Abstract
Objective: To evaluate safety, tolerability, and preliminary activity of inotuzumab ozogamicin (InO) plus rituximab, gemcitabine, dexamethasone, and cisplatin (R-GDP) in patients with relapsed/refractory CD22+ B-cell non-Hodgkin lymphoma (NHL).
Methods: Patients received InO plus R-GDP (21-day cycle; six-cycle maximum) using up-and-down dose-escalation schema for gemcitabine and cisplatin to define the highest dosage regimen(s) with acceptable toxicity (Part 1; n = 27). Part 2 (n = 10) confirmed safety and tolerability; Part 3 (n = 18) evaluated preliminary efficacy.
Results: Among 55 patients enrolled, 42% were refractory at baseline (median 2 [range, 1–6] prior therapies); 38% had diffuse large B-cell lymphoma (DLBCL). The highest dosage regimen with acceptable toxicity was InO 0.8 mg/m2, rituximab 375 mg/m2, cisplatin 50 mg/m2, gemcitabine 500 mg/m2 (day 1 only) and dexamethasone 40 mg (days 1–4); this was confirmed in Part 2, in which three patients had dose-limiting toxicities (grade 4 thrombocytopenia [n = 2], febrile neutropenia [n = 2]). Most frequent treatment-related adverse events were thrombocytopenia (any grade, 85%; grade ≥3, 75%) and neutropenia (69%; 62%). Overall (objective) response rate (ORR) was 53% (11 complete, 18 partial responses); ORR was 71%, 33%, and 62% in patients with follicular lymphoma (n = 14), DLBCL (n = 21), and mantle cell lymphoma (n = 13), respectively.
Conclusions: InO 0.8 mg/m2 plus R-GDP was associated with manageable toxicity, although gemcitabine and cisplatin doses were lower than in the standard R-GDP regimen due to hematologic toxicity. Evidence of antitumor activity was observed; however, these exploratory data should be interpreted with caution due to the small sample size and short follow-up duration (Clinicaltrials.gov number: NCT01055496).
Introduction
Non-Hodgkin lymphomas (NHL) are a heterogeneous group of malignancies that typically express B-cell antigens, including CD22, which is an attractive therapeutic target for B-cell NHL because it is expressed by most (∼90%) aggressive and indolent lymphomas [Citation1,Citation2], and its expression is confined to B-cells [Citation3].
Inotuzumab ozogamicin (InO; CMC-544) is a humanized immunoglobulin G4 (IgG4) CD22 monoclonal antibody (mAb) conjugated to a derivative of calicheamicin, a potent cytotoxic antitumor antibiotic [Citation3–6]. InO has demonstrated manageable toxicity and preliminary antitumor activity in relapsed/refractory B-cell NHL, both as a single agent and combined with other treatments [Citation7–9]. The maximum tolerated dose (MTD) of single-agent InO was determined to be 1.8 mg/m2 when administered intravenously every 4 weeks [Citation7]. InO 1.8 mg/m2 combined with the CD20 mAb rituximab (R) has demonstrated preliminary activity and a safety profile similar to that reported for InO alone in patients with relapsed/refractory B-cell NHL [Citation9–11].
Preclinical evidence supports the use of InO combined with chemotherapeutics [Citation12]; in xenograft models, InO combined with gemcitabine (G) or cisplatin (P) has demonstrated greater antitumor activity versus each agent alone [Citation13]. This phase 1, three-part study explored the safety, tolerability, pharmacokinetics (PK), and preliminary antitumor activity of InO combined with R, G, dexamethasone (D), and P (R-GDP) in patients with relapsed/refractory CD22+ B-cell NHL.
