, all amino acids except glycine have four different substituent groups at the α-carbon atom. For this reason, the α-carbon atom is said to have molecular asymmetry or chirality (from the Greek – χειρ – ‘hand’). It is the specularity phenomenon, i.e. the property of a molecule not to be superposed in space with its mirror image. Due to this structural feature amino acid molecules can exist in different stereoisomeric forms differing only in the orientation of radical groups at the α-carbon atom.
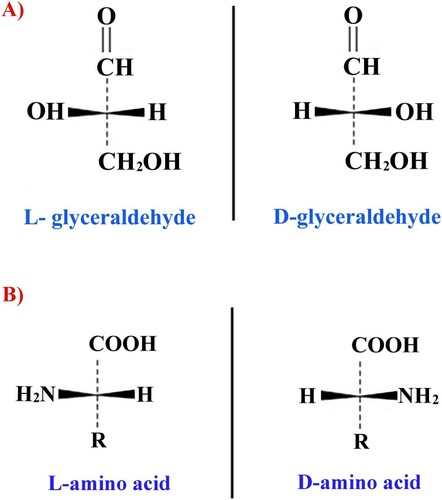
Historically, the spatial configuration of radicals at the α-carbon atom was compared with that of a reference compound which was glyceraldehyde. For glyceraldehyde, it was initially decided to divide these positions into L- and D-configurations. Therefore, stereoisomers of amino acids corresponding in their configuration to the α-carbon atom of L, D-glyceraldehyde, are also labeled with the letters L or D, although the stereoisomerism of amino acids is defined by the location of the amino group at the chiral atom. If in the Fischer projection formula an amino group is located to the left of the chirality center, it corresponds to an L-amino acid, otherwise – to its D-stereoisomer (see Figure ).
At the present time, amino acids for agriculture and biomedicine are obtained by microbiological synthesis, hydrolysis of connective tissues or chemical synthesis (Cartus Citation2012; Keating et al. Citation2018). Whereas in the first and second cases mainly L-isomers of amino acids are produced, in the latter case only racemic L, D-mixtures are produced (for example, by the ammonolysis of alpha halo carboxylic acids). Due to the fact that the industry requires mainly L-forms, there is a need to separate such mixtures. To separate amino acids onto optical antipodes, chemical, microbiological and especially enzymatic methods are used.
Chemical methods include basic or acidic interactions of isomeric forms of amino acids with optically active auxiliary compounds (derivatives of tartaric acid, camphor, sulfonic acid, etc.) with the formation of optically active salts and their subsequent separation and refining by fractional crystallization. Enzymatic methods are based on the use of a number of free or immobilized enzymes (acylases, esterases) to selectively cleave a specific kind of the enantiomeric forms of amino acids (Cartus Citation2012).
In recent years, chromatography on selective chiral sorbents has been increasingly used for separation of racemic mixtures (Keating et al. Citation2018).
However, 100% separation efficiency of racemic mixtures or complete conversion of one stereoisomer into another is difficult. This is due to the fact that the vast majority of optically active substances, including amino acids, have limited stability over time, which is expressed in a gradual tendency of the system to some optical equilibrium, i.e. to the racemization which is most often accelerated under the influence of some physical and chemical factors, for example, in the presence of acid or alkaline admixtures (Martínez-Rodríguez et al. Citation2010) (Figure ).
Figure 2. L, D- racemization reaction of amino acids (A- Acid-catalyzed racemization. B – Base-catalyzed racemization).
The tendency to such equilibrium is most probably dependent on many factors, since at the constant temperature, pressure and pH of the medium, the entropy of molecules of a chirally pure system was found to be zero (S = 0), while for a racemic mixture it takes a completely different form: S = kLn2, where ‘k’ is the Boltzmann constant (Tverdislov and Yakovenko Citation2008).
It should be noted that the value of spontaneous racemization varies for different amino acids. Its frequency for free and bound amino acids has been experimentally shown to decrease in the following order (Cartus Citation2012): Cys > Asp > Pro > Glu > Met > His > Leu > Lys > Phe > Ala > Tyr > Arg > Trp> Val > Ile > Ser > Thr.
Aspects of D-amino acid metabolism
Numerous enzyme-catalyzed reactions involving D-amino acids occur in major types of cells: prokaryotic and eukaryotic. D-amino acids act not only as regulatory molecules, but in some cases they can be included in catabolism or have toxic effects, thus leading to the need for the involvement of detoxification mechanisms.
Enzymes involved in the biosynthesis, degradation or modification of D-amino acids or their derivatives may be specific to a particular amino acid or, in contrast, may have a broad spectrum of action relative to substrates. The list of these enzymes includes racemases, transaminases, oxidoreductases, dehydrogenases, etc. In this review, we will not go deeply into the enzymology and metabolism of D-amino acids, confining ourselves to general data regarding the main enzymes involved in the metabolism and reactions catalyzed by them (see Table ).
Table 1. The main enzymes involved in the metabolism of D-amino acids.
There is a number of spatial barriers for the active involvement of D-amino acids in cell metabolism and protein synthesis (more details in the following section). But in spite of steric limitations, the translational machinery can erroneously, probably due to partial antagonism with L-competitors, accept D-aminoacyl (aa)-tRNAs into the ribosomal aa-tRNA binding (A) site, use the A-site D-aa-tRNA as a peptidyl-transfer acceptor, and translocate the resulting peptidyl-D-aa-tRNA into the ribosomal peptidyl-tRNA binding (P) site (Englander et al. Citation2015). These facts may partly explain the isomer-toxic effects of D-amino acids on cells of some living organisms (Soutourina et al. Citation2000; Takayama et al. Citation2005; Bardaweel et al. Citation2013; Hener et al. Citation2018).
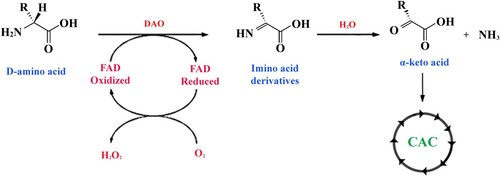
Thus, the cells of a multicellular organism throughout its life meet unusual and even toxic variants of amino acids. Detoxification of D-isomers of amino acids seems to be closely related to the process of oxidative deamination that generates α-keto acids (D’Mello Citation2003; Bardaweel et al. Citation2013) that can be used later in the citric acid cycle (CAC) (see Figure ).
Figure 3. Reaction of oxidative deamination of D-amino acids. The amino acid undergoes FAD-dependent enzymatic oxidation with the help of D-amino-acid oxidase (DAO) to produce an imino-derivative as the intermediate product. Subsequently, the imino acid undergoes non-enzymatic hydrolysis that converts the substrate to the corresponding α-keto acid and ammonia.
Evolutionary aspects of the origin of D-amino acids
In nature, L-enantiomers primarily prevail, which are otherwise called proteinogenic amino acids. There is still debate about where and how the molecular asymmetry of amino acids has first appeared and why L-isomers have become proteinogenic.
