Abstract
Positive transcription factor b (P-TEFb) has emerged as a general and essential factor to release RNA polymerase II from promoter-proximal pausing. Recruitment of P-TEFb by various master developmental regulators seems to be a common theme to allow for coordinated gene expression. The biological outcome is highly dependent on the cellular context as well as the nature of the transcription factor that recruits P-TEFb. Understanding the physiological functions of P-TEFb and its regulators will certainly have implications in human diseases such as cancer.
KEYWORDS:
1. Introduction
Precise and coordinated gene expression lies at the heart of many biological processes such as organismal development and stress responses to harmful environmental stimuli (Little et al. Citation2013; Pani and Nudler Citation2017). Like unicellular organisms, multicellular organisms control the expression of many protein-coding genes at the step of initiation, the process by which RNA polymerase II (RNAP II) assembles at the promoter with the aid of general as well as various tissue-specific transcription factors. Unwanted gene expression is shut down without a RNAP II bound to the promoter (Goodrich and Tjian Citation2010). In addition to initiation, recent studies have demonstrated that transcription elongation is also a key regulatory step in multicellular organisms including humans, that is, RNAP II is paused at the promoter-proximal region without generating full-length mRNA (Guo and Price Citation2013; Scheidegger and Nechaev Citation2016). At a glance, it seems to be energetically unfavorable to assemble RNAP II at the promoter without further transcription. Although the logic behind this type of control is not entirely clear, current evidence strongly suggests that preassembly of RNAP II at the promoter may be the molecular underpinnings of highly dynamic yet precise regulation of gene expression during animal development (Smith and Shilatifard Citation2013). The sites of promoter-proximal pausing are co-occupied by RNAP II and negative elongation factors, DRB Sensitivity Inducing Factor (DSIF) as well as Negative Elongation Factor (NELF) (Rahl et al. Citation2010). And recent studies have shown that promoter-proximal pausing is primarily for 5′ capping, partially facilitated by interaction between DSIF and capping enzyme (Wen and Shatkin Citation1999; Mandal et al. Citation2004). Multicellular organisms may also have evolved to exploit transcription elongation control to allow for rapid and efficient induction of gene expression to sense environmental stimuli such as mounting immunological responses to fight infections (Freaney et al. Citation2013).
Cellular factors that regulate transcription elongation of RNAP II have been identified (Guo and Price Citation2013; Smith and Shilatifard Citation2013; Scheidegger and Nechaev Citation2016). One of the key factors central to this control is Positive Elongation Factor b (P-TEFb). P-TEFb, composed of cyclin-dependent kinase 9 (CDK9) and cyclin T, releases paused RNAP II into productive elongation via phosphorylation of the carboxyl-terminal domain (CTD) of the largest subunit of RNAP II, DSIF as well as NELF (Bartholomeeusen et al. Citation2012). In cells, the kinase activity of P-TEFb is tightly regulated by reversible association with the 7SK small nuclear ribonucleoprotein complex (snRNP). Dysregulation of P-TEFb activity has implications in human diseases, exemplified by HIV-1 transcription in which viral protein Tat usurps cellular P-TEFb normally sequestered by 7SK snRNP to active transcription from the HIV promoter (Peterlin and Price Citation2006). In this review, we summarize findings in recent literature on roles of P-TEFb and its regulators in animal development. Understanding the physiological functions of P-TEFb and its regulators will shed light on etiology of human diseases such as cancer because the developmental process and tumorigenesis are inextricably linked at the molecular level. For those who are interested in the biochemical aspects of P-TEFb and its roles in human diseases, please refer to other articles in this issue as well as recent excellent review articles on these topics (Guo and Price Citation2013; Smith and Shilatifard Citation2013; Yu et al. Citation2015; Franco et al. Citation2018).
2. Regulation of P-TEFb: small and large forms of P-TEFb
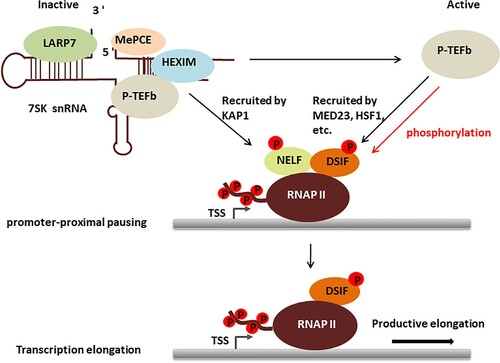
In mammalian cells, most cellular P-TEFb is sequestered by the 7SK snRNP, composed of 7SK small nuclear RNA (snRNA) (Yang et al. Citation2001) and at least three protein components including hexamethylene bis-acetamide inducible 1 or 2 (HEXIM1 or HEXIM2) (Yik et al. Citation2003), methylphosphate capping enzyme (MEPCE) (Jeronimo et al. Citation2007) and La ribonucleoprotein domain family, member 7 (LARP7) (He et al. Citation2008; Krueger et al. Citation2008). In this state, termed large form, the kinase activity of P-TEFb is inhibited. Biochemical studies demonstrate that 7SK snRNA and HEXIM proteins together are sufficient to inhibit P-TEFb kinase activity in vitro (Li et al. Citation2005). LARP7 directly binds to 7SK snRNA and appears to maintain its steady level in cells while not required to inhibit P-TEFb in vitro (He et al. Citation2008; Krueger et al. Citation2008). MEPCE modifies the 5′ end of 7SK snRNA and is also not required to inhibit P-TEFb in vitro (Jeronimo et al. Citation2007). A small portion of cellular P-TEFb is free of 7SK snRNP. In this state, termed small form, P-TEFb can be recruited by various factors to the promoter region to release paused RNAP II (Peterlin and Price Citation2006).