Methods
Patients
Eligible patients were aged ≥18 years with a diagnosis of CD20+ and CD22+ B-cell NHL (CD22 positivity was assessed by the local laboratory as per standard of care). Patients were required to have had ≥1 prior anticancer treatment (including prior R and chemotherapy) and an Eastern Cooperative Oncology Group performance status ≤2; adequate bone marrow, hepatic, and renal function (absolute neutrophil count ≥1.0 × 109/L and platelet count ≥100 × 109/L; aspartate and alanine aminotransferase levels ≤2.5 × upper limit of normal [ULN]; total bilirubin ≤ ULN [unless patient has Gilbert’s disease]); serum creatinine ≤1.5 mg/dL and a urine protein to creatinine ratio of ≤0.5; and ≥1 measurable lesion (diameter >1 cm) with a product diameter of ≥2.25 cm2 by computed tomography or magnetic resonance imaging. Key exclusion criteria included >3 previous chemotherapy regimens consisting of ≥2 cytotoxic agents; treatment with CD22 antibodies, radioimmunotherapy, or autologous transplant within 6 months before the first dose; or chemotherapy, cancer immunosuppressive therapy, radiotherapy, growth factors other than erythropoietin, or investigational agents <28 days before the first dose.
The study was performed in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. The protocol was approved by the institutional review board and/or independent ethics committee at each participating center. Written informed consent was obtained from each patient before trial-specific activities.
Study design and treatment
This prospective, open-label, phase 1 study (Clinicaltrials.gov, NCT01055496) investigated the tolerability profile and the recommended phase 2 dose (RP2D) regimen of InO plus R-GDP in patients with relapsed/refractory CD22+ B-cell NHL. Additional objectives included characterizing the PK and determining the preliminary antitumor activity of InO plus R-GDP. Therefore this study was undertaken in three parts: Part 1, identification of the highest dose regimen(s) with acceptable toxicity (MTD); Part 2, confirmation of the RP2D regimen(s); Part 3, RP2D expansion.
Patients received R 375 mg/m2 intravenously (IV), G 500–1000 mg/m2 IV, and P 37.5–75 mg/m2 IV on day 1 plus oral D 40 mg on days 1–4, followed by InO 0.8 mg/m2 IV on day 2 of each 21-day cycle (±2 days). Treatment was continued for up to six cycles unless progressive disease (PD) or intolerable toxicity occurred.
Determination of recommended phase 2 dose regimen
The MTD regimen(s) was defined as the highest dose regimen(s) for which <33% of patients experienced a dose-limiting toxicity (DLT); because two agents were escalated, multiple regimens could have been identified. The MTD regimen(s) was determined using an adaptive up-and-down dose-escalation method, which allowed evaluation of up to 11 dose combinations (four levels of P and three levels of G, excluding 0,0; Supplementary Table I) [Citation14–16]. Doses of either G or P could be increased from cohort-to-cohort. Initially, InO and R concentrations were fixed; P and G dose levels were varied with the intention to escalate InO to 1.3 mg/m2 if the full G and P doses were tolerated.
The starting cohort of two patients received P 37.5 mg/m2 and G 500 mg/m2; the subsequent dose escalation method is described in detail in Supplementary Table II. Only DLTs occurring during the first cycle were evaluated; two patients had to complete cycle 1 before new dosing cohorts were considered. Only up to six patients could be enrolled per cohort, except for cohort 1 where ≤12 patients could be enrolled. The final dosing cohorts and treatment schedules are shown in Supplementary Table I.
Ten additional patients for each identified MTD regimen in Part 1 were enrolled in Part 2 to confirm safety, which required a DLT rate of <33% in cycle 1 and <33% of the patients discontinuing treatment before cycle 3 due to treatment-related toxicity. For each confirmed RP2D regimen, approximately 20 additional patients were to be enrolled in Part 3 to further assess the safety and preliminary antitumor activity of InO plus R-GDP.
Safety, antitumor activity, pharmacokinetic, and immunogenicity analysis
Adverse events (AEs) in the safety population (patients who received ≥1 dose of study drug) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0 and were monitored for 4–6 weeks after the last dose through and including the end-of-treatment visit.
Tumor response and progression status were evaluated objectively using a modification of the International Working Group Criteria [Citation17] using CT scans or MRI performed at screening, every 9 weeks during the active period (or earlier if there was evidence of tumor response), at the end-of-treatment visit, and every 12–24 weeks during long-term follow-up visits (conducted up to 1 year or until March 12, 2014 ± 7 days) until disease progression, new anti-cancer therapy or death, whichever occurred first.