It should be noted that the study of the Murchison meteorite (a large meteorite that fell to the Earth near Murchison, Victoria, Australia, in 1969) has shown that its material contains both isomers of amino acids with L-enantiomers prevailing (Cronin and Pizzarello Citation1997; Elsila et al. Citation2016). Since carbonaceous chondrites, which this meteorite belongs to, have formed 4.5 billion years ago, the authors (Cronin and Pizzarello Citation1997) came up with a bold hypothesis that the molecular asymmetry of amino acids have appeared long before the emergence of life on Earth.
Despite these data, there is still a mystery about how and why L-amino acids have become the dominant form on the planet. The preferential use of L-enantiomers for the construction of cell proteins obviously has deep evolutionary roots. In the course of molecular evolution, major mechanisms have been selected, apparently at random, that exclude D-amino acids from protein synthesis and thus support the homochirality of peptides.
However, various organisms still produce biologically active peptides containing D-amino acids. They are synthesized by direct incorporation of free D-amino acids, which is carried out by large enzyme complexes called nonribosomal peptide synthetases (NRPSs) or by means of D-modification of a peptide precursor constructed from L-amino acids, otherwise called the post-translational L-amino acid epimerization. The first mechanism is specific for prokaryotes, while the second occurs in both pro- and eukaryotic cells. D-amino acids in the natural state are practically not found among receptors, structural proteins and proteins of the immune system. Nonetheless, they can still be seen in the composition of peptide antibiotics, hormones, neuropeptides, hepatotoxins and opioids.
Among the main reasons for such evolutionary selectivity, energy effects and D-nucleotide influence are particularly prominent. For instance, the studies (Fisun and Savin Citation1992) and (Soares et al. Citation1997) have shown that peptide α-helix constructed from D-amino acids is less stable from the energy point of view than a similar helix constructed from L-isomers. Theoretical calculations have shown that the energy differences between D- and L-amino acids are on the order of 10−19 eV per molecule (Lins and Ferreira Citation2006).
Some answers to these questions can also be obtained from the analysis of protein–nucleic acid interactions that constantly occur between RNA, DNA and proteins involved in nucleic acid metabolism. Polynucleotides containing the standard D-sugars have been shown to react differently with D- or L-amino acids. Calculations show that primitive tRNAs formed from several ribonucleotides and capable of specific binding to an L-amino acid by hydrogen bonds are in most cases unable to recognize D-amino acids with the same specificity, because their lateral substituent groups are sterically difficult to fit into the amino acid binding site of tRNA (Balasubramanian Citation1983). In addition, this process is complicated by a special enzyme D-aminoacyl-tRNA deacylase (DTD) that possesses a proofreading activity and removes D-isomers of amino acids accidentally binding with tRNA (Kuncha et al. Citation2018). Thus, the coexistence of D-ribonucleotides and L-amino acids is necessary for certain types of biological interactions. If we take into account a popular hypothesis that ribonucleic acids preceded proteins in the ‘prebiotic soup’ (Joyce Citation1989), it becomes obvious that the evolutionary choice in favor of L-amino acids could be a result of the basic properties of D-ribonucleotides and RNA molecules constructed from them (Bailey Citation1998).
Distribution of D-amino acids in the nature
D-amino acids were previously called ‘non-native’ because in the vast majority of cases they are not used as the building blocks of structural proteins of cellular and non-cellular forms of life. At the same time, these compounds are present in prokaryotic and eukaryotic cells, in free form or as a part of other substances.
Da Silva and Da Silva (Citation2009) suggested the hypothesis that D-amino acids in different species have different effects: toxic, protective and signaling.
One of the best-studied examples of the presence of D-amino acids in the composition of natural biological structures is peptidoglycans of some microorganisms. Amino acids such as D-alanine and D-glutamate are important components of the cell walls of Eubacteria, contributing to their resistance to hydrolysis by peptidases. D-amino acids are characteristic of yeast cells as well, providing resistance of their spores to the influence of proteolytic enzymes.
Among peptides that in natural state contain D-amino acids can be found not only peptidoglycans, but also antibiotics, hepatotoxins, hormones, opioid peptides, neuropeptides, etc.
Peptide antibiotics containing D-amino acids and possessing antimicrobial properties were found in many microorganisms. In particular, the structure and synthesis of peptide antibiotics are well studied in some members of the genus Bacillus (Peypoux et al. Citation1985; Jack and Jung Citation1998). For example, the therapeutic index of such cyclic antibiotics as gramicidin S and polymyxin B is strongly modulated by D-phenylalanine contained in their structure. All of these peptides are synthesized independently of ribosomes. Their effect is based on the formation of transmembrane channels or inhibition of cell wall synthesis.
Biologically active peptides containing D-amino acids are also found in the cells of higher eukaryotic organisms; the only difference is that they are primarily formed as a result of post-translational modification of a precursor consisting of L-amino acids.
D-amino acid containing peptides with opioid or antimicrobial activity have been isolated from the skin secretions of invertebrates and amphibians. Among such compounds are achatins of snails, dermorphins, deltorphins and bombinins of amphibian species (Mignogna et al. Citation1998; Volkmann and Heck Citation1998; Jilek et al. Citation2005; Negri et al. Citation2005; Checco et al. Citation2018). Carnivorous marine gastropod mollusks of the family Conidae produce secretions with venom peptides containing D-tryptophan or D-leucine called conotoxins, which can result in fish kill and also be fatal even to mammals by blocking neuromuscular transmission (Volkmann and Heck Citation1998; Jimenez et al. Citation2001; Bai et al. Citation2013; Miyamoto and Homma Citation2018).
The presence of D-amino acid residues has also been detected in peptide hormones of some arthropods. For example, many crustaceans contain two isoforms of a special hyperglycemic hormone (Chung et al. Citation2010). The biological effect of this hormone consists in increasing the concentration of glucose in the hemolymph in response to stress or molting (Chung et al. Citation2010).
Another example is the venom of the North American funnel-web spider Agelenopsis aperta containing a variety of proteinaceous toxins capable of blocking calcium channels (Heck et al. Citation1994; Murkin and Tanner Citation2002). These peptides called agatoxins differ only by the presence of D-serine in the amino acid sequence. The toxin that contains D-amino acid (ω – agatoxin IV) is more effective than its L-analogue (agatoxin IVC) (Heck et al. Citation1994; Murkin and Tanner Citation2002).
A large number of studies conducted on mammals and birds showed that free D-amino acids are present in tissues and biological fluids of vertebrates (Errico et al. Citation2008; Hamase et al. Citation2009; Lin et al. Citation2017). Among the D-amino acids, D-aspartate and D-serine occur at the highest concentrations. D-aspartate is widely distributed throughout the CNS and in the endocrine peripheral organs of rats, humans and chicken embryos. The localization of D-aspartate and D-serine in specific parts of the brain suggests that these D-amino acids act as a kind of neurotransmitters or neuromodulators (Wolosker et al. Citation2008; Errico et al. Citation2011, Citation2015; Horio et al. Citation2011).
