Abstract
Objective: The objective of the study was to systematically investigate the outcomes of Liposomal Bupivacaine following major colorectal resections.
Patients and methods: We conducted a comprehensive literature search of PubMed, Medline, Google scholar, Cochrane Central Registry and clinical trials.gov databases through May 2017 for studies published regarding liposomal bupivacaine. Studies were filtered based on relevance to perioperative analgesia in colorectal resections. Data comparing type of study, techniques of resection, mode of administration of liposomal bupivacaine, details of control group, outcomes were collected.
Results: A total of 1008 patients from seven studies were included in this systematic review and meta-analysis. The studies were mostly retrospective or prospective cohort studies with one randomized controlled trial (RCT). Meta-analysis showed that liposomal bupivacaine was associated with decreased length of stay, standard mean difference in days (SMD) − 0.34, (95% confidence intervals [CI] − 0.56, −0.13, p = .001) and decreased IV opioid use (expressed as intravenous morphine equivalent in milligrams) in the first 48–72 h, SMD −0.49 (95% CI −0.69, −0.28, p < .00001). Pain scores were also significantly low in patients who received liposomal bupivacaine, SMD −0.56 (95% CI −1.07, −0.06, p = .03]. There was no significant difference in hospitalization costs between the two groups.
Conclusions: Use of liposomal bupivacaine is associated with decreased IV opioid use, length of stay and lower pain scores. However, our data needs to be interpreted cautiously given the relative paucity of randomized controlled trials.
Introduction
Multimodal approach is recommended as a primary modality for management of postoperative pain by the American Pain Society and American Society of Anesthesiologists [Citation1]. Using a combination of multiple agents targeting various receptors within central and peripheral nervous systems allows additive or synergistic effects of different medications. Multimodal analgesia after abdominal operations including colorectal resections includes opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), local anesthetic injection, and other adjuncts.
NSAIDs are limited by daily dosage and side effects, including renal injury, platelet inhibition, increased risk of bleeding, and negative impacts on intestinal anastomoses [Citation2]. Local anesthetic injections, lidocaine or bupivacaine hydrochloride, have short half-lives and do not provide long-term pain control postoperatively. Neuraxial anesthesia techniques such as epidural anesthesia can provide efficient pain control, but are associated with disadvantages of hypotension, respiratory depression, and possible spinal cord complications [Citation3]. Regional blocks such as transversus abdominis plane (TAP) block is an attractive option for patients who undergo laparotomy or laparoscopic colectomy, but is limited by the half-life of the drug injected and lack of widespread use.
Intravenous and oral opioids have become the predominant agents used for postoperative pain control. Increasing dosage of opioids provides efficacious pain control at the cost of increased side effects, including respiratory depression, delirium, nausea, vomiting, ileus, tolerance, constipation, and urinary retention [Citation4]. More importantly though, the use of opioids postoperatively has been shown to be associated with chronic opioid use resulting in addiction of epidemic proportions. Opioid-related adverse events (ORAE) significantly impact patients’ recovery postoperatively and prolong length of stay (LOS). Thus, the search continues for an opioid sparing analgesic that provides long-lasting pain control without the side effects.
Liposomal formulations have been used in the delivery of antifungals, antineoplastics, and antibiotics [Citation5]. Encapsulating an aqueous core containing the drug with a non-concentric phospholipid bilayer gives a multivesicular structure to the liposome with unique properties. Liposomal bupivacaine (LB) was introduced as an alternative to standard bupivacaine with the advantage of providing long-lasting pain relief due to a 9.8-fold increase in the terminal half-life [Citation6]. Formulating bupivacaine in multivesicular liposomes provides characteristic drug release patterns, leading to increased stability and longer duration of drug release.
Though liposomal bupivacaine was introduced many years ago, data on its efficacy in colorectal resections is limited. While multiple RCTs have been published investigating the use of liposomal bupivacaine for abdominal hysterectomy, data on colorectal resections is limited [Citation7–9]. The available literature on liposomal bupivacaine in colorectal resection is limited by mostly retrospective nature and small sample size [Citation10,Citation11]. We performed a systematic review and meta-analysis to evaluate the efficacy of liposomal bupivacaine compared to other analgesic approaches in the management of postoperative pain following colorectal resections. Specifically, the outcomes evaluated were length of stay, postoperative intravenous opioid use expressed in morphine equivalents, pain score and hospitalization costs.