For PK analyses, blood samples were collected in Parts 2 and 3 for the measurement of InO, total calicheamicin (conjugated plus unconjugated forms), and unconjugated calicheamicin serum concentrations. Analyzed samples were available during cycle 1 prior to dosing and cycle 3 at 0 hours (predose), 1 hour (immediately before end of infusion), 3, 24, and 144 hours. Analyses were performed using validated ELISA (limits of quantitation for PK assessments: InO, 52.2 ng/mL; total calicheamicin, 5 ng/mL; unconjugated calicheamicin, 1.25 ng/mL). Serum samples for the assessment of anti-InO and anti-R antibodies were collected 24 hours predose during cycles 1, 4, and at the end of treatment.
Statistical analysis
The primary aim of this study was to identify an InO plus R-GDP regimen with acceptable toxicity for further evaluation. An analysis was performed based on the 10 patients in the RP2D confirmation cohort(s). The decision to proceed to Part 3 was based on information available from the unlocked database at the time of this analysis, which required complete response (CR) or partial response (PR) in ≥2 patients, <33% of patients experiencing a DLT, and <33% unable to receive at least three cycles due to treatment-related toxicity. Outcomes reported are based on final data for all treated patients. The Kaplan–Meier method was used to summarize distributions of progression-free survival (PFS), overall survival (OS), and duration of response (DOR). Patients were followed for survival for up to 2 years from the date of first dose or until March 12, 2014, or death, whichever occurred first.
Results
Patients
Overall, 55 patients were enrolled (Part 1, n = 27; Part 2, n = 10; Part 3, n = 18). Most patients had diffuse large B-cell lymphoma (DLBCL; 38%), follicular lymphoma (FL; 25%), or mantle cell lymphoma (MCL; 24%), with a median of 2 prior therapies (range, 1–6) (). Twenty-three patients (42%) had refractory disease at baseline (i.e. best response of stable disease [SD] or PD during most recent prior anticancer therapy).
Table 1. Patient characteristics.
A median of four (range, 1–6) treatment cycles were completed, with a total of 21 (38%) patients completing all six cycles. The median follow-up time was 22.6 (range, 1.0–29.6) months. Overall, 34 (62%) patients discontinued treatment; 19 (35%) due to AEs, nine (16%) due to PD, two (4%) due to investigator-request, and three (5%) due to withdrawal of consent. One additional patient discontinued at cycle 4 because cycle 5 had to be delayed >28 days to allow platelet recovery to meet dosing criteria.
Recommended phase 2 dose determination and confirmation
No DLTs occurred at dose levels 1 and 3; DLTs occurred in two of three patients at dose level 2, and in two of four patients at dose level 5 (Supplementary Table III). Two of eight patients enrolled at dose level 4 were not evaluable for DLTs: one died from PD before the end of cycle 1 and another had prophylactic G-CSF treatment during cycle 1. Of the six evaluable patients, only one experienced DLTs (grade 4 neutropenia lasting ≥7 days and grade 3 febrile neutropenia requiring G-CSF); therefore, dose level 4, which had the highest P and G dose with acceptable toxicity, was further evaluated in Part 2. Among the 10 patients dosed in Part 2, three had DLTs (grade 4 thrombocytopenia lasting >7 days, n = 2; grade 4 febrile neutropenia, n = 2), two discontinued before cycle 3 due to AEs (one of them also had a DLT), and three reported PR as a best overall response. The RP2D regimen was thus confirmed to be InO 0.8 mg/m2 (day 2), R 375 mg/m2 (day 1), G 500 mg/m2 (day 1), D 40 mg (days 1–4), P 50 mg/m2 (day 1) every 21 days (dose level 4) and the decision was made to move forward with Part 3.