One of the most exotic examples of the presence of D-amino acid isoforms in mammalian proteins is the defensin-like peptide (DLP-2) isolated from the platypus venom (Torres et al. Citation2002; Whittington et al. Citation2009). Male platypus (Ornithorhynchus anatinus) has calcaneus spurs on each hind limb reaching 1.5 cm in length by the time of puberty. Each spur is connected by a duct with a special femoral gland that produces a poisonous secretion. The poison of the platypus is quite capable of killing a small animal like dingo. The poisonousness of the DLP-2 has been found to be associated with the presence of D-methionine in the second position (Torres et al. Citation2002, Citation2007; Whittington et al. Citation2009).
D-amino acid derivatives are also present in algal thalli and seedlings of higher plants, where they appear mainly in the form of dipeptides and malonic acid esters (Brückner and Westhauser Citation2003; Yokoyama et al. Citation2003; Herrero et al. Citation2007; Bouillaut et al. Citation2013; Gholizadeh Citation2015; Strauch et al. Citation2015). Different plants have been found to respond to stress by D-tryptophan production with subsequent synthesis of its malonyl derivatives (Yu et al. Citation2014). According to some studies, D-tryptophan derivatives can be precursors of plant hormones auxins that have a powerful effect on the growth of fruits and shoots of higher plants (Rekoslavskaya Citation1986).
D-amino acids in agriculture and fodder production
Farm animals remain the basis of the global food supply. It is obvious that almost all functions of a living organism are to some degree related to their protein components. Proteins perform a variety of functions such as catalytic, structural, regulatory, receptor, molecular transport, protective, and respiratory ones.
The proteins of the body are built exclusively from the proteins and L-amino acids of the food. Therefore these compounds are very important in the regulation of nutrition of industrially important livestock and poultry to create the basis for animal health and for the intravital formation of animal-derived foods with specified technological and consumer attributes (Liu et al. Citation2016).
The long-term deficiency of protein or any of the essential L-amino acids can lead to thermoregulation disorders, reduced productivity or the emergence of a number of serious diseases, the most important of which are immune disorders, since immunoglobulins being the main structural and functional components of the immune system are proteins themselves. Thus, for the normal growth, development, resistance to infectious agents and adverse environmental factors, as well as for the formation of animal-derived products with the desired properties, farm animals and poultry need a constant intake of the L-amino acid complex in a certain proportion and digestible form. Crude protein and some essential amino acids are often deficient in diets and are routinely added synthetically to commercial feed mixes.
To improve the nutritional value of diets in terms of crude protein, they are balanced by protein-containing fodder supplements or free amino acids. Some of these amino acids are produced by microbiological synthesis in certain types of autotrophic soil bacteria, while others are produced by chemical synthesis in the form of chlorinated racemic mixtures requiring subsequent separation procedures (see above). In most cases this procedure is not used because of a significant increase in the cost of the final fodder product, thus for farm animals L, D-racemates are frequently given.
The removal of D-isomers of amino acids from fodder is necessary for several reasons: they have low metabolic and nutritional value (D’Mello Citation2003) (Table ), reduce the availability of L-amino acids (Csapo et al. Citation2009) and require the involvement of at least two energy-consuming pathways for their utilization in animal cells. The first pathway is oxidative deamination into the corresponding α-keto acid, the second – L-specific reamination that involves special aminotransferases (D’Mello Citation2003; Bhagavan and Ha Citation2015). It should be noted that, for instance, the poultry organisms simply do not have such aminotransferases for the L-specific reamination of many D-amino acids, therefore they remain metabolically intact and subsequently find themselves in the environment in the form of various ballast nitrogen compounds together with the poultry droppings, which can have adverse environmental effects in areas of intensive poultry farming (Maheshwari Citation2013).
Table 2. Nutritional value of D-isomers of amino acids as a percentage of that of the L-isomers.
Previously, a number of D-isomers of amino acids have been shown to have toxic effects for some mammals and poultry, which is manifested in the weakening of the immune status, nephrotoxicity, reduced productivity and weight loss (Cherkin et al. Citation1978; Baker Citation2006; Csapo et al. Citation2009; Cartus Citation2012). Although the exact mechanism of this toxicity is still unknown, it can be assumed to be associated with the above-mentioned effect of competition of D- and L-isomers and the erroneous inclusion of D-aa-tRNAs into the translational machinery (Englander et al. Citation2015).
It should also be noted that not only D- but also free forms of some L-amino acids may be toxic for the body. For example, studies on chickens have shown that the consumption of an excess amount of free sulfur-containing L-amino acids can significantly increase their mortality due to malfunctions of the spleen or the central nervous system (CNS) and retinal degeneration. The authors of the study suggest that these amino acids may act as chelating agents that bind a number of important micronutrients leading to their deficiencies (Persia et al. Citation2003, Baker Citation2006).
It is obvious that in animal husbandry and poultry farming free D-amino acids as well as their L-enantiomers sometimes have negative impact, thus finding ideal variants of supplements for balancing the crude protein and essential L-amino acids is still important.
In terms of the above, it is interesting that a number of studies have shown that the effectiveness of the absorption of L-amino acids from incomplete polypeptide hydrolysates in the digestive tract is sometimes higher than their absorption from mixtures of free L, D-amino acids (Fairclough et al. Citation1980; Silk et al. Citation1980, Citation1985; Freeman et al. Citation1983; Grimble et al. Citation1986; Rérat et al. Citation1988; Boza et al. Citation1995; Tutel’yan et al. Citation2003; Timofeeva et al. Citation2005; Jung et al. Citation2007; Ten Have et al. Citation2007). For this reason, to obtain amino acid-balanced rations, at the present time alternative methods of crude protein balancing are actively developed, where the main role is given not to free, but to peptide-contained amino acids. Previously, the authors of this review proposed a biotechnological approach for the creation of thermostable fodder peptide cassettes enriched in the required proportion with L-amino acid residues important for the metabolism of various groups of poultry (Grishin et al. Citation2017, Citation2018).
D-amino acids in biomedicine and food industry
Despite the fact that in agriculture and fodder production D-amino acids play a rather negative role, in the field of biomedicine everything is exactly opposite. Since D-amino acids are frequently detected in the composition of natural antibiotics isolated from microorganisms, invertebrates and amphibians, they are often used as a basis for the development of medicinal and veterinary antimicrobial agents.
Among antibiotic drugs based on L, D-amino acids, widely known are tyrothricin (Bacillus brevis), D-cycloserine (Streptomyces orchidaceus), daptomycin (Streptomyces roseosporus), benzylpenicillin (Penicillium chrysogenum), actinomycin D (Streptomyces parvullus), capreomycin (Streptomyces capreolus), polymyxin (Bacillus polymyxa) (Martínez-Rodríguez et al. Citation2010; Robbel and Marahiel Citation2010; Grishin and Sokolov Citation2014). Among them, there are bactericidal, bacteriostatic agents and even compounds with antitumor activity.