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [Citation12]. We conducted a comprehensive search of PubMed, Medline, Google scholar, Cochrane Central Registry of controlled trials, clinical trials.gov databases for studies published in English language, through 1 May 2017, that investigated the use of liposomal bupivacaine (Exparel, Pacira pharmaceuticals, Parsippany, NJ). Search terms included ‘liposomal bupivacaine’, ‘Exparel’, ‘liposomal bupivacaine and colectomy’, ‘liposomal bupivacaine and colon resection’, ‘liposomal bupivacaine and colorectal surgery’ with Boolean operators “AND” or “OR.” We did not set a time limit to the period when the articles were published, although most were within the last 10 years.
Articles were filtered by relevance to colorectal surgery, and included if they involved colorectal resections. Studies were included only if LB was utilized as a local anesthetic in the study population irrespective of the method of administration (TAP block or local injection) and was compared to a control group, with or without local anesthesia. Excluded articles were: studies relating to pure anorectal procedures (hemorrhoidectomy), ileostomy closures, review articles, case reports, published abstracts, combination surgeries (colorectal resections with hernia surgery), gynecological procedures, and the like. Pooled studies of individual articles were excluded but individual articles were considered and included if related to colorectal resection. Studies evaluating the pharmacokinetic profile, safety profile, and animal studies of liposomal bupivacaine were excluded.
The clinical trials.gov website was searched using the same search terms. Only completed studies were considered, while open studies or terminated studies were excluded. Abstracts of potential studies were evaluated, and full texts of articles meeting inclusion criteria were obtained and reviewed; further articles were selected from references. There was no limit on age or sample size while screening the manuscripts for inclusion. Disagreement between the reviewers was resolved by discussion and consensus.
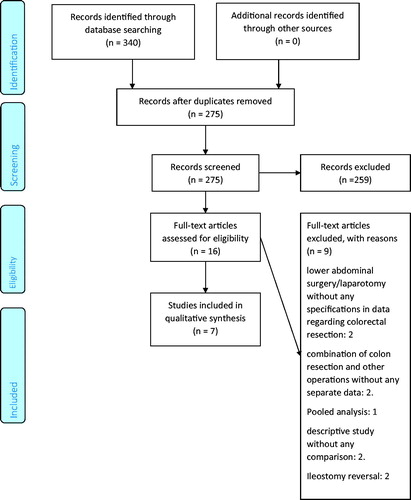
The retrieval process is presented in . Data from individual studies was extracted independently; we sought to clarify from investigators via email regarding incomplete data. Data extracted were author names, type of colorectal resection, number of patients in experimental and control group, demographic variables, drugs utilized in each group, mode of administration. Main outcome measures studied were mean difference in LOS, amount of intravenous opioid used, pain score and costs, expressed in US $. ORAE, and other relevant data were extracted. Individual studies were evaluated using the Methodological Index for Nonrandomized Studies (MINORS) for nonrandomized trials [Citation13] and modified Jadad score [Citation14] was used for RCT. A ‘risk of bias’ table to assess RCT was created containing the following points: random sequence creation, allocation concealment, blinding, incomplete outcome data, free of selective reporting and other bias [Citation15]. Consensus was reached through discussion.
Statistical analysis was performed using Review Manager (RevMan. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration). For continuous outcomes, mean differences with 95% confidence intervals (CI) with inverse variance were used. p value less than .05 was considered statistically significant. Statistical heterogeneity was evaluated with the values of Q statistic, p and I2. If the I2 <50% and p > .1, studies were considered to be of low heterogeneity. To derive pooled estimates of outcomes with 95% CI, random effects model was used. In studies where means and or standard deviation were not reported, we estimated them from reported medians and ranges as described by Hozo et al. [Citation16]. In studies where means were reported without standard deviation, the standard deviation was imputed from the mean of the SDs, as previously described in other studies [Citation17]. Amount of opioids consumed was converted to intravenous morphine equivalent and expressed in milligrams. Some studies reported the cumulative amount of opioids consumed white others reported this is a mean daily dose. In this situation the earliest reported dose was taken into consideration for calculating the amount of IV opioid used. Further sensitivity analysis was performed to analyze the results of removal of any single study and compare the differences between local and TAP infiltration. Also, we compared patients undergoing open operations and laparoscopic operations separately by sub analysis of data from the studies. This systematic review was prospectively registered with PROSPERO #CRD42017062683.