Stress signals such as ultraviolet (UV) radiation and conditions inducing hypertrophy in cardiomyocytes rapidly release P-TEFb from 7SK snRNP (Sano et al. Citation2002). The large form of P-TEFb reforms once stress signals are removed. P-TEFb is also freed from 7SK snRNP when cells are treated with transcription inhibitors such as flavopiridol and actinomycin D (Biglione et al. Citation2007). Presumably it is a cellular mechanism to compensate for decreased transcriptional activity. Interestingly, inhibitors of histone deacetylases (HDACis) and bromodomain and extra terminal domain (BETis) can also release P-TEFb from 7SK snRNP, followed by increased synthesis of HEXIM1, reassembly of the 7SK snRNP, and ultimately leading to P-TEFb inhibition and subsequently reduced cell growth and differentiation (Bartholomeeusen et al. Citation2012; Bartholomeeusen et al. Citation2013). P-TEFb freed from 7SK snRNP may be involved in cellular processes other than releasing paused RNAP II. These studies have shown that P-TEFb activity is important to mount full DNA damage response (Zhang et al. Citation2013; Nepomuceno et al. Citation2017). It will be of interest in future to identify other cellular targets and pathways regulated by P-TEFb to fully understand the physiological functions of the inactive, large form of P-TEFb.
The cellular mechanisms controlling assembly and disassembly of the large form of P-TEFb are not well understood. Interestingly, more than 80% of HEXIM1 proteins are not in complex with 7SK snRNA or P-TEFb (Li et al. Citation2005, Citation2007). The amount of 7SK snRNP in cells is likely more than P-TEFb (∼2 × 105 7SK molecules per HeLa cell) (Wassarman and Steitz Citation1991). Yet not all P-TEFb are in complex with 7SK snRNP or HEXIM1, indicating that there may be unidentified regulatory factors that control the interaction between P-TEFb and 7SK snRNP. This also indicates that HEXIM proteins may have functions other than controlling P-TEFb. Indeed recent studies have shown that HEXIM1 is required for the innate immune response to detect foreign DNA (Morchikh et al. Citation2017), and regulates leptin function involved in maintaining whole-body energy balance (Dhar-Mascareno et al. Citation2016). Furthermore, HEXIM1 can act as a sensor to nucleotide stress and function as a tumour suppressor in melanoma and prostate cancer respectively (Yeh et al. Citation2014; Tan et al. Citation2016).
Recent genome sequencing studies have identified mutations in LARP7 to be associated with various solid tumors: https://cancer.sanger.ac.uk/cosmic. Specifically, several loss-of-function mutations (frameshift and nonsense mutations) are predicted to generate C-terminal truncated LARP7 proteins. As the C-terminus of LARP7 is required to assemble 7SK snRNP, the P-TEFb activity in these cells are likely to be higher than that in normal cells. Sequencing studies also identified a large portion of missense mutations in cancer cell, scattering all over LARP7 protein. Whether these missense mutations have functional outcomes, if any, awaits elucidation (Table ).
Table 1. Summary of P-TEFb regulators.
3. P-TEFb is required during early embryogenesis
P-TEFb is an essential factor during early embryogenesis in animals. Knockdown of CDK9 in C. elegans embryos arrests development at about 100 cells without signs of differentiation. Same phenotype is observed when the largest subunit of RNAP II is knocked down. Like humans, C. elegans genome encodes two cyclin T genes, homologous to human cyclin T1 and cyclin T2 (Peng et al. Citation1998). Knockdown of each cyclin T individually does not impair embryogenesis or further development. However, combined knockdown of both cyclin T genes leads to highly similar phenotypes as inhibition of CDK9 (Shim et al. Citation2002). Knockdown of P-TEFb also causes diminished Ser-2 phosphorylation of RNAP II CTD. Thus P-TEFb as well as its kinase activity is required for C. elegans development (Shim et al. Citation2002). In Drosophila, CDK9 depletion disrupts embryo development, and results in a variety of patterning defects in the wing and notum (Chopra et al. Citation2009). On the other hand, knockdown of HEXIM1 is embryonic lethal and exhibits classical wing as well as leg defects. (Nguyen et al. Citation2012). These results indicate that P-TEFb activity must be tightly regulated for proper metazoan development.
Genetic ablation of P-TEFb subunits in mice also reveals its essential role in early embryogenesis. Cyclin T2 −/− embryos cease to develop likely before the 4-cell stage (Kohoutek et al. Citation2009). Somewhat surprisingly, cyclin T1–/– mice develop normally except for minor immunological defects (Oven et al. Citation2007). This could indicate that cyclin T2 may functionally replace cyclin T1. Alternatively, leaky expression of cyclin T1 observed in knockout mice may be sufficient for development (Oven et al. Citation2007). Cyclin K −/− embryos dies before the blastocyst stage (Blazek et al. Citation2011). Although recombinant cyclin K interacts with CDK9 in vitro (Fu et al. Citation1999), recent studies have shown that cyclin K interacts with CDK12 and CDK13 instead of CDK9 in cells (Blazek et al. Citation2011). Lethal phenotypes observed in cyclin T2 −/− embryos predict that inhibition of P-TEFb will lead to developmental arrest before 4-cell stage. Indeed, pharmaceutical inhibition of P-TEFb by flavopiridol results in developmental arrest at the two-cell stage in mice (Oqani et al. Citation2016). Collectively, these results demonstrate that P-TEFb is required for normal development at the earliest stage.