Such antibiotics can be divided into predominantly containing proteinogenic or non-proteinogenic amino acids. For example, antibacterial polymyxins and antitumor antibiotic actinomycin D consist mainly of proteinogenic amino acids, only some of which have a D-configuration. Such antibiotics can have both linear and cyclic structural organization.
Antibiotics that are comprised mainly of non-proteinogenic amino acids in most cases have a completed cyclic form. At the same time, they are quite diverse in molecular mechanisms of action which vary from the dephosphorylation of phospholipids of the bacterial plasma membrane (bacitracin A) to the formation of pseudo-ionic channels that disrupt the ion transport across membranes (tyrothricin). Long-term cytotoxic effects of such peptide antibiotics may be associated with both isomer-toxic effects and resistance of peptides built from D-isomers of amino acids to the cleavage by proteolytic enzymes.
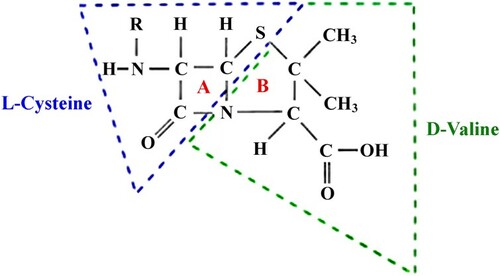
It should be noted that many popular antibiotics such as penicillin group members contain a residue of secondary D-valine that forms the basis of the nucleus of 6-aminopenicillanic acid (see Figure ).
Figure 4. The core of penicillin derivatives is a cyclic dipeptide consisting of two amino acids (D-valine and L-cysteine) (A – β-lactam ring. B – thiazolidine ring).
As previously mentioned, D-amino acids and related compounds play a certain role in the work of CNS. For instance, D-serine is the endogenous co-agonist of ionotropic glutamate receptors (NMDAR; NMDA-receptor) in entorhinal cortex, acting as a neuromodulator. The amount of this serine isoform is regulated by the D-amino acid oxidase (DAO) that belongs to the family of flavoproteins catalyzing the oxidative deamination of amino acids. The level of expression and enzymatic activity of DAO in the brain of people with neuropsychiatric disorders (schizophrenia, Alzheimer’s disease, autism) has been found to be much higher than in healthy controls. For this reason, the system is considered a promising new target in the treatment of these neuropsychiatric disorders. Currently, one of the possible therapeutic approaches is a combination of D-serine and DAO inhibitors (Wolosker Citation2007; Rojas et al. Citation2016; Szilágyi et al. Citation2018).
Recently, promising results have been published concerning in vivo uptake of D-amino acids in tumors; 18F-fluoromethyl-labelled D-tyrosine has been shown to be suitable for positron emission tomography (PET) imaging of normal and tumor tissues (Murayama et al. Citation2009; Urakami et al. Citation2009; Martínez-Rodríguez et al. Citation2010).
Scientific advances have made it possible to design, with the help of chemical synthesis, peptides built exclusively from D-amino acids. This fact allowed to compare properties of D-peptides with those of their L-analogues. One of the first such experiments was carried out with the protease of the retrovirus HIV-1 (Nakatani et al. Citation2008; Torbeev et al. Citation2011). Both enantiomers of this protein contain 99 amino acids and have opposite specificities for their substrates. The second example is a synthetic theta defensins constructed only of D-versions of amino acids. A number of studies show that these cationic peptides are more stable and exhibit anti-retroviral activity in vitro, binding to their corresponding receptors (Owen et al. Citation2004; Wang Citation2012; Falanga et al. Citation2017).
In addition to the above, the age-specific increase in the frequency of spontaneous racemization of aspartic acid in some cell proteins has also been found. At the same time, its D-isomer can appear in a number of polypeptides of the body as it ages (osteocalcin, collagen, myelin, β-amyloid, etc.), in which it does not normally occur (Fujii Citation2005; Kaji et al. Citation2010; Chervyakov et al. Citation2011; Fujii et al. Citation2018). Its appearance can affect the functions of proteins and proteides, having a destabilizing effect on the tertiary and quaternary structures of the proteins. For example, the proportion of D-aspartic acid has been observed to increase in the crystalline lens of the eye and the CNS of elderly people, which some researchers associate with incidence rates of clinically diagnosed Alzheimer’s disease and cataracts. Thus, it is obvious that D-aspartic acid has a strong potential to become a biological marker of vertebrate aging (Fujii and Saito Citation2004; Fujii Citation2005; Kaji et al. Citation2010; Chervyakov et al. Citation2011; Fujii et al. Citation2018).
In terms of biomedicine, it is reasonable to note that small amounts of free D-amino acids are found in fruits and vegetables comprising the daily diet of humans, where their content varies from 0.7 to 3.4% of the total amount of amino acids (Cartus Citation2012). However, this proportion significantly increases with the use of a number of preserving agents, with various kinds of intensive processing of raw food, as well as with their microbial contamination. For example, in the process of wine and juice making, such a characteristic as the content of D-alanine is considered as a marker of bacterial contamination occurring before or during wine and juice producing (Gandolfi et al. Citation1994; Cartus Citation2012; Mutaguchi et al. Citation2013).
Conclusions
D-amino acid optical isomers are rather ambiguous compounds with various effects and various potentials in diverse fields of science and industry.
On the one hand, in the field of animal husbandry and poultry farming they are mostly a ballast component of fodder that is not only poorly utilized by the organism of most animals and significantly reduces the digestibility and nutritional value of crude fodder protein, but also has a number of toxic effects (oxidative stress, nephrotoxicity, immunodeficiency, emaciation, etc.). That is why in the field of agriculture and fodder production the importance of the development of fodder supplements with a reduced proportion of these stereoisomers will increase every year.
On the other hand, it should be remembered that the same D-amino acids in trace amounts perform important functions in the work of the CNS, can serve as markers of various pathophysiological processes or become the basis for next generation medical remedies. Therefore, in contrast to agriculture, the production of these amino acid variants for biomedicine is likely to increase in the coming decades.
Additional information
No additional information is available for this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arakawa N, Igarashi M, Kazuoka T, Oikawa T, Soda K. 2003. D-arginase of Arthrobacter sp. KUJ 8602: characterization and its identity with Zn(2+)-guanidinobutyrase. J Biochem. 133(1):33–42. https://doi.org/https://doi.org/10.1093/jb/mvg016.
- Ariyoshi M, Katane M, Hamase K, Miyoshi Y, Nakane M, Hoshino A, Okawa Y, Mita Y, Kaimoto S, Uchihashi M, et al. 2017. D-Glutamate is metabolized in the heart mitochondria. Sci Rep. 7:43911. doi:https://doi.org/10.1038/srep43911.
- Bai L, Livnat I, Romanova EV, Alexeeva V, Yau PM, Vilim FS, Weiss KR, Jing J, Sweedler JV. 2013. Characterization of GdFFD, a D-amino acid-containing neuropeptide that functions as an extrinsic modulator of the Aplysia feeding circuit. J Biol Chem. 288(46):32837–32851. doi:https://doi.org/10.1074/jbc.M113.486670.