Results
A total of 340 studies were identified by searching through databases, of which 275 were screened after removing duplicates. There were 259 records excluded after applying exclusion criteria, and 16 full text articles were assessed for eligibility. Of these, 9 articles were excluded, leaving 7 studies to be included in the qualitative assessment, as shown in . shows studies included in the final analysis. There was 1 randomized double-blind trial [Citation18], while others were cohort studies [Citation10,Citation11,Citation19–22]. Of these 6 cohort studies, there were 2 retrospective matched studies [Citation11,Citation22], 2 prospective cohort studies [Citation10,Citation21], and 2 retrospective cohort studies [Citation19,Citation20]; except the last study [Citation19], all had prospectively collected data.
Table 1. Studies included in the systematic review.
Cumulatively, 1008 total patients underwent either open or laparoscopic colorectal resections, of which 538 underwent infiltration of LB either locally or via TAP block, while 470 patients served as controls. Number of study participants in each study ranged from 39 to 407. details the type of study, surgical intervention, demographic outcomes and quality assessment.
Quality assessment
Quality assessment of the nonrandomized studies is shown in (). The risk of bias table evaluating the quality of the RCT showed that the trial was of good quality, as shown in (). There was adequate random sequence generation, allocation concealment, appropriate blinding and incomplete outcome data, and avoidance of selective reporting. The MINORS evaluation for the nonrandomized studies demonstrated that mostly they were of high quality. Due to the nature of the studies, participants were not blinded, resulting in bias in assessing the study endpoint. Only one study [Citation22] provided prospective calculation of study size.
Table 2. Risk of bias table and modified Jadad score evaluating the quality of randomized controlled trial by Knudson et al. [16].
Modified Jadad score for randomized controlled trial.
Main outcomes
Length of stay
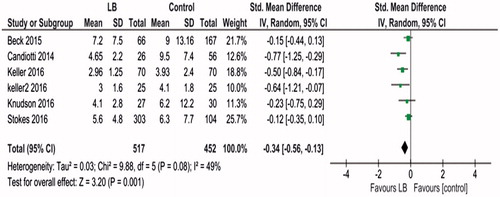
Data regarding length of stay from six studies [Citation11,Citation16–20] involving 969 patients were included in the meta-analysis. Results of meta-analysis showed heterogeneity: Tau2 = 0.03; Chi2 = 9.88, df = 5 (p = .08); I2 = 49%. A random effects model was used for analysis. This showed that use of liposomal bupivacaine was associated with significantly decreased length of stay, standard mean difference (SMD) − 0.34, (95% CI −0.56, −0.13, p = .001; ).
Amount of IV opioid consumed
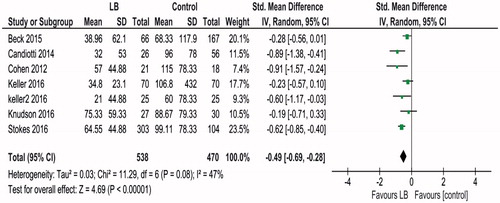
Data about amount of IV opioid consumed from seven studies [Citation10,Citation11,Citation16–20] involving 1008 patients were included in the meta-analysis. Heterogeneity was low, Tau2 = 0.03; Chi2=11.29, df = 6 (p = .08); I2=47%. Random effects model was used. The results of meta-analysis showed that use of liposomal bupivacaine was associated with decreased IV opioid used (expressed in IV morphine equivalent) SMD −0.49 (95% CI −0.69, −0.28, p < .00001; )
Pain score
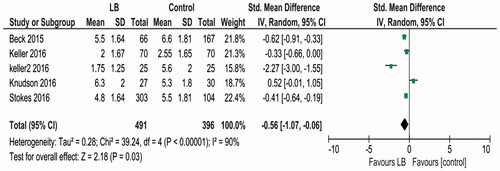
Information regarding earliest documented postoperative pain score that could be used for meta-analysis was available from five studies [Citation11,Citation16–18,Citation20], involving a total of 887 patients. Heterogeneity was high, Tau2 = 0.28; Chi2 = 39.24, df = 4 (p < .00001); I2 = 90%. Random effects model was used. Liposomal bupivacaine was associated with lower pain scores, expressed in visual analog scale, SMD −0.56 [95% CI −1.07, −0.06, p = .03; )
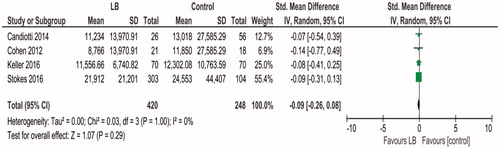
Cost
Data regarding cost was available from four studies [Citation10,Citation18–20], involving 668 patients. Meta-analysis using the random effects model was performed. Heterogeneity was low, Tau2 = 0.00; Chi2 = 0.27, df = 3 (p = .97); I2 = 0%. Liposomal bupivacaine was associated with decreased cost of hospitalization, SMD −0.09 (95% CI −0.26, −0.08). However, this was not statistically significant, p = .29 ().