In contrast, regulators of P-TEFb such as LARP7 and HEXIM proteins do not seem to be important for early development. Surprisingly germline loss-of-function mutations in LARP7 lead to primordial dwarfism and microcephaly in humans (Najmabadi et al. Citation2011). Consistently, Larp7 −/− mice embryos are also smaller than their wild-type counterparts (Okamura et al. Citation2012). It is quite paradoxical because loss of LARP7 releases P-TEFb from the large form, and is expected to increase P-TEFb activity as well as cell proliferation, while primordial dwarfism is a condition characterized by global growth failure throughout life span (Klingseisen and Jackson Citation2011). Several explanations could potentially account for this paradoxical observation. When LARP7 is knocked down, there is a significant concomitant decrease in P-TEFb protein level (He et al. Citation2008; Krueger et al. Citation2008; Dai et al. Citation2014). This likely will offset the otherwise increased activity. Indeed, transcription from HIV-1 LTR, a promoter sensitive to P-TEFb activity, is only slightly upregulated after LARP7 knockdown (Krueger et al. Citation2008). Thus, loss of LARP7 may not significantly increase cellular P-TEFb activity due to a significant concomitant decrease in P-TEFb protein level. It is also possible that P-TEFb regulation may not be the main function of LARP7 as LARP7 interacts with other proteins (Jeronimo et al. Citation2007; Krueger et al. Citation2008). Finally, ectopic differentiation caused by LARP7 null-mutations may explain the etiology for primordial dwarfism. However, increased P-TEFb may not be involved. Instead, loss of LARP7 reduces LIN28 expression independent of P-TEFb, which in turn seem to cause untimely differentiation (Dai et al. Citation2014). No Larp7 knockout adult mice are available yet simply because the newborn might be eaten (Okamura et al. Citation2012).
Similarly, genetic ablation of HEXIM1 in mice does not result in overt cell overproliferation except in hearts (Huang et al. Citation2004). This could be because HEXIM2 can compensate for HEXIM1 loss in most organs (Byers et al. Citation2005). It is not clear why HEXIM2 fails to rescue HEXIM1 deficiency specifically in hearts. Alternatively, this may also indicate that HEXIM1 has P-TEFb-independent functions in hearts as most HEXIM1 are not in complex with P-TEFb (Oakley et al. Citation2013). Huang et al. have reported that defect of HEXIM1 not only lead to cardiac hypertrophy but also even be lethal in late fetal stages (Huang et al. Citation2004). Cardiac hypertrophy-like conditions may not fully explain this lethal phenotype because animals can tolerate hypertrophic conditions for an extended time (Maillet et al. Citation2013). It will be of interest to try to generate HEXIM1 −/− animals in future to fully understand the physiological functions of this protein.
Embryonic stem cells (ESCs) are self-renewing, pluripotent cells derived from the inner cell mass of blastocysts. Under optimal conditions, ESCs can be propagated in culture indefinitely (Martello and Smith Citation2014). Like germ cells, ESCs need to suppress expression of all somatic programs (Young Citation2011). Recent studies have shown that ESCs cultured in serum can exist in two interchangeable states (Weinberger et al. Citation2016). One state, termed naïve state, resembles the inner cell mass. The other more differentiated state, termed the primed state, resembles developmentally more advanced epiblast. Self-renewal and pluripotency in both states are maintained by the same set of core pluripotency transcription factors while key differences exist. Of note, ESCs in primed state express detectable lineage-affiliated transcripts (Lee et al. Citation2014). It is a common observation that ESCs cultured in serum exhibit spontaneous differentiation, likely because those in the primed state are prone to differentiation.
Early studies have shown that many lineage-affiliated genes have paused RNAP II around their promoters, and propose regulation of transcription elongation is an important mechanism to maintain pluripotency (Lin et al. Citation2011). These studies are carried out with ESCs cultured in serum, in which naïve and primed states of ESCs coexist (Ying et al. Citation2008). Interestingly promoter pausing is more prevalent in the culture condition termed 2i, which maintains ESCs in naïve state. Precocious transcription of lineage-affiliated genes in naïve state is restrained by RNA polymerase II promoter-proximal pausing. In mouse ESCs, 7SK snRNA may function as a multifaceted regulator to repress the expression of lineage-affiliated genes in a manner both dependent and independent of P-TEFb (Castelo-Branco et al. Citation2013). These observations strongly suggest that P-TEFb activity is tightly controlled in naïve state, and in the primed state unknown factors recruit P-TEFb to promoters of lineage-affiliated genes. Little is known about the functions of HEXIM1 and LARP7 in ESCs. However, knockout studies show that both are not essential for early embryogenesis in mice (Huang et al. Citation2004; Okamura et al. Citation2012).
In summary, P-TEFb is required for animal development at the earliest stage, consistent with its function as an essential, general factor for transcription elongation. In contrast, the physiological functions of P-TEFb regulators are less clear. Current data suggest that regulation of P-TEFb may not be essential for embryonic development, and these regulators may have P-TEFb independent functions.
4. Repression of P-TEFb in germ cell development
In contrast to its essential role in somatic cells during early embryogenesis, P-TEFb activity seems to be inhibited in primordial germ cells (PGCs). It is a daunting task for germ cells to keep their pluripotent potentials and at the same time not to activate any somatic genetic programs. Precocious activation of any specific cell lineages in germ cells would reduce the number of germ cells and therefore compromise reproduction. Remarkably a global inhibition of Ser2 phosphorylation of RNAP II CTD seems to be a common thread in different species to suppress somatic programs and keep their germ cell identify (Robert et al. Citation2015). Interestingly P-TEFb activity is interfered although underlying molecular mechanisms are different. In C. elegance, PIE-1 protein is critical for germline specification (Mello et al. Citation1996). Part of PIE-1 function is carried out by binding and inhibiting P-TEFb through its CTD-mimic motif YAPMAPT (Ghosh and Seydoux Citation2008). Loss of CDK9 from germ cells has little effect on Ser2 phosphorylation. It seems that CDK12 is mainly responsible for the low but detectable Ser2 phosphorylation in germ cells (Bowman et al. Citation2013). Consistently knockdown of CDK9 does not compromise germ cell specification by PIE-1 during embryogenesis, in contrast to cease of proliferation in somatic cells (Shim et al. Citation2002). In flies, the polar granule component (Pgc) binds P-TEFb and sequesters it away from the chromatin. However, Pgc does not inhibit the kinase activity of P-TEFb. Loss of Pgc increases Ser2 phosphorylation in germ cells and leads to germ cell degeneration. Ectopic expression of Pgc in somatic cells is sufficient to suppress Ser2 phosphorylation in these cells (Hanyu-Nakamura et al. Citation2008). Lastly, a study identified posterior end mark (PEM), a key protein in the germline of sea pineapple Halocynthia roretzi, interacts with P-TEFb to suppress Ser2 phosphorylation globally (Kumano et al. Citation2011). Low level of Ser2 phosphorylation is also observed in primordial germ cells in mice (Seki et al. Citation2007). However, it is currently unknown whether there is a specific P-TEFb inhibitor in mammalian germ cells. Remarkably, PIE-1, Pgc and PEM do not share any detectable sequence similarities, and therefore must have evolved independently. Yet they converge at interference with P-TEFb functions in diverse species.