- Bailey JM. 1998. RNA-directed amino acid homochirality. FASEB J. 12:503–507. https://doi.org/https://doi.org/10.1096/fasebj.12.6.503.
- Baker DH. 2006. Comparative species utilization and toxicity of sulfur amino acids. J Nutr. 136(6):1670S–1675S. doi:https://doi.org/10.1093/jn/136.6.1670S.
- Balasubramanian R. 1983. Possible mechanism for origin of chiral specificity during origins of life. Orig Life. 13:109–112. https://doi.org/https://doi.org/10.1007/BF00928888.
- Baldassarre M, Scirè A, Fiume I, Tanfani F. 2011. Insights into the structural properties of D-serine dehydratase from Saccharomyces cerevisiae: an FT-IR spectroscopic and in silico approach. Biochimie. 93:542–548. doi:https://doi.org/10.1016/j.biochi.2010.11.009.
- Bardaweel SK, Abu-Dahab R, Almomani NF. 2013. An in vitro based investigation into the cytotoxic effects of D-amino acids. Acta Pharm. 63(4):467–478. doi:https://doi.org/10.2478/acph-2013-0032.
- Bhagavan NV, Ha C-E. 2015. Essentials of medical biochemistry: with clinical cases. 2nd ed. USA: Academic Press. p. 752.
- Bouillaut L, Self WT, Sonenshein AL. 2013. Proline-dependent regulation of Clostridium difficile Stickland metabolism. J Bacteriol. 195(4):844–854. doi:https://doi.org/10.1128/JB.01492-12.
- Boza JJ, Martínez-Augustin O, Baró L, Suarez MD, Gil A. 1995. Protein v. enzymic protein hydrolysates. nitrogen utilization in starved rats. Br J Nutr. 73(1):65–71. https://doi.org/https://doi.org/10.1079/BJN19950009.
- Brückner H, Westhauser T. 2003. Chromatographic determination of L- and D-amino acids in plants. Amino Acids. 24:43–55. https://doi.org/https://doi.org/10.1007/s00726-002-0322-8.
- Cartus AT. 2012. D-Amino acids and cross-linked amino acids as food contaminants. In: Schrenk D, editor. Chemical contaminants and residues in food. Cambridge: Woodhead Publishing; p. 286–319. doi:https://doi.org/10.1533/9780857095794.2.286.
- Checco JW, Zhang G, Yuan WD, Yu K, Yin SY, Roberts-Galbraith RH, Yau PM, Romanova EV, Jing J, Sweedler JV. 2018. Molecular and physiological characterization of a receptor for D-amino acid-containing neuropeptides. ACS Chem Biol. 13(5):1343–1352. doi:https://doi.org/10.1021/acschembio.8b00167.
- Cherkin A, Davis JL, Garman MW. 1978. D-Proline: stereospecificity and sodium chloride dependence of lethal convulsant activity in the chick. Pharmacol Biochem Behav. 8(5):623–625. https://doi.org/https://doi.org/10.1016/0091-3057(78)90399-4.
- Chernobrovkin MG, Anan’eva IA, Shapovalova EN, Shpigun OA. 2004. Determination of amino acid enantiomers in pharmaceuticals by reversed-phase high-performance liquid chromatography. J Anal Chem. 59(1):55–63. https://doi.org/https://doi.org/10.1023/B:JANC.0000011669.08932.d8.
- Chervyakov AV, Gulyaeva NV, Zakharova MN. 2011. D-amino acids in normal ageing and pathogenesis of neurodegenerative diseases. Neurochemical Journal. 5(2):100–114. https://doi.org/https://doi.org/10.1134/S1819712411020036.
- Chung JS, Zmora N, Katayama H, Tsutsui N. 2010. Crustacean hyperglycemic hormone (CHH) neuropeptidesfamily: functions, titer, and binding to target tissues. Gen Comp Endocrinol. 166(3):447–454. doi:https://doi.org/10.1016/j.ygcen.2009.12.011.
- Cronin JR, Pizzarello S. 1997. Enantiomeric excesses in meteoritic amino acids. Science. 275(5302):951–955. doi:https://doi.org/10.1126/science.275.5302.951.
- Csapo J, Albert C, Csapo-Kiss Z. 2009. The D-amino acid content of foodstuffs (A review). Acta Univ Sapientiae, Alimentaria. 2:5–30. http://www.acta.sapientia.ro/acta-alim/C2-1/alim2-1.pdf.
- Da Silva JJRF, Da Silva JAL. 2009. D-Amino acids in biology – more than one thinks, (D-amino acidos em biologia – mais do que se julga). Quim Nova. 32:554–561. https://doi.org/http://doi.org/10.1590/S0100-40422009000200046).
- D’Mello JPF. 2003. Amino acids as multifunctional molecules. In: D’Mello JPF, editor. Amino acids in animal nutrition. 2nd ed. CABI Publishing; p. 1–14. doi:https://doi.org/10.1079/9780851996547.0001.
- Elsila JE, Aponte JC, Blackmond DG, Burton AS, Dworkin JP, Glavin DP. 2016. Meteoritic amino acids: diversity in compositions reflects parent body histories. ACS Cent Sci. 2(6):370–379. doi:https://doi.org/10.1021/acscentsci.6b00074.
- Englander MT, Avins JL, Fleisher RC, Liu B, Effraim PR, Wang J, Schulten K, Leyh TS, Jr GR, Cornish VW. 2015. The ribosome can discriminate the chirality of amino acids within its peptidyl-transferase center. PNAS. 112(19):6038–6043. doi:https://doi.org/10.1073/pnas.1424712112.
- Errico F, Mothet JP, Usiello A. 2015. D-Aspartate: an endogenous NMDA receptor agonist enriched in the developing brain with potential involvement in schizophrenia. J Pharm Biomed Anal. 116:7–17. doi:https://doi.org/10.1016/j.jpba.2015.03.024.
- Errico F, Nisticò R, Napolitano F, Mazzola C, Astone D, Pisapia T, Giustizieri M, D’Aniello A, Mercuri NB, Usiello A. 2011. Increased D-aspartate brain content rescues hippocampal age-related synaptic plasticity deterioration of mice. Neurobiol Aging. 32(12):2229–2243. doi:https://doi.org/10.1016/j.neurobiolaging.2010.01.002.
- Errico F, Nisticò R, Palma G, Federici M, Affuso A, Brilli E, Topo E, Centonze D, Bernardi G, Bozzi Y, et al. 2008. Increased levels of D-aspartate in the hippocampus enhance LTP but do not facilitate cognitive flexibility. Mol Cell Neurosci. 37(2):236–246. doi:https://doi.org/10.1016/j.mcn.2007.09.012.
- Fairclough PD, Hegarty JE, Silk DB, Clark ML. 1980. Comparison of the absorption of two protein hydrolysates and their effects on water and electrolyte movements in the human jejunum. Gut. 21(10):829–834. https://gut.bmj.com/content/gutjnl/21/10/829.full.pdf.