Safety and tolerability
The most common treatment-related AEs (any grade AEs occurring in ≥25% of all patients) were hematologic including thrombocytopenia (any grade, 85%; grade ≥3, 75%), neutropenia (69%; 62%), anemia (38%; 18%), lymphopenia (29%; 22%), and leukopenia (25%; 24%) (). Common non-hematologic treatment-related AEs (any grade AEs occurring in ≥20% of all patients) included fatigue (any grade, 36%; grade ≥3, 7%), nausea (33%; 2%), and constipation (22%; 0%). Treatment-related aspartate aminotransferase elevations of grade 1/2 severity were also reported (16%).
Table 2. Treatment-related AEs.Table Footnotea
The most common AEs leading to dose reductions were thrombocytopenia (n = 10/18 [56%]) and neutropenia (6/18 [33%]); the most frequent AEs leading to dose delays were also neutropenia (n = 19/37 [51%]) and thrombocytopenia (n = 16/37 [43%]). Among patients with AEs leading to treatment discontinuation, most were due to thrombocytopenia (n = 12/21 [57%]; Supplementary Table IV). One patient with ascites, that was documented prior to receiving study drug and deemed possibly disease-related, discontinued treatment on day 20 (cycle 4) due to treatment-related, grade 3 venoocclusive liver disease/sinusoidal obstruction syndrome (VOD/SOS). A liver biopsy showed VOD/SOS and iron overload; this patient died due to sepsis while VOD/SOS, although improving, was still ongoing. Another patient discontinued due to tumor lysis syndrome on day 4 (cycle 1) and later died due to PD on day 11 (cycle 1); this death was not considered treatment-related.
Patients were monitored for survival for up to 2 years after the first dose or until March 12, 2014, whichever occurred first. Overall, 23 deaths were reported, with 21 occurring >28 days after the last dose (or during follow up). The primary cause of death was PD (n = 18); other causes included graft-versus-host disease, toxicity after allograft, metastatic urothelial cancer, sequelae of subdural hematoma-pneumonia, and intra-abdominal sepsis secondary to gastric ulcer perforation (all n = 1).
Antitumor activity
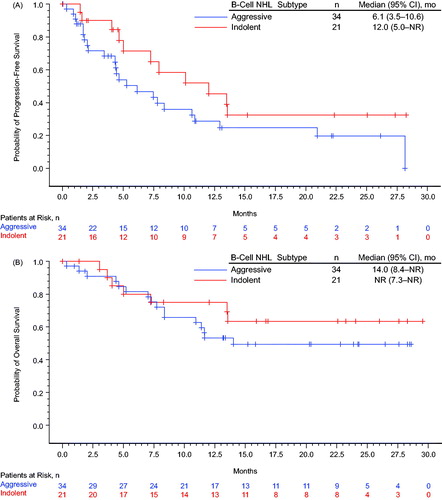
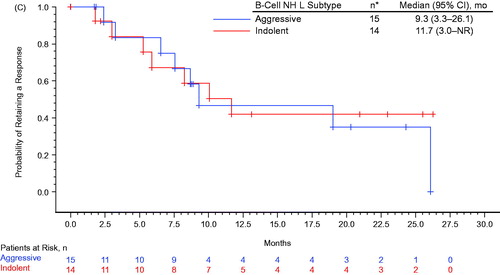
Among the treated population (n = 55), the overall response rate (ORR) was 53% (including 11 CRs); among patients receiving the RP2D regimen (n = 36), the ORR was 50% (including five CRs; ). The ORR among all treated patients with indolent (n = 21) and aggressive (n = 34) B-cell NHL was 67% and 44% (including 4 and 7 CRs), respectively; for patients with FL (n = 14), DLBCL (n = 21), and MCL (n = 13), the ORR was 71%, 33%, and 62% (including 4, 4, and 3 CRs), respectively. Among 23 patients who were refractory to their most recent prior therapy, the ORR was 35% (n = 8; including 2 CRs); among 8 and 15 patients with refractory aggressive or indolent B-cell NHL, the ORR was 50% (n = 4) and 27% (n = 4), respectively. Kaplan–Meier’s estimated probabilities of being event-free for PFS at 24 months was 0.24 (95% CI, 0.12–0.38) for all treated patients; 0.32 (95% CI, 0.12–0.55) and 0.20 (95% CI, 0.07–0.37) for patients with indolent and aggressive lymphoma, respectively (). The Kaplan–Meier estimated probability of being event free for OS at 24 months was 0.55 (95% CI, 0.40–0.67) for all treated patients; 0.63 (95% CI, 0.38–0.81) and 0.49 (95% CI, 0.31–0.65) for patients with indolent lymphoma and aggressive lymphoma, respectively (). The median (95% CI) DOR was 10.1 (6.5 – not yet reached [NYR]) months ().