5. Roles of P-TEFb and its regulators in heart, skeletal muscle, and blood cells
In the context of cardiomyocytes, regulation of P-TEFb is tightly linked to cellular growth. Various signals inducing hypertrophic growth converge at releasing P-TEFb from the large form. Constitutive overexpression of cyclin T in hearts also induces cardiac hypertrophy (Sano et al. Citation2002). In another transgenic mouse model in which cardiac hypertrophy condition is induced by overexpression of calcineurin, dissociation of HEXIM1 from P-TEFb is observed. Conversely genetic ablation of HEXIM1 leads to cardiac hypertrophy-like conditions, and further enhances susceptibility to hypertrophy induced by constitutive overexpression of cyclin T in transgenic mice (Espinoza-Derout et al. Citation2009). And Huang et al. have demonstrated that defect of HEXIM1 also led to be lethal in late fetal stages (Huang et al. Citation2004). Finally, overexpression of HEXIM1 prevents endothelin-1-induced cardiac hypertrophy (Yoshikawa et al. Citation2012). It is intriguing that in other cellular contexts HEXIM2 can replace HEXIM1 to repress P-TEFb, while in hearts it fails to do so (Espinoza-Derout et al. Citation2007). Another unsolved issue is that in other cell types, CDK9 protein level is reduced if P-TEFb is released from the large complex for an extended period. It does not seem to be the case in hearts. Lastly, it is not known how increased P-TEFb activity induces cellular growth in hearts. P-TEFb does not bind DNA or RNA by itself. It is a recurring theme that P-TEFb needs to be recruited by transcription factors and chromatin remodeling protein such as KAP1 and MED23 (Wang et al. Citation2013; Di Micco et al. Citation2014; McNamara et al. Citation2016) (Figure ).
In a closely related cellular context, the regulation of P-TEFb is shown to be important for skeletal muscle biology. SMYD3, a histone methyltransferase, mediates dexamethasone-induced skeletal muscle atrophy in mice via activation of myostatin and c-Met, potent inhibitors of muscle cell growth. Mechanistically SMYD3 recruits BRD4 and subsequently P-TEFb to release paused RNAP II from the promoters of myostain and c-Met. JQ1, a small molecule inhibitor of BRD4 (Filippakopoulos et al. Citation2010), or deletion of SMYD3 is sufficient to ameliorate dexamethasone-induced myotube atrophy (Proserpio et al. Citation2013). Conversely, activation of P-TEFb from the large form seems to be required to maintain muscle homeostasis after injury. Upon injuries, quiescent muscle stem cells, termed satellite cells, are activated to proliferate in order to regenerate muscle mass (Dumont et al. Citation2015). Overexpression of HEXIM1 seems to reduce the rate of satellite cell expansion, and conversely genetic ablation of HEXIM1 increases the proliferation of satellite cells. These effects seem to be mediated by the regulation of P-TEFb (Hong et al. Citation2012). It is not clear whether HEXIM2 is expressed in satellite cells, and if it does, why it fails to rescue the defect of HEXIM1 in this cellular context. Cyclin T2 is the major isoform expressed in muscle cells (Marchesi et al. Citation2013). Interestingly, MyoD, the master regulator of skeletal muscles (Pownall et al. Citation2002), directly interacts with cyclin T2, and recruits P-TEFb to activate muscle-specific genes (Galatioto et al. Citation2010). In addition to stimulate transcription elongation, P-TEFb directly phosphorylates histone H1 and dissociates it from myogenin gene promoter regions during differentiation (O'Brien et al. Citation2012). Thus, the activity of P-TEFb needs to be tightly controlled to maintain skeletal muscle homeostasis.
Several master regulators of hematopoiesis seem to recruit P-TEFb to regulate their respective genetic programs. GATA1 is important for the specification and maintenance of erythroid and megakaryocytic cells (Kaneko et al. Citation2010). Mechanistically, GATA1 collaborates with other regulators such as Ikaros and Ldb1 to recruit P-TEFb (Song et al. Citation2010; Bottardi et al. Citation2011). Another regulator of erythroid cell development, TIF1gamma, also interacts with P-TEFb to promote the transcription elongation of erythroid genes (Bai et al. Citation2013). In human T cells, transcription of immediate early genes requires rapid assembly of p300 and RNAP II at their promoters. p300 further recruits BRD4 and P-TEFb to stimulate transcription elongation. BRD4 and P-TEFb leaves the promoter once the stimulation signal is removed, while p300 and RNAP II remain bound at the promoter-proximal region. These bookmarked genes by p300 and RNAP II can be readily reactivated by other unrelated stimuli later on (Byun et al. Citation2009). Defects in master regulators of hematopoiesis frequently lead to human disorders such as leukemia (Shimizu and Yamamoto Citation2012). Of note, Table provides P-TEFb inhibitors that have shown promising effects on leukemia and various neoplasm in clinical trials (Guha Citation2013; Boffo et al. Citation2018).
Table 2. CDK9 inhibitors in clinical trials.