- Falanga A, Nigro E, De Biasi MG, Daniele A, Morelli G, Galdiero S, Scudiero O. 2017. Cyclic peptides as novel therapeutic microbicides: engineering of human defensin mimetics. Molecules. 22(7):E1217. doi:https://doi.org/10.3390/molecules22071217.
- Fisun OI, Savin AV. 1992. Homochirality and long-range transfer in biological systems. Biosystems. 27(3):129–135. https://doi.org/https://doi.org/10.1016/0303-2647(92)90068-A.
- Freeman HJ, Sleisinger MH, Kim YS. 1983. Human protein digestion and absorption: normal mechanisms and protein-energy malnutrition. Clin Gastroenterol. 12(2):357–378. https://www.ncbi.nlm.nih.gov/pubmed/6409468.
- Fujii N. 2005. D-amino acid in elderly tissues. Biol Pharm Bull. 28(9):1585–1589. https://doi.org/https://doi.org/10.1248/bpb.28.1585.
- Fujii N, Saito T. 2004. Homochirality and life. Chem Rec. 4:267–278. doi:https://doi.org/10.1002/tcr.20020.
- Fujii N, Takata Т, Fujii N, Aki K, Sakaue H. 2018. D-Amino acids in protein: the mirror of life as a molecular index of aging. Biochim Biophys Acta. 1866(7):840–847. doi:https://doi.org/10.1016/j.bbapap.2018.03.001.
- Gandolfi I, Palla G, Marchelli R, Dossena A, Puelli S, Salvadori C. 1994. D-Alanine in fruit juices: a molecular marker of bacterial activity, heat treatments and shelf-life. J Food Sci. 59(1):152–154. https://doi.org/https://doi.org/10.1111/j.1365-2621.1994.tb06921.x.
- Gholizadeh A. 2015. The possible involvement of D-amino acids or their metabolites in Arabidopsis cysteine proteinase/cystatin N-dependent proteolytic pathway. Tsitol Genet. 49(2):73–79. https://doi.org/https://doi.org/10.3103/S0095452715020036.
- Gogami Y, Ito K, Kamitani Y, Matsushima Y, Oikawa T. 2009. Occurrence of D-serine in rice and characterization of rice serine racemase. Phytochemistry. 70(3):380–387. doi:https://doi.org/10.1016/j.phytochem.2009.01.003.
- Grimble GK, Keohane PP, Higgins BE, Kaminski MV Jr., Silk DB. 1986. Effect of peptide chain length on amino acid and nitrogen absorption from two lactalbumin hydrolysates in the normal human jejunum. Clin Sci. 71(1): 65–69. doi:https://doi.org/10.1042/cs0710065.
- Grishin DV, Gladilina YA, Aleksandrova SS, Pokrovskaya MV, Podobed OV, Pokrovskii VS, Zhdanov DD, Sokolov NN. 2017. Creation of thermostable polypeptide cassettes for amino acid balancing in farm animal rations. Appl Biochem Microbiol. 53(6):688–698. https://doi.org/https://doi.org/10.1134/S0003683817060072.
- Grishin DV, Sokolov NN. 2014. Defensins are natural peptide antibiotics of higher eukaryotes. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry. 8(1):11–18. https://doi.org/https://doi.org/10.1134/S1990750814010077.
- Grishin DV, Zhdanov DD, Gladilina J, Pokrovsky VS, Podobed OV, Pokrovskaya MV, Aleksandrova SS, Milyushkina AL, Vigovskiy MA, Sokolov NN. 2018. Construction and Characterization of a Recombinant Mutant Homolog of the CheW protein from Thermotoga petrophila RKU-1. Biochem (Moscow) Suppl S B Biomed Chem. 12(2):143–150. https://doi.org/https://doi.org/10.1134/S1990750818020051.
- Hamase K, Morikawa A, Etoh S, Tojo Y, Miyoshi Y, Zaitsu K. 2009. Analysis of small amounts of D-amino acids and the study of their physiological functions in mammals. Anal Sci. 25(8):961–968. https://doi.org/https://doi.org/10.2116/analsci.25.961.
- Heck SD, Siok CJ, Krapcho KJ, Kelbaugh PR, Thadeio PF, Welch MJ, Williams RD, Ganong AH, Kelly ME, Lanzetti AJ, et al. 1994. Functional consequences of posttranslational isomerization of Ser46 in a calcium channel toxin. Science. 266(5187):1065–1068. doi:https://doi.org/10.1126/science.7973665.
- Hener C, Hummel S, Suarez J, Stahl M, Kolukisaoglu Ü. 2018. D-Amino acids Are Exuded by Arabidopsis thaliana roots to the Rhizosphere. Int J Mol Sci. 19(4):E1109. doi:https://doi.org/10.3390/ijms19041109.
- Herrero M, Ibáñez E, Fanali S, Cifuentes A. 2007. Quantitation of chiral amino acids from microalgae by MEKC and LIF detection. Electrophoresis. 28(15):2701–2709. doi:https://doi.org/10.1002/elps.200600599.
- Horio M, Kohno M, Fujita Y, Ishima T, Inoue R, Mori H, Hashimoto K. 2011. Levels of D-serine in the brain and peripheral organs of serine racemase (Srr) knock-out mice. Neurochem Int. 59(6):853–859. doi:https://doi.org/10.1016/j.neuint.2011.08.017.
- Jack RW, Jung G. 1998. Natural peptides with antimicrobial activity. Chimia. 52:48–55. http://www.ingentaconnect.com/contentone/scs/chimia/1998/00000052/f0020001/art00006?crawler=true.
- Jenkinson CP, Grody WW, Cederbaum SD. 1996. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 114(1):107–132. https://doi.org/https://doi.org/10.1016/0305-0491(95)02138-8.
- Jilek A, Mollay C, Tippelt C, Grassi J, Mignogna G, Müllegger J, Sander V, Fehrer C, Barra D, Kreil G. 2005. Biosynthesis of a D-amino acid in peptide linkage by an enzyme from frog skin secretions. PNAS. 102(12):4235–4239. doi:https://doi.org/10.1073/pnas.0500789102.
- Jimenez EC, Watkins M, Juszczak LJ, Cruz LJ, Olivera BM. 2001. Contryphans from Conus textile venom ducts. Toxicon. 39(6):803–808. https://doi.org/https://doi.org/10.1016/S0041-0101(00)00210-5.
- Jones H, Venables WA. 1983. Effects of solubilisation on some properties of the membrane-bound respiratory enzyme D-amino acid dehydrogenase of Escherichia coli. FEBS Lett. 151(2):189–192. https://www.ncbi.nlm.nih.gov/pubmed/6131836.
- Joyce G. 1989. RNA evolution and the origins of life. Nature. 338:217–224. doi:https://doi.org/10.1038/338217a0.
- Jung E, Kim J, Kim M, Jung DH, Rhee H, Shin JM, Choi K, Kang SK, Kim MK, Yun CH, et al. 2007. Artificial neural network models for prediction of intestinal permeability of oligopeptides. BMC Bioinformatics. 8:1–9. doi:https://doi.org/10.1186/1471-2105-8-245.