Figure 1. Kaplan–Meier’s estimate of (A) progression-free survival, (B) overall survival, and (C) duration of response. Progression-free survival (PFS) was defined as the time between first dose and earliest date of disease progression (including symptomatic deterioration), new anti-lymphoma therapy, or death from any cause, which ever occurred first. Patients without a PFS event were censored at the last valid post baseline tumor assessment date; patients without a PFS event and without a post baseline tumor assessment were censored at the date of first dose. Overall survival (OS) was defined as the time between the first dose and death from any cause, censored at the date the patient was last known to be alive. Duration of response (DOR) was defined as the time between the date of first response and the earliest date of disease progression (including symptomatic deterioration), new anti-lymphoma therapy, or death, which ever occurred first. Patients without an event were censored at the last valid tumor assessment date. Indolent non-Hodgkin lymphoma includes follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, lymphoplasmacytic lymphoma, Waldenstrom’s macroglobulinemia, and low-grade B-cell lymphoma favoring follicular lymphoma. Aggressive non-Hodgkin lymphoma includes diffuse large B-cell lymphoma and mantle cell lymphoma. CI: confidence interval.
Table 3. Antitumor activity.
Pharmacokinetics and immunogenicity
PK observations at the beginning of cycle 3 were available for 19 patients treated with the RP2D regimen; mean concentrations during cycle 3 are shown in Supplementary Table V. Peak serum concentrations of InO and total calicheamicin were observed 1–3 hours after the start of the cycle 3 infusion. By day 8 of cycle 3, concentrations were below the limit of quantitation. The vast majority of PK measures for unconjugated calicheamicin were below or near the limit of quantification.
Antibodies to InO were detected in 6% (7/113) of the serum samples from 9% (5/53) of patients. Most of these observations were observed in pre-cycle 1 samples and were not considered to be InO treatment-related. Antibodies to InO were detected in one patient after treatment. The patient did not exhibit clinical symptoms definitively attributable to anti-InO antibody formation. No antibodies to R were detected in 124 serum samples from 54 patients.
Discussion
Standard second-line treatments for FL and DLBCL include chemotherapy with or without R [Citation18]. However, several studies have demonstrated the benefit of adding R to chemotherapy when treating patients with relapsed/refractory disease [Citation19–22]. Given the potential for additive or synergistic effects, this study assessed the combination of two targeted agents, InO and R, with GDP, an outpatient chemotherapy regimen commonly used to treat patients with relapsed/refractory B-cell NHL. In the original publication describing the GDP regimen, G was administered in patients with relapsed/refractory B-cell NHL at 1000 mg/m2 on days 1 and 8, P at 75 mg/m2 on day 1, and D 40 mg orally in divided doses on days 1–4 of a 21-day cycle [Citation23]. In this study, a high proportion of patients experienced grade 3/4 hematologic toxicities, including neutropenia (63%), thrombocytopenia (27%), and febrile neutropenia (14%) [Citation23]. As grade 3/4 thrombocytopenia and neutropenia were also reported in patients with relapsed/refractory B-cell NHL receiving InO alone or in combination with R [Citation7,Citation11], it was anticipated that reduced doses of P and/or G would be required when coadministered with InO, to reduce the risk of hematologic toxicities. Thus, the starting cohort evaluated the combination with G at 25% of the standard dose and P at 50% of the standard dose (500 and 37.5 mg/m2, respectively, both administered on day 1 of a 21-day cycle). InO was also administered at a lower dose (0.8 mg/m2 once every 3 weeks) than the MTD (1.8 mg/m2) reported for InO as a single agent or in combination with R in patients with relapsed/refractory NHL.