6. Concluding remarks
Research in the last decade has firmly established P-TEFb as an essential factor in RNAP II elongation regulation. Its activity is mainly regulated by dynamic association with 7SK snRNP. Recent studies begin to reveal the roles of P-TEFb and its regulators under physiological and diseased conditions. A common theme has emerged from these studies, that is, P-TEFb is recruited by a variety of master developmental regulators, mainly transcription and epigenetic factors to control genetic programs. This highlights the importance of P-TEFb in development because transcription factors function together with epigenetic factors to determine cell identity. Activation or repression of gene transcription depends on the nature of the protein that recruits P-TEFb. Thus, it is important to address the specific cellular context in order to understand the physiological role of P-TEFb. Regulators of P-TEFb such as HEXIM1 and LARP7 also have P-TEFb-independent functions, which are less understood. Despite the initial discovery that P-TEFb is sequestered by the 7SK RNP almost two decades ago, it is still unclear how the dynamic association of P-TEFb with 7SK RNP is regulated in cells. Cellular factors that regulate dissociation and reassembly of 7SK RNP-P-TEFb complex remain elusive. Unbiased omics approaches may provide the solution to this question. The developmental process and human disease progression are intertwined at many levels. Understanding the physiological functions of P-TEFb and its regulators will certainly shed light on etiology of disorders such as cancer and cardiac hypertrophy and facilitate the discovery of novel therapeutic strategies.
Acknowledgment
This work was supported by West China Second University Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arter J, Wegner M. 2015. Transcription factors Sox10 and Sox2 functionally interact with positive transcription elongation factor b in Schwann cells. J Neurochem. 132(4):384–393.
- Bai X, Trowbridge JJ, Riley E, Lee JA, DiBiase A, Kaartinen VM, Orkin SH, Zon LI. 2013. TiF1-gamma plays an essential role in murine hematopoiesis and regulates transcriptional elongation of erythroid genes. Dev Biol. 373(2):422–430.
- Bartholomeeusen K, Fujinaga K, Xiang Y, Peterlin BM. 2013. Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription. J Biol Chem. 288(20):14400–14407.
- Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM. 2012. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem. 287(43):36609–36616.
- Biglione S, Byers SA, Price JP, Nguyen VT, Bensaude O, Price DH, Maury W. 2007. Inhibition of HIV-1 replication by P-TEFb inhibitors DRB, seliciclib and flavopiridol correlates with release of free P-TEFb from the large, inactive form of the complex. Retrovirology. 4:47.
- Blazek D, Kohoutek J, Bartholomeeusen K, Johansen E, Hulinkova P, Luo Z, Cimermancic P, Ule J, Peterlin BM. 2011. The Cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev. 25(20):2158–2172.
- Boffo S, Damato A, Alfano L, Giordano A. 2018. CDK9 inhibitors in acute myeloid leukemia. J Exp Clin Cancer Res. 37(1):36.
- Bottardi SA, Mavoungou L, Pak H, Daou S, Bourgoin V, Lakehal Y, Affar El B, Milot E. 2014. The IKAROS interaction with a complex including chromatin remodeling and transcription elongation activities is required for hematopoiesis. PLoS Genet. 10(12).
- Bottardi S, Zmiri FA, Bourgoin V, Ross J, Mavoungou L, Milot E. 2011. Ikaros interacts with P-TEFb and cooperates with GATA-1 to enhance transcription elongation. Nucleic Acids Res. 39(9):3505–3519.
- Bowman EA, Bowman CR, Ahn JH, Kelly WG. 2013. Phosphorylation of RNA polymerase II is independent of P-TEFb in the C. elegans germline. Development. 140(17):3703–3713.
- Byers SA, Price JP, Cooper JJ, Li Q, Price DH. 2005. HEXIM2, a HEXIM1-related protein, regulates positive transcription elongation factor b through association with 7SK. J Biol Chem. 280(16):16360–16367.
- Byun JS, Wong MM, Cui W, Idelman G, Li Q, De Siervi A, Bilke S, Haggerty CM, Player A, Wang YH, et al. 2009. Dynamic bookmarking of primary response genes by p300 and RNA polymerase II complexes. Proc Natl Acad Sci U S A. 106(46):19286–19291.
- Castelo-Branco G, Amaral PP, Engstrom PG, Robson SC, Marques SC, Bertone P, Kouzarides T. 2013. The non-coding snRNA 7SK controls transcriptional termination, poising, and bidirectionality in embryonic stem cells. Genome Biol. 14(9):R98.
- Chopra VS, Hong JW, Levine M. 2009. Regulation of Hox gene activity by transcriptional elongation in Drosophila. Curr Biol. 19(8):688–693.
- Dai Q, Luan G, Deng L, Lei T, Kang H, Song X, Zhang Y, Xiao ZX, Li Q. 2014. Primordial dwarfism gene maintains Lin28 expression to safeguard embryonic stem cells from premature differentiation. Cell Rep. 7(3):735–746.
- Dhar-Mascareno M, Ramirez SN, Rozenberg I, Rouille Y, Kral JG, Mascareno EJ. 2016. Hexim1, a novel regulator of leptin function, modulates obesity and glucose disposal. Mol Endocrinol. 30(3):314–324.
- Di Micco R, Fontanals-Cirera B, Low V, Ntziachristos P, Yuen SK, Lovell CD, Dolgalev I, Yonekubo Y, Zhang G, Rusinova E, et al. 2014. Control of embryonic stem cell identity by BRD4-dependent transcriptional elongation of super-enhancer-associated pluripotency genes. Cell Rep. 9(1):234–247.
- Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. 2015. Satellite cells and skeletal muscle regeneration. Compr Physiol. 5(3):1027–1059.
- Ebmeier CCLARD, Erickson B, Allen B, Allen M, Kim H, Fong N, Jacobsen J, Liang K, Shilatifard A, Dowell R. 2017. Human TFIIH Kinase CDK7 Regulates Transcription-Associated Chromatin Modifications. Cell Rep. 20(5):1173–1186.