Other outcomes
Opioid related adverse events and/or medications needed for treating them were reported in four studies [Citation10,Citation18,Citation19,Citation21]. Candiotti et al. [Citation21] reported that there was a significant difference in opioid-related adverse events in the liposomal bupivacaine group. [0.1 vs. 0.6 events, p = .002]. No opioid related adverse events were noted by Cohen et al. [Citation10]. Significant difference in use of antipruritic, antiemetic and anti-constipation medication, favouring Exparel was reported by Beck et al. [Citation19], while antinausea medication use was not significant in the study by Knudson et al. [Citation18].
Sensitivity analysis
Sensitivity analysis demonstrated similar overall outcomes for length of stay, IV opioid used and costs with the removal of any single study. However, sensitivity analysis for pain score showed that removal of the randomized controlled trial resulted in significant change in SMD −0.8 (95% CI −1.29, −0.3, p = .002), favoring LB. Sensitivity analysis evaluating length of stay for patients receiving local infiltration only showed that LB was associated with significantly decreased length of stay, SMD −0.39 (95% CI −0.66, −0.12, p = .005). Sensitivity analysis was not performed for TAP block as there was only one study that utilized purely a TAP Block [Citation20]. The study that included a combination of local and TAP block was excluded from this sensitivity analysis [Citation11]. Similarly, IV opioid use in patients receiving local infiltration only was significantly lower among LB patients, SMD −0.44 (95% CI −0.72, −0.16, p = .002). When evaluating pain score among patients receiving local infiltration, there was no significant difference between the two groups: SMD −0.19 (95% CI −0.75, 0.38, p = .52). Similarly, there was no difference in costs between the two groups in patients receiving local infiltration only: SMD −0.09 (95% CI −0.34, 0.16, p = .48).
We also studied the use of IV opioid in patients undergoing open procedures only, utilizing data from three studies [Citation10,Citation18,Citation20]. This showed that LB was not associated with significant difference in IV opioid expressed as IV morphine equivalent, SMD −0.45 (95% CI −1.08, 0.18), p = .16. However, when analyzing laparoscopic procedures only [Citation11,Citation18,Citation20–22], LB was associated with significant decrease in IV opioid use: SMD –0.59 (95% CI −0.88, −0.29), p < .0001. We could not study the difference between open and laparoscopic approaches for other outcomes of interest as this data was not available from the studies.
Discussion
To our knowledge, this is the first systematic review and meta-analysis evaluating the efficacy of LB in patients undergoing both open and laparoscopic colectomy. In this meta-analysis of six cohort studies and one prospective randomized trial including a total of 1008 patients, we found that use of liposomal bupivacaine was associated with decreased length of stay, decreased IV opioid used postoperatively and lower pain scores. However, there was no significant difference in costs associated with hospitalization when comparing the two groups. LB was associated with an acceptable safety and tolerance profile across all studies, with no reported complications. Sensitivity analysis showed that LB was associated with improved outcomes with local infiltration. Laparoscopic procedures showed significantly lower IV opioid use when compared to open procedures.
Although LB was introduced more than 20 years ago [Citation23], studies evaluating its efficacy have been published only within the last 7–10 years [Citation24]. Although ∼600,000 colon resections are performed annually in the United States [Citation25], there is limited data available to evaluate LB in postoperative analgesia following colorectal surgery. Introduction of LB came with it the promise of providing long-lasting analgesia, sparing opioids that have significant negative impact on postoperative recovery, especially in intestinal surgery.