Based on the observed DLTs in the current study, which were primarily hematologic as expected, the RP2D regimen of InO plus R-GDP was InO 0.8 mg/m2 (day 2), R 375 mg/m2 (day 1), G 500 mg/m2 (day 1), D 40 mg (days 1 − 4), and P 50 mg/m2 (day 1) every 21 days (i.e. only 25% of the G dose and 67% of the P dose used in the original GDP regimen). The primary treatment-related grade ≥3 toxicities were thrombocytopenia (in 75% of patients) and neutropenia (62%). However, all patients had received prior chemotherapy (80% had received ≥2 regimens), potentially predisposing them to hematologic toxicities. Non-hematologic grade ≥3 toxicities were less common overall, with fatigue (7%) occurring most frequently; one patient developed VOD/SOS.
In this heavily pretreated population with relapsed/refractory B-cell NHL, InO plus R-GDP demonstrated preliminary antitumor activity, with an ORR of 53%, including responses in patients refractory to their prior therapy. Complete responses were achieved in four (29%) patients with FL, four (19%) patients with DLBCL, and three (23%) patients with MCL. However, these exploratory efficacy data should be interpreted with caution due to the limitations imposed by a relatively small sample size, short follow-up period, and the constraints of a phase 1 trial, which is primarily designed to investigate safety and feasibility.
Comparisons between response rates observed here and those reported elsewhere are difficult because of differences in study designs and patient characteristics across studies (e.g. proportions of heavily pretreated and/or refractory patients), the doses of chemotherapy and combinations administered, and because of the small sample size and population heterogeneity of the present study. In the original study of the GDP regimen in 51 eligible patients with relapsed/refractory B-cell NHL who had received 1 prior anthracycline-based regimen (40 patients with DLBCL and four patients with refractory disease), a response rate (CR plus PR) of 49% was observed (16% with CR), similar to the findings of the current study [Citation23]. In a smaller study of 18 patients with relapsed/refractory MCL who received a GDP regimen (with P at a higher dose of 100 mg/m2), similar findings were reported, with an ORR of 44%, including four confirmed and unconfirmed CRs (22%) and four PRs (22%) [Citation24]. In contrast to the present study, patients in both the original GDP study and the smaller MCL study were less heavily pretreated and fewer were refractory.
Prior studies of InO, either as a single agent or in combination therapies, have shown antitumor activity in patients with relapsed/refractory NHL [Citation7,Citation11]. In a phase 1 study of single-agent InO at the MTD of 1.8 mg/m2, ORRs of 68% for FL and 15% for DLBCL were observed [Citation7]. In a phase 1/2 combination study of InO plus R, at the MTD of InO 1.8 mg/m2 plus R 375 mg/m2, ORRs reported for R plus InO were 87% for relapsed FL, 74% for relapsed DLBCL and 20% for refractory aggressive NHL [Citation11]. However, patients in the current study were more heavily pretreated, and included more refractory patients versus the FL and relapsed DLBCL subgroups of the InO plus R study, further complicating any comparison.
The addition of R to standard chemotherapy has improved the outcome for patients with MCL [Citation25,Citation26], but a limited number of salvage therapies are available for patients with relapsed/refractory disease. These preliminary findings of three CRs and five PRs among the 13 patients with MCL receiving InO plus R-GDP in the present study suggest that this regimen has some antitumor activity in these patients. The mean serum concentrations of InO, total, and unconjugated calicheamicin following concomitant treatment with R in patients with NHL were consistent with previously reported values [Citation11]; only one patient tested positive for antibodies to InO after treatment. However, no clinical symptoms attributed to anti-InO antibodies were reported.