- Espinoza-Derout J, Wagner M, Salciccioli L, Lazar JM, Bhaduri S, Mascareno E, Chaqour B, Siddiqui MA. 2009. Positive transcription elongation factor b activity in compensatory myocardial hypertrophy is regulated by cardiac lineage protein-1. Circ Res. 104(12):1347–1354.
- Espinoza-Derout J, Wagner M, Shahmiri K, Mascareno E, Chaqour B, Siddiqui MA. 2007. Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy. Cardiovasc Res. 75(1):129–138.
- Faust TBWMWJD, Li Y, Bacon C, Jang G, Weiss A, Jayaraman B, Newton B, Krogan N, 'orso D, Frankel I, A. 2018. The HIV-1 Tat protein recruits a ubiquitin ligase to reorganize the 7SK snRNP for transcriptional activation.
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. 2010. Selective inhibition of BET bromodomains. Nature. 468(7327):1067–1073.
- Franco LC, Morales F, Boffo S, Giordano A. 2018. CDK9: a key player in cancer and other diseases. J Cell Biochem. 119(2):1273–1284.
- Freaney JE, Kim R, Mandhana R, Horvath CM. 2013. Extensive cooperation of immune master regulators IRF3 and NFkappaB in RNA Pol II recruitment and pause release in human innate antiviral transcription. Cell Rep. 4(5):959–973.
- Fu TJ, Peng J, Lee G, Price DH, Flores O. 1999. Cyclin K functions as a CDK9 regulatory subunit and participates in RNA polymerase II transcription. J Biol Chem. 274(49):34527–34530.
- Galatioto J, Mascareno E, Siddiqui MA. 2010. CLP-1 associates with MyoD and HDAC to restore skeletal muscle cell regeneration. J Cell Sci. 123(Pt 21):3789–3795.
- Galli GGCS, Carrara M, Yuan W, Valdes-Quezada C, Gurung B, Pepe-Mooney B, Zhang T, Geeven G, Gray N, De Laat W. 2015. YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers. Mol Cell. 60(2):328–337.
- Ghosh D, Seydoux G. 2008. Inhibition of transcription by the Caenorhabditis elegans germline protein PIE-1: genetic evidence for distinct mechanisms targeting initiation and elongation. Genetics. 178(1):235–243.
- Goodrich JA, Tjian R. 2010. Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat Rev Genet. 11(8):549–558.
- Guha M. 2013. Blockbuster dreams for Pfizer's CDK inhibitor. Nat Biotechnol. 31(3):187.
- Guo J, Price DH. 2013. RNA polymerase II transcription elongation control. Chem Rev. 113(11):8583–8603.
- Hanyu-Nakamura K, Sonobe-Nojima H, Tanigawa A, Lasko P, Nakamura A. 2008. Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature. 451(7179):730–733.
- Hashiguchi TJWY, Bruss N, Best S, Lam V, Danilova O, Paiva C, Wolf J, Gilbert E, Okada C, Kaur P. 2019. Cyclin-Dependent Kinase-9 Is a Therapeutic Target in MYC-Expressing Diffuse Large B-Cell Lymphoma. Molecular cancer therapeutics. 18(9):1520–1532.
- He N, Jahchan NS, Hong E, Li Q, Bayfield MA, Maraia RJ, Luo K, Zhou Q. 2008. A La-related protein modulates 7SK snRNP integrity to suppress P-TEFb-dependent transcriptional elongation and tumorigenesis. Mol Cell. 29(5):588–599.
- Hong P, Chen K, Huang B, Liu M, Cui M, Rozenberg I, Chaqour B, Pan X, Barton ER, Jiang XC, Siddiqui MA. 2012. HEXIM1 controls satellite cell expansion after injury to regulate skeletal muscle regeneration. J Clin Invest. 122(11):3873–3887.
- Huang F, Wagner M, Siddiqui MA. 2004. Ablation of the CLP-1 gene leads to down-regulation of the HAND1 gene and abnormality of the left ventricle of the heart and fetal death. Mech Dev. 121(6):559–572.
- Jang MKSN, Mochizuki K, Zhou M, Jeong H, Brady J, Ozato K. 2005. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 19(4):523–534.
- Jeronimo C, Forget D, Bouchard A, Li Q, Chua G, Poitras C, Therien C, Bergeron D, Bourassa S, Greenblatt J, et al. 2007. Systematic analysis of the protein interaction network for the human transcription machinery reveals the identity of the 7SK capping enzyme. Mol Cell. 27(2):262–274.
- Kaneko H, Shimizu R, Yamamoto M. 2010. GATA factor switching during erythroid differentiation. Curr Opin Hematol. 17(3):163–168.
- Klingseisen A, Jackson AP. 2011. Mechanisms and pathways of growth failure in primordial dwarfism. Genes Dev. 25(19):2011–2024.
- Kohoutek J, Li Q, Blazek D, Luo Z, Jiang H, Peterlin BM. 2009. Cyclin T2 is essential for mouse embryogenesis. Mol Cell Biol. 29(12):3280–3285.
- Krueger BJ, Jeronimo C, Roy BB, Bouchard A, Barrandon C, Byers SA, Searcey CE, Cooper JJ, Bensaude O, Cohen EA, et al. 2008. LARP7 is a stable component of the 7SK snRNP while P-TEFb, HEXIM1 and hnRNP A1 are reversibly associated. Nucleic Acids Res. 36(7):2219–2229.
- Kumano G, Takatori N, Negishi T, Takada T, Nishida H. 2011. A maternal factor unique to ascidians silences the germline via binding to P-TEFb and RNAP II regulation. Curr Biol. 21(15):1308–1313.
- Lee JH, Lee JB, Shapovalova Z, Fiebig-Comyn A, Mitchell RR, Laronde S, Szabo E, Benoit YD, Bhatia M. 2014. Somatic transcriptome priming gates lineage-specific differentiation potential of human-induced pluripotent stem cell states. Nat Commun. 5:5605.