In this era of enhanced recovery protocols following colorectal resection, ORAE such as constipation and ileus have undesirable effects, prolonging LOS. If indeed the promise of LB holds true, it will be an indispensable component of enhanced recovery pathways by its ability to minimize opioid requirement. We found that use of liposomal bupivacaine was associated with significantly decreased IV opioid use and length of stay. The magnitude of statistical significance for pain score though, was somewhat smaller. This possibly is due to factors such as only five studies reporting pain scores and not all of them reporting pain scores at the same interval. Of note, in the randomized controlled trial, patients receiving liposomal bupivacaine had a higher mean pain score on postoperative day 4 as compared to those in the control group. Also, while stratifying open and laparoscopic approaches, the decrease in IV opioid use was significant for laparoscopic operations as compared to open operations. We surmise that this is due to the inherent significant difference in pain between the two approaches that gets magnified with the utilization of LB.
Hospitalization costs trended lower in patients receiving liposomal bupivacaine but this did not reach statistical significance. This can be due to various reasons – only four studies reporting costs, equipment used, type of surgery (lap vs. open), adjustment of hospital charges to calendar year dollars, and so on. Opioid side effects are also associated with costs – the direct cost of prolonged LOS, nursing costs, medication costs from managing side effects and such. In addition, identifying soft costs from inadequate pain control is very challenging. It is pertinent to note that a single vial of LB costs $315 [Citation26] and is not a trivial expense. But this expense is a justifiable one if liposomal bupivacaine significantly decreases length of stay and avoids the opioid side effects following laparoscopic or open colorectal resections.
The results of our systematic review remain consistent with previous reviews exploring LB in various surgical domains. Vyas et al. [Citation27] systematically reviewed the efficacy of LB in plastic surgery and found comparable or better favorability than control groups across multiple outcomes. Another meta-analysis [Citation28] evaluating the role of local infiltration of LB after total hip arthroplasty found better pain control at 24 h and decreased length of stay.
Although our systematic review and meta-analysis included cohort studies that were of good quality and a randomized controlled trial that had minimal risk of bias, we acknowledge that multiple limitations exist in this and other studies we evaluated. There was some imbalance in study design between LB and control groups. LB formed a component of multimodal analgesia that was compared to an opioid-only group. NSAIDs were freely used among LB patients, while controls were exposed only to opioids. While most studies used a standardized dose of liposomal bupivacaine, (20 ml vial of LB = 266 mg of freebase bupivacaine =300 mg of bupivacaine hydrochloride) [Citation29], there was variation in the local anesthetic used in the control group and in mode of administration.
Also, opioid related adverse events were not systematically reported across all studies. We were unable to perform subgroup analysis or metaregression for other outcomes as studies that included both open and laparoscopic operations did not report breakdown by numbers in such groups. Lastly, there are limitations to the systematic review/meta-analysis because of potential errors in search methodology, selection, and reporting bias. An ideal study to evaluate the efficacy of LB in colectomy should be prospective, randomized, multi-centre, double-blinded, include both laparoscopic and open approaches, have a standardized postoperative pathway, preferably including enhanced recovery protocol and utilize a control group with equivalent dose of bupivacaine hydrochloride. Perioperative outcome measures should be rigorously studied and specific attention paid to ORAE, as opioid-sparing is the central premise of utilizing LB.
Conclusions
In conclusion, our meta-analysis shows that use of liposomal bupivacaine may be advantageous in shortening the length of stay, and decreasing IV opioid use following colorectal resections. Patients receiving liposomal bupivacaine report lower pain scores. However, considering the paucity of randomized controlled trials, our results need to be cautiously interpreted. Further studies are needed to identify patient subgroups that will truly benefit from liposomal bupivacaine.
Transparency
Declaration of funding
No funding to declare.
Declaration of financial/other relationships
None of the authors has any financial or other relationships to declare. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
SR: conception and design, analysis and interpretation of data, drafting and critical revision, helped with final approval and agree to be accountable for all aspects of work. ML: conception and design, drafting of the article, final approval and agree to be accountable. NK: analysis and interpretation of data, critical revision of article, helped with final approval and agree to be accountable.
Acknowledgements
Marcie White, Librarian at Mercy Medical Center, for help in procuring the articles.
References
- Chou R, Gordon DB, de Leon-Casasola OA. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157.
- Bakker N, Deelder JD, Richir MC, et al. Risk of anastomotic leakage with nonsteroidal anti-inflammatory drugs within an enhanced recovery program. J Gastrointest Surg. 2016;20:776–782.
- Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008;107:1026–1040.
- George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. JOM. 2010; 6:47–54.
- Ostro MJ, Cullis PR. Use of liposomes as injectable-drug delivery systems. Am J Hosp Pharm. 1989;46:1576–1587.
- Chahar P, Cummings KC. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257–264.
- Barron KI, Lamvu GM, Schmidt RC, et al. Wound infiltration with extended-release versus short-acting bupivacaine before laparoscopic hysterectomy: a randomized controlled trial. J Minim Invasive Gynecol. 2017;24:286–292.
- Hutchins J, Delaney D, Vogel RI, et al. Ultrasound guided subcostal transversus abdominis plane (TAP) infiltration with liposomal bupivacaine for patients undergoing robotic assisted hysterectomy: a prospective randomized controlled study. Gynecol Oncol. 2015;138:609–613.
- Mohling SI, Elkattah R, Furr RS, et al. Comparison of laparoscopically assisted transversus abdominis plane block using liposomal bupivacaine with pre-incisional bupivacaine for post-operative pain control in patients undergoing laparoscopic or robotic-assisted laparoscopic hysterectomy: a randomized controlled trial. J Minim Invasive Gynecol. 2015;22:S36
- Cohen SM. Extended pain relief trial utilizing infiltration of Exparel((R)), a long-acting multivesicular liposome formulation of bupivacaine: a Phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567–572.
- Keller DS, Tahilramani RN, Flores-Gonzalez JR, et al. Pilot study of a novel pain management strategy: evaluating the impact on patient outcomes. Surg Endosc. 2016;30:2192–2198.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716.
- Oremus M, Wolfson C, Perrault A, et al. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer's disease drug trials. Dement Geriatr Cogn Disord. 2001;12:232–236.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;20:5.
- Barendse RM, van den Broek FJ, Dekker E, et al. Systematic review of endoscopic mucosal resection versus transanal endoscopic microsurgery for large rectal adenomas. Endoscopy. 2011;43:941–949.
- Knudson RA, Dunlavy PW, Franko J, et al. Effectiveness of liposomal bupivacaine in colorectal surgery: a pragmatic nonsponsored prospective randomized double blinded trial in a community hospital. Dis Colon Rectum. 2016;59:862–869.
- Beck DE, Margolin DA, Babin SF, et al. Benefits of a multimodal regimen for postsurgical pain management in colorectal surgery. Ochsner J. 2015;15:408–412.
- Stokes AL, Adhikary SD, Quintili A, et al. Liposomal bupivacaine use in transversus abdominis plane blocks reduces pain and postoperative intravenous opioid requirement after colorectal surgery. Dis Colon Rectum. 2017;60:170–177.
- Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp. 2014;76:1–6.
- Keller DS, Pedraza R, Tahilramani RN, et al. Impact of long-acting local anesthesia on clinical and financial outcomes in laparoscopic colorectal surgery. Am J Surg. 2017;214:53–58.
- Mowat JJ, Mok MJ, MacLeod BA, et al. Liposomal bupivacaine. Extended duration nerve blockade using large unilamellar vesicles that exhibit a proton gradient. Anesthesiology. 1996;85:635–643.
- Candiotti K. Liposomal bupivacaine: an innovative nonopioid local analgesic for the management of postsurgical pain. Pharmacotherapy. 2012;32:19S–26S.
- https://www.sages.org/publications/patient-information/patient-information-for-laparoscopic-colon-resection-from-sages/ Accessed 8 January 2017.
- Beachler JA, Kopolovich DM, Tubb CC, et al. Liposomal bupivacaine in total hip arthroplasty: do the results justify the cost? J Orthop. 2017;14:161–165.
- Vyas KS, Rajendran S, Morrison SD, et al. Systematic review of liposomal bupivacaine (exparel) for postoperative analgesia. Plast Reconstr Surg. 2016;138:748e–756e.
- Ma TT, Wang YH, Jiang YF, et al. Liposomal bupivacaine versus traditional bupivacaine for pain control after total hip arthroplasty: a meta-analysis. Medicine. 2017;96:e7190.
- Smoot JD, Bergese SD, Onel E, et al. The efficacy and safety of DepoFoam bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammaplasty: a randomized, double-blind, active-control study. Aesthet Surg J. 2012;32:69–76.