Conclusions
In conclusion, these results show that treatment of patients with relapsed/refractory CD22+ B-cell NHL with InO 0.8 mg/m2 in combination with R-GDP is feasible. However, lower doses of G and P compared with the standard GDP regimen were necessary, primarily due to hematologic toxicity. Evidence of antitumor activity was observed, with responses in patients with indolent and aggressive lymphoma (including FL, DLBCL and MCL); however, the heterogeneity and relatively small number of patients enrolled preclude an adequate comparison to other treatments for NHL. The lower doses of G and P used in this regimen may have also impacted any potential efficacy. Given that InO could not be combined with the full dose of R-GDP due to hematologic toxicities, alternative approaches or combinations should be examined with InO. Therefore, there are currently no plans for further investigation of InO plus R-GDP in NHL. However, studies of InO in combination with other chemotherapeutics and/or other biologics are currently ongoing in order to better understand the potential benefit of combination therapies in patients with hematologic malignancies.
Transparency
Declaration of funding
This study was sponsored by Pfizer Inc.
Declaration of interest
RS received honoraria from Boehringer Ingelheim, AstraZeneca, Pfizer, Lundbeck, Roche, and Eli Lily; has been a consultant/advisor for Boehringer Ingelheim, AstraZeneca, Roche, and Eli Lily; and received research funding from AbbVie, Novartis, Agensys, Roche, Celgene, and Amgen. AD has received honoraria from Roche, Gilead, Mundipharma, CTI BioPharma, and Janssen; has been a consultant/advisor for Takeda, Roche, Karyopharm, and ADC Therapeutics; received research funding from Gilead, Roche, Bayer, GlaxoSmithKline, Karyopharm, and Takeda; and received reimbursement for travel, accommodations, or expenses from Roche, Gilead, Mundipharma, CTI BioPharma, and Takeda. NHD’s institution received research funding from Pfizer. MO has received honoraria from Takeda; has been a consultant/advisor for Meiji Seika Pharma, Mundipharma, Mediphysics, Novartis, and Celltrion; and received research funding from Takeda, Janssen, Symbio, Pfizer, Solasia, Mundipharma, Eisai, and Chugai. DAM has been a consultant/advisor for Roche, Lundbeck, Celgene, Gilead, Janssen, and Amgen; received research funding from Lundbeck; and received reimbursement for travel, accommodations, or expenses from Roche and Lundbeck. YTG has received honoraria from Janssen, Celgene, Roche, Bristol-Myers Squibb, and Sanofi-Aventis; has been a consultant/advisor for Janssen, Celgene, Onyx, Pfizer, and Sanofi-Aventis; and received research funding from Novartis and Janssen. RA, MLP, EV, and JB are employees of and own stock in Pfizer. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
RS, AD, NHD, MO, and DAM were involved in the design and planning of the study, collected data, interpreted the results, provided substantive input, and critically reviewed the manuscript. RA and MLP performed analyses, interpreted the results, provided substantive input, and critically reviewed the manuscript. EV was involved in the design and planning of the study, performed analyses, interpreted the results, provided substantive input, and critically reviewed the manuscript. JB performed analyses, interpreted the results, provided substantive input, and critically reviewed the manuscript. YTG was involved in the design and planning of the study, collected data, interpreted the results, provided substantive input, and critically reviewed the manuscript.
Previous presentations
These data were presented, in part, at the Annual Meeting of the American Society of Clinical Oncology (ASCO), May 31–June 4, 2013, Chicago, IL, and at the 75th Annual Meeting of the Japanese Society of Hematology (JSH), October 11–13, 2013, Sapporo, Japan.
Supplementary_Files.zip
Download Zip (92.5 KB)Acknowledgements
Editorial support is provided by Johna Van Stelten, PhD, and Simon J. Slater, PhD, of Complete Healthcare Communications, LLC, and funded by Pfizer Inc.
References
- Leonard JP, Coleman M, Ketas JC, et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: phase I/II clinical trial results. Clin Cancer Res. 2004;10(16):5327-5334.
- Cesano A, Gayko U. CD22 as a target of passive immunotherapy. Semin Oncol. 2003;30(2):253-257.
- Vaickus L, Ball ED, Foon KA. Immune markers in hematologic malignancies. Crit Rev Oncol Hematol. 1991;11(4):267-297.