- Li Q, Cooper JJ, Altwerger GH, Feldkamp MD, Shea MA, Price DH. 2007. HEXIM1 is a promiscuous double-stranded RNA-binding protein and interacts with RNAs in addition to 7SK in cultured cells. Nucleic Acids Res. 35(8):2503–2512.
- Li Q, Price JP, Byers SA, Cheng D, Peng J, Price DH. 2005. Analysis of the large inactive P-TEFb complex indicates that it contains one 7SK molecule, a dimer of HEXIM1 or HEXIM2, and two P-TEFb molecules containing Cdk9 phosphorylated at threonine 186. J Biol Chem. 280(31):28819–28826.
- Lin C, Garrett AS, De Kumar B, Smith ER, Gogol M, Seidel C, Krumlauf R, Shilatifard A. 2011. Dynamic transcriptional events in embryonic stem cells mediated by the super elongation complex (SEC). Genes Dev. 25(14):1486–1498.
- Lis JTH, Mason P, Peng J, Price D, Werner J. 2000. P-TEFb kinase recruitment and function at heat shock loci. Genes Dev. 14(7):792–803.
- Little SC, Tikhonov M, Gregor T. 2013. Precise developmental gene expression arises from globally stochastic transcriptional activity. Cell. 154(4):789–800.
- Liu X, Gao Y, Ye H, Gerrin S, Ma F, Wu Y, Zhang T, Russo J, Cai C, Yuan X. 2017. Positive feedback loop mediated by protein phosphatase 1alpha mobilization of P-TEFb and basal CDK1 drives androgen receptor in prostate cancer. Nucleic Acids Res. 45(7):3738–3751.
- Liu L, Xu Y, He M, Zhang M, Cui F, Lu L, Yao M, Tian W, Benda C, Zhuang Q. 2014. Transcriptional pause release is a rate-limiting step for somatic cell reprogramming. Cell Stem Cell. 15(5):574–588.
- Maillet M, van Berlo JH, Molkentin JD. 2013. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 14(1):38–48.
- Mandal SS, Chu C, Wada T, Handa H, Shatkin AJ, Reinberg D. 2004. Functional interactions of RNA-capping enzyme with factors that positively and negatively regulate promoter escape by RNA polymerase II. Proc Natl Acad Sci U S A. 101(20):7572–7577.
- Marchesi I, Nieddu V, Caracciolo V, Maioli M, Gaspa L, Giordano A, Bagella L. 2013. Activation and function of murine cyclin T2A and cyclin T2B during skeletal muscle differentiation. J Cell Biochem. 114(3):728–734.
- Martello G, Smith A. 2014. The nature of embryonic stem cells. Annu Rev Cell Dev Biol. 30:647–675.
- McNamara RP, Reeder JE, McMillan EA, Bacon CW, McCann JL, D'Orso I. 2016. KAP1 recruitment of the 7SK snRNP complex to promoters enables transcription elongation by RNA polymerase II. Mol Cell. 61(1):39–53.
- Mello CC, Schubert C, Draper B, Zhang W, Lobel R, Priess JR. 1996. The PIE-1 protein and germline specification in C. elegans embryos. Nature. 382(6593):710–712.
- Morchikh M, Cribier A, Raffel R, Amraoui S, Cau J, Severac D, Dubois E, Schwartz O, Bennasser Y, Benkirane M. 2017. HEXIM1 and NEAT1 long non-coding RNA form a multi-subunit complex that regulates DNA-mediated innate immune response. Mol Cell. 67(3):387–399.e5.
- Najmabadi H, Hu H, Garshasbi M, Zemojtel T, Abedini SS, Chen W, Hosseini M, Behjati F, Haas S, Jamali P, et al. 2011. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 478(7367):57–63.
- Nepomuceno TC, Fernandes VC, Gomes TT, Carvalho RS, Suarez-Kurtz G, Monteiro AN, Carvalho MA. 2017. BRCA1 recruitment to damaged DNA sites is dependent on CDK9. Cell Cycle. 16(7):665–672.
- Nguyen D, Krueger BJ, Sedore SC, Brogie JE, Rogers JT, Rajendra TK, Saunders A, Matera AG, Lis JT, Uguen P, Price DH. 2012. The Drosophila 7SK snRNP and the essential role of dHEXIM in development. Nucleic Acids Res. 40(12):5283–5297.
- Oakley RH, Ren R, Cruz-Topete D, Bird GS, Myers PH, Boyle MC, Schneider MD, Willis MS, Cidlowski JA. 2013. Essential role of stress hormone signaling in cardiomyocytes for the prevention of heart disease. Proc Natl Acad Sci U S A. 110(42):17035–17040.
- O'Brien SK, Knight KL, Rana TM. 2012. Phosphorylation of histone H1 by P-TEFb is a necessary step in skeletal muscle differentiation. J Cell Physiol. 227(1):383–389.
- Okamura D, Maeda I, Taniguchi H, Tokitake Y, Ikeda M, Ozato K, Mise N, Abe K, Noce T, Izpisua Belmonte JC, Matsui Y. 2012. Cell cycle gene-specific control of transcription has a critical role in proliferation of primordial germ cells. Genes Dev. 26(22):2477–2482.
- Oqani RK, Lin T, Lee JE, Kim SY, Sa SJ, Woo JS, Jin DI. 2016. Inhibition of P-TEFb disrupts global transcription, oocyte maturation, and embryo development in the mouse. Genesis. 54(9):470–482.
- Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM. 2007. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 27(24):8815–8823.
- Pani B, Nudler E. 2017. Mechanistic insights into transcription coupled DNA repair. DNA Repair (Amst). 56:42–50.
- Peng J, Zhu Y, Milton JT, Price DH. 1998. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 12(5):755–762.
- Peterlin BM, Price DH. 2006. Controlling the elongation phase of transcription with P-TEFb. Mol Cell. 23(3):297–305.