- Kaji Y, Oshika T, Takazawa Y, Fukayama M, Fujii N. 2010. Pathological role of D-amino acid-containing proteins and advanced glycation end products in the development of age-related macular degeneration. Anti-Aging Med. 7(10):107–111. https://doi.org/https://doi.org/10.3793/jaam.7.107.
- Keating JJ, Bhattacharya S, Belfort G. 2018. Separation of D, L-amino acids using ligand exchange membranes. J Membr Sci. 555:30–37. https://doi.org/https://doi.org/10.1016/j.memsci.2018.03.030.
- Kuncha SK, Mazeed M, Singh R, Kattula B, Routh SB, Sankaranarayanan R. 2018. A chiral selectivity relaxed paralog of DTD for proofreading tRNA mischarging in Animalia. Nat Commun. 9(1):511. doi:https://doi.org/10.1038/s41467-017-02204-w.
- Lin C-H, Yang H-T, Chiu C-C, Lane H-Y. 2017. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci Rep. 7:14849. https://doi.org/https://doi.org/10.1038/s41598-017-13951-7.
- Lins RD, Ferreira R. 2006. The stability of right- and left-handed alpha-helices as a function of monomer chirality. Quim Nova. 29(5):997–998. https://doi.org/http://doi.org/10.1590/S0100-40422006000500020.
- Liu Y, Wang X, Wu H, Chen S, Zhu H, Zhang J, Hou Y, Hu CA, Zhang G. 2016. Glycine enhances muscle protein mass associated with maintaining Akt-mTOR-FOXO1 signaling and suppressing TLR4 and NOD2 signaling in piglets challenged with LPS. Am J Physiol Regul Integr Comp Physiol. 311(2):R365–R373. doi:https://doi.org/10.1152/ajpregu.00043.2016.
- Maheshwari S. 2013. Environmental impacts of poultry production. Poult Fish Wildl Sci. 1:101. doi:https://doi.org/10.4172/pfw.1000101.
- Martínez-Rodríguez S, Martínez-Gómez AI, Rodríguez-Vico F, Clemente-Jiménez JM, Las Heras-Vázquez FJ. 2010. Natural occurrence and industrial applications of D-amino acids: an overview. Chem Biodivers. 7(6):1531–1548. doi:https://doi.org/10.1002/cbdv.200900245.
- Mignogna G, Simmaco M, Barra D. 1998. Occurrence and function of D-amino acid-containing peptides and proteins: antimicrobial peptides. EXS. 85:29–36. https://www.ncbi.nlm.nih.gov/pubmed/9949866.
- Miyamoto T, Homma H. 2018. Detection and quantification of d -amino acid residues in peptides and proteins using acid hydrolysis. BBA Proteins Proteom. 1866(7):775–782. doi:https://doi.org/10.1016/j.bbapap.2017.12.010.
- Murayama C, Harada N, Kakiuchi T, Fukumoto D, Kamijo A, Kawaguchi AT, Tsukada H. 2009. Evaluation of D-18F-FMT, 18F-FDG, L-11C-MET, and 18F-FLT for monitoring the response of tumors to radiotherapy in mice. J Nucl Med. 50(2):290–295. doi:https://doi.org/10.2967/jnumed.108.057091.
- Murkin AS, Tanner ME. 2002. Dehydroalanine-based inhibition of a peptide epimerase from spider venom. J Org Chem. 67(24):8389–8394. doi:https://doi.org/10.1021/jo0204653.
- Mutaguchi Y, Ohmori T, Akano H, Doi K, Ohshima T. 2013. Distribution of D-amino acids in vinegars and involvement of lactic acid bacteria in the production of D-amino acids. Springerplus. 2:691. doi:https://doi.org/10.1186/2193-1801-2-691.
- Nakatani S, Hidaka K, Ami E, Nakahara K, Sato A, Nguyen JT, Hamada Y, Hori Y, Ohnishi N, Nagai A, et al. 2008. Combination of non-natural D-amino acid derivatives and allophenylnorstatine-dimethylthioproline scaffold in HIV protease inhibitors have high efficacy in mutant HIV. J Med Chem. 51(10):2992–3004. doi:https://doi.org/10.1021/jm701555p.
- Negri L, Lattanzi R, Giannini E, Colucci MA, Mignogna G, Barra D, Grohovaz F, Codazzi F, Kaiser A, Kreil G, Melchiorri P. 2005. Biological activities of Bv8 analogues. Br J Pharmacol. 146(5):625–632. doi:https://doi.org/10.1038/sj.bjp.0706376.
- Owen SM, Rudolph D, Wang W, Cole AM, Sherman MA, Waring AJ, Lehrer RI, Lal RB. 2004. A theta-defensin composed exclusively of D-amino acids is active against HIV-1. J Pept Res. 63(6):469–476. doi:https://doi.org/10.1111/j.1399-3011.2004.00155.x.
- Persia ME, Parsons CM, Baker DH. 2003. Amelioration of oral copper toxicity in chicks by dietary additions of ascorbic acid, cysteine and zinc. Nutr Res. 23:1709–1718. https://eurekamag.com/pdf/004/004034268.pdf.
- Peypoux F, Marion D, Maget-Dana R, Ptak M, Das BC, Michel G. 1985. Structure of bacillomycin F, a new peptidolipid antibiotic of the iturin group. Eur J Biochem. 153(2):335–340. https://doi.org/https://doi.org/10.1111/j.1432-1033.1985.tb09307.x.
- Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G. 2007. Physiological functions of D-amino acid oxidases: from yeast to humans. CMLS. 64:1373–1394. doi:https://doi.org/10.1007/s00018-007-6558-4.
- Rekoslavskaya NI. 1986. Possible role of N-malonyl-D-tryptophan as an auxin precursor. Biologia Plantarum. 28:62–67. https://doi.org/https://doi.org/10.1007/BF02885326.
- Rérat A, Vaissade P, Vaugelade P. 1988. Absorption kinetics of dietary hydrolysis products in conscious pigs given diets with different amounts of fish protein 1. Amino-nitrogen and glucose. Br J Nutr. 60:91–104. https://doi.org/https://doi.org/10.1079/BJN19880080.
- Robbel L, Marahiel MA. 2010. Daptomycin, a bacterial lipopeptide synthesized by a nonribosomal machinery. J Biol Chem. 285(36):27501–27508. doi:https://doi.org/10.1074/jbc.R110.128181.
- Rojas C, Alt J, Ator NA, Thomas AG, Wu Y, Hin N, Wozniak K, Ferraris D, Rais R, Tsukamoto T, Slusher BS. 2016. D-amino-acid oxidase inhibition increases D-serine plasma levels in mouse but not in monkey or dog. Neuropsychopharmacology. 41(6):1610–1619. doi:https://doi.org/10.1038/npp.2015.319.
- Silk DB, Fairclough PD, Clark ML, Hegarty JE, Marrs TC, Addison JM, Burston D, Clegg KM, Matthews DM. 1980. Use of a peptide rather than free amino acid nitrogen source in chemically defined “elemental” diets. JPEN J Parenter Enteral Nutr. 4(6):548–553. https://doi.org/https://doi.org/10.1177/0148607180004006548.