- Hinman LM, Hamann PR, Wallace R, et al. Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res. 1993;53(14):3336-3342.
- Hamann PR, Hinman LM, Hollander I, et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody–calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug Chem. 2002;13(1):47-58.
- DiJoseph JF, Armellino DC, Boghaert ER, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103(5):1807-1814.
- Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. J Clin Oncol. 2010;28(12):2085-2093.
- Ogura M, Tobinai K, Hatake K, et al. Phase I study of inotuzumab ozogamicin (CMC-544) in Japanese patients with follicular lymphoma pretreated with rituximab-based therapy. Cancer Sci. 2010;101(8):1840-1845.
- Ogura M, Hatake K, Ando K, et al. Phase I study of anti-CD22 immunoconjugate inotuzumab ozogamicin plus rituximab in relapsed/refractory B-cell non-Hodgkin lymphoma. Cancer Sci. 2012;103(5):933-938.
- Dang NH, Ogura M, Castaigne S, et al. Randomized, phase 3 trial of inotuzumab ozogamicin plus rituximab (R-InO) versus chemotherapy for relapsed/refractory aggressive B-cell non-Hodgkin lymphoma (B-NHL). J Clin Oncol. 2014;32(15 Suppl):8529.
- Fayad L, Offner F, Smith MR, et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-Hodgkin lymphoma: results of a phase I/II study evaluating the immunoconjugate inotuzumab ozogamicin with rituximab. J Clin Oncol. 2013;31(5):573-583.
- DiJoseph JF, Dougher MM, Evans DY, et al. Preclinical anti-tumor activity of antibody-targeted chemotherapy with CMC-544 (inotuzumab ozogamicin), a CD22-specific immunoconjugate of calicheamicin, compared with non-targeted combination chemotherapy with CVP or CHOP. Cancer Chemother Pharmacol. 2011;67(4):741-749.
- Data on file. New York, NY: Pfizer Inc; 2014.
- Gandhi L, Bahleda R, Tolaney SM, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol. 2014;32(2):68-75.
- Ivanova A, Wang K. A non-parametric approach to the design and analysis of two-dimensional dose-finding trials. Stat Med. 2004;23(12):1861-1870.
- Isakoff SJ, Wang D, Campone M, et al. Bosutinib plus capecitabine for selected advanced solid tumours: results of a phase 1 dose-escalation study. Br J Cancer. 2014;111(11):2058-2066.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586.
- Halwani AS, Link BK. Chemotherapy and antibody combinations for relapsed/refractory non-Hodgkin's lymphoma. Expert Rev Anticancer Ther. 2011;11(3):443-455.
- Griffin MM, Morley N. Rituximab in the treatment of non-Hodgkin's lymphoma—a critical evaluation of randomized controlled trials. Expert Opin Biol Ther. 2013;13(5):803-811.
- Hagemeister F. Rituximab for the treatment of non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2010;70(3):261-272.
- Keating GM. Spotlight on rituximab in chronic lymphocytic leukemia, low-grade or follicular lymphoma, and diffuse large B-cell lymphoma. BioDrugs. 2011;25(1):55-61.
- Tarella C, Zanni M, Magni M, et al. Rituximab improves the efficacy of high-dose chemotherapy with autograft for high-risk follicular and diffuse large B-cell lymphoma: a multicenter Gruppo Italiano Terapie Innnovative nei linfomi survey. J Clin Oncol. 2008;26(19):3166-3175.
- Crump M, Baetz T, Couban S, et al. Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma: a Phase II study by the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG). Cancer. 2004;101(8):1835-1842.
- Morschhauser F, Depil S, Jourdan E, et al. Phase II study of gemcitabine-dexamethasone with or without cisplatin in relapsed or refractory mantle cell lymphoma. Ann Oncol. 2007;18(2):370-375.
- Griffiths R, Mikhael J, Gleeson M, et al. Addition of rituximab to chemotherapy alone as first-line therapy improves overall survival in elderly patients with mantle cell lymphoma. Blood. 2011;118(18):4808-4816.
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210.