- Pownall ME, Gustafsson MK, Emerson, Jr CP. 2002. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 18:747–783.
- Proserpio V, Fittipaldi R, Ryall JG, Sartorelli V, Caretti G. 2013. The methyltransferase SMYD3 mediates the recruitment of transcriptional cofactors at the myostatin and c-Met genes and regulates skeletal muscle atrophy. Genes Dev. 27(11):1299–1312.
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. 2010. c-Myc regulates transcriptional pause release. Cell. 141(3):432–445.
- Robert VJ, Garvis S, Palladino F. 2015. Repression of somatic cell fate in the germline. Cell Mol Life Sci. 72(19):3599–3620.
- Sano M, Abdellatif M, Oh H, Xie M, Bagella L, Giordano A, Michael LH, DeMayo FJ, Schneider MD. 2002. Activation and function of cyclin T-Cdk9 (positive transcription elongation factor-b) in cardiac muscle-cell hypertrophy. Nat Med. 8(11):1310–1317.
- Scheidegger A, Nechaev S. 2016. RNA polymerase II pausing as a context-dependent reader of the genome. Biochem Cell Biol. 94(1):82–92.
- Scholz B, Kowarz E, Rossler T, Ahmad K, Steinhilber D, Marschalek R. 2015. AF4 and AF4N protein complexes: recruitment of P-TEFb kinase, their interactome and potential functions. Am J Blood Res. 5(1):10–24.
- Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, Saga Y, Tachibana M, Shinkai Y, Saitou M. 2007. Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development. 134(14):2627–2638.
- Shan JAS, Zhang F, Sharkey J, Tang T, Ord T, Kilberg M. 2016. The C/ebp-Atf response element (CARE) location reveals two distinct Atf4-dependent, elongation-mediated mechanisms for transcriptional induction of aminoacyl-tRNA synthetase genes in response to amino acid limitation. Nucleic Acids Res. 44:9719–9732.
- Shii LE, Song L, Maurer K, Zhang Z, Sullivan K. 2017. SERPINB2 is regulated by dynamic interactions with pause-release proteins and enhancer RNAs. Mol Immunol. 88:20–31.
- Shim EY, Walker AK, Shi Y, Blackwell TK. 2002. CDK-9/cyclin T (P-TEFb) is required in two postinitiation pathways for transcription in the C. elegans embryo. Genes Dev. 16(16):2135–2146.
- Shimizu R, Yamamoto M. 2012. Contribution of GATA1 dysfunction to multi-step leukemogenesis. Cancer Sci. 103(12):2039–2044.
- Smith E, Shilatifard A. 2013. Transcriptional elongation checkpoint control in development and disease. Genes Dev. 27(10):1079–1088.
- Song SH, Kim A, Ragoczy T, Bender MA, Groudine M, Dean A. 2010. Multiple functions of Ldb1 required for beta-globin activation during erythroid differentiation. Blood. 116(13):2356–2364.
- Tan JL, Fogley RD, Flynn RA, Ablain J, Yang S, Saint-Andre V, Fan ZP, Do BT, Laga AC, Fujinaga K, et al. 2016. Stress from nucleotide depletion activates the transcriptional regulator HEXIM1 to suppress melanoma. Mol Cell. 62(1):34–46.
- Wagner AHSH, Conzelmann M, Fitzer F, Giese T, Gulow K, Falk C, Kramer O, Dietrich S, Hecker M, Luft T. 2015. JAK1/STAT3 activation directly inhibits IL-12 production in dendritic cells by preventing CDK9/P-TEFb recruitment to the p35 promoter. Biochem Pharmacol. 96(1):52–64.
- Wang W, Yao X, Huang Y, Hu X, Liu R, Hou D, Chen R, Wang G. 2013. Mediator MED23 regulates basal transcription in vivo via an interaction with P-TEFb. Transcription. 4(1):39–51.
- Wassarman DA, Steitz JA. 1991. Structural analyses of the 7SK ribonucleoprotein (RNP), the most abundant human small RNP of unknown function. Mol Cell Biol. 11(7):3432–3445.
- Weinberger L, Ayyash M, Novershtern N, Hanna JH. 2016. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol. 17(3): 155–169.
- Wen Y, Shatkin AJ. 1999. Transcription elongation factor hSPT5 stimulates mRNA capping. Genes Dev. 13(14):1774–1779.
- Yang Z, Zhu Q, Luo K, Zhou Q. 2001. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature. 414(6861):317–322.
- Yeh IJ, Song K, Wittmann BM, Bai X, Danielpour D, Montano MM. 2014. HEXIM1 plays a critical role in the inhibition of the androgen receptor by anti-androgens. Biochem J. 462(2):315–327.
- Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q. 2003. Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell. 12(4):971–982.
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. 2008. The ground state of embryonic stem cell self-renewal. Nature. 453(7194):519–523.
- Yoshikawa N, Shimizu N, Maruyama T, Sano M, Matsuhashi T, Fukuda K, Kataoka M, Satoh T, Ojima H, Sawai T, et al. 2012. Cardiomyocyte-specific overexpression of HEXIM1 prevents right ventricular hypertrophy in hypoxia-induced pulmonary hypertension in mice. PLoS One. 7(12):e52522.
- Young RA. 2011. Control of the embryonic stem cell state. Cell. 144(6):940–954.
- Yu M, Yang W, Ni T, Tang Z, Nakadai T, Zhu J, Roeder RG. 2015. RNA polymerase II-associated factor 1 regulates the release and phosphorylation of paused RNA polymerase II. Science. 350(6266):1383–1386.
- Zhang H, Park SH, Pantazides BG, Karpiuk O, Warren MD, Hardy CW, Duong DM, Park SJ, Kim HS, Vassilopoulos A, et al. 2013. SIRT2 directs the replication stress response through CDK9 deacetylation. Proc Natl Acad Sci U S A. 110(33):13546–13551.