- Silk DBA, Grimble GK, Rees RG. 1985. Protein digestion and amino acid and peptide absorption. Proc Nutr Soc. 44(1):63–72. https://doi.org/https://doi.org/10.1079/PNS19850011.
- Soares TA, Lins RD, Longo R, Garratt R, Ferreira R. 1997. Plural origins of molecular homochirality in our biota part II. The relative stabilities of homochiral and mixed oligoribotides and peptides. Z. Naturforsch. 52:89–96. http://zfn.mpdl.mpg.de/data/Reihe_C/52/ZNC-1997-52c-0089.pdf.
- Sorokina ON, Sumina EG, Shtykov SN, Atayan VZ, Barysheva SV. 2010. TLC separation of D-, L -of amino acids in the 2-hydroxypropyl-ß- cyclodextrin aqueous mobile phase. Sorption and Chromatographic Processes. 10(1):135–141. http://www.sorpchrom.vsu.ru/?l=en&p=6&y=2010&vol=10&num=1 (in Russian)
- Soutourina J, Blanquet S, Plateau P. 2001. Role of D-cysteine desulfhydrase in the adaptation of Escherichia coli to D-cysteine. J Biol Chem. 276(44):40864–40872. doi:https://doi.org/10.1074/jbc.M102375200.
- Soutourina J, Plateau P, Blanquet S. 2000. Metabolism of D-aminoacyl-tRNAs in Escherichia coli and Saccharomyces cerevisiae cells. J Biol Chem. 275(42):32535–32542. doi:https://doi.org/10.1074/jbc.M005166200.
- Strauch RC, Svedin E, Dilkes B, Chapple C, Li X. 2015. Discovery of a novel amino acid racemase through exploration of natural variation in Arabidopsis thaliana. PNAS. 112(37):11726–11731. doi:https://doi.org/10.1073/pnas.1503272112.
- Szilágyi B, Kovács P, Ferenczy GG, Rácz A, Németh K, Visy J, Szabó P, Ilas J, Balogh GT, Monostory K, et al. 2018. Discovery of isatin and 1H-indazol-3-ol derivatives as D-amino acid oxidase (DAAO) inhibitors. Bioorg Med Chem. 26(8):1579–1587. doi:https://doi.org/10.1016/j.bmc.2018.02.004.
- Takayama T, Ogawa T, Hidaka M, Shimizu Y, Ueda T, Masaki H. 2005. Esterification of Escherichia coli tRNAs with D-histidine and D-lysine by aminoacyl-tRNA synthetases. Biosci Biotechnol Biochem. 69(5):1040–1041. doi:https://doi.org/10.1271/bbb.69.1040.
- Ten Have GA, Engelen MP, Luiking YC, Deutz NE. 2007. Absorption kinetics of amino acids, peptides, and intact proteins. Int J Sport Nutr Exerc Metab. 17:S23–S36. https://pdfs.semanticscholar.org/8344/b4e504ebd0c21a97a495560c6c58dcac795a.pdf.
- Timofeeva NM, Khavinson VKh, Malinin VV, Nikitina AA, Egorova VV. 2005. Effect of peptide Livagen on activity of digestive enzymes in gastrointestinal tract and non-digestive organs in rats of different ages. Adv Gerontol. 16:92–96. https://www.ncbi.nlm.nih.gov/pubmed/16075683 (in Russian).
- Torbeev VY, Raghuraman H, Hamelberg D, Tonelli M, Westler WM, Perozo E, Kent SB. 2011. Protein conformational dynamics in the mechanism of HIV-1 protease catalysis. PNAS. 108(52):20982–20987. doi:https://doi.org/10.1073/pnas.1111202108.
- Torres AM, Menz I, Alewood PF, Bansal P, Lahnstein J, Gallagher CH, Kuchel PW. 2002. D-Amino acid residue in the C-type natriuretic peptide from the venom of the mammal, Ornithorhynchus anatinus, the Australian platypus. FEBS Lett. 524(1–3):172–176. https://doi.org/https://doi.org/10.1016/S0014-5793(02)03050-8.
- Torres AM, Tsampazi M, Kennett EC, Belov K, Geraghty DP, Bansal PS, Alewood PF, Kuchel PW. 2007. Characterization and isolation of L-to-D-amino-acid-residue isomerase from platypus venom. Amino Acids. 32(1):63–68. doi:https://doi.org/10.1007/s00726-006-0346-6.
- Tutel’yan VA, Khavinson V, Malinin VV. 2003. Physiological role of short peptides in nutrition. Bull Exp Biol Med. 135(1):1–5. https://doi.org/https://doi.org/10.1023/A:1023467622252.
- Tverdislov VA, Yakovenko LV. 2008. Physical aspects of the emergence of living cell precursors: the ion and chiral asymmetries as two fundamental asymmetry types. Mosc Univ Phys Bull. 63:151–163. https://doi.org/https://doi.org/10.3103/S0027134908030016.
- Urakami T, Sakai K, Asai T, Fukumoto D, Tsukada H, Oku N. 2009. Evaluation of O-[18F]fluoromethyl-d-tyrosine as a radiotracer for tumor imaging with positron emission tomography. Nucl Med Biol. 36(3):295–303. doi:https://doi.org/10.1016/j.nucmedbio.2008.12.012.
- Volkmann RA, Heck SD. 1998. Biosynthesis of D-amino acid-containing peptides: exploring the role of peptide isomerases. EXS. 85:87–105. https://www.ncbi.nlm.nih.gov/pubmed/9949870.
- Wang G. 2012. Natural antimicrobial peptides as promising anti-HIV candidates. Curr Top Pept Protein Res. 13:93–110. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4730921/pdf/nihms727000.pdf.
- Whittington CM, Koh JM, Warren WC, Papenfuss AT, Torres AM, Kuchel PW, Belov K. 2009. Understanding and utilising mammalian venom via a platypus venom transcriptome. J Proteomics. 72(2):155–164. doi:https://doi.org/10.1016/j.jprot.2008.12.004.
- Wolosker H. 2007. NMDA receptor regulation by D-serine: New findings and perspectives. Mol Neurobiol. 36(2): 152–164. doi:https://doi.org/10.1007/s12035-007-0038-6.
- Wolosker H, Dumin E, Balan L, Foltyn VN. 2008. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 275(14):3514–3526. doi:https://doi.org/10.1111/j.1742-4658.2008.06515.x.
- Yokoyama T, Kan-no N, Ogata T, Kotaki Y, Sato M, Nagahisa E. 2003. Presence of free D-amino acids in microalgae. Biosci Biotechnol Biochem. 67(2):388–392. https://doi.org/https://doi.org/10.1271/bbb.67.388.
- Yu P, Hegeman AD, Cohen JD. 2014. A facile means for the identification of indolic compounds from plant tissues. Plant J. 79(6):1065–1075. doi:https://doi.org/10.1111/tpj.12607.