Abstract
Aim: Purple urine bag syndrome (PUBS) is rarely seen in clinical practice. Several studies have reported that PUBS is relatively benign in its clinical course, but this study aimed to identify risk factors for mortality related to PUBS.
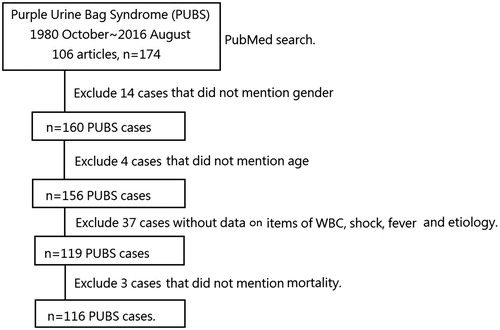
Materials and methods: In a PubMed search from October 1980 to August 2016, using the search term “Purple urine bag syndrome (PUBS)”, 106 articles (n = 174 cases) were identified. This study excluded 58 cases. Among them, 14 cases did not include information on patient sex and four cases did not include information on patient age. Thirty-seven cases did not report the white blood cell (WBC) count, shock, fever, or etiology. Three cases did not report patient survival. This study considered 116 PUBS cases. Chi-square tests were used to compare the survival and mortality groups.
Results: In relative risk analysis, uremia (17.8), shock (14.4), diabetes (4.8), leukocytosis (1.1), and female sex (1.1) were significant risk factors for mortality after PUBS. However, it is possible that PUBS cases are under-reported worldwide.
Conclusions: PUBS is a warning sign of a urinary tract infection, and it often follows a relatively benign clinical course. This study found that female sex, leukocytosis, shock at presentation, comorbidity with diabetes, and uremia are risk factors for mortality associated with PUBS.
Introduction
Purple urine bag syndrome (PUBS) was first described in 1978Citation1. It is very rarely seen in clinical practice and is indicative of urinary tract infection (UTI)Citation2. It occurs when a decrease in bowel motility causes bacterial overgrowth in the intestine, leading to increased metabolism of tryptophan to indole, resulting in high levels of indigo and indirubin in the urineCitation3–11. The majority of PUBS patients are bed ridden, primarily female, with an indwelling urinary catheter, experiencing constipation and a UTI with a urine alkaline pHCitation3–5,Citation8–12. Most studies have reported that PUBS is benign, harmless, and even asymptomaticCitation3,Citation4,Citation7,Citation13. However, there are some reports that it does not always have a harmless courseCitation12. We aimed to identify the risk factors for mortality associated with PUBS. We collected all PubMed articles describing PUBS from October 1980 to August 2016 in order to analyze the risk of mortality after experiencing PUBS. We have adopted items that related to comparability and adjustment (which are not associated with studies) and retained items that focused on our selection, representativeness of cases, and ascertainment of exposure and outcomesCitation14.
Materials and methods
In our PubMed search from October 1980 to August 2016 for “Purple urine bag syndrome (PUBS)”, we identified 106 articles (174 patients). We excluded 58 cases of PUBS. Among these, 14 studies did not describe patient sex, and age was not reported in four cases. Thirty-seven studies did not report the white blood cell (WBC) count, shock, fever, or etiology. Three studies did not report survival data. We finally included 116 PUBS cases (). We recorded age, sex, the presence of fever, shock, WBC count, urine pH, indwelling urinary catheter status, constipation, nursing home residency, history of diabetes, history of uremia, diabetes mellitus (DM), culture results from urine and blood and survival. We defined shock as the systolic blood pressure < 90 mmHg. Nursing home residency was defined as patients who lived in a home care unit rather than their home.
Comparisons are made between survival group and mortality group, and relative risks to mortality are also analyzed. Chi-square tests were used to compare the survival and mortality groups. Data were analyzed using IBM SPSS Statistics v. 20, 2011 (New York, NY).
Results
Female sex, higher WBC count, presenting with shock, a history of diabetes, and uremia were risk factors significantly correlated with mortality. However, age, urine pH values, fever, indwelling urinary catheter, constipation, and nursing home residency did not influence mortality. Mortality from PUBS was correlated with female sex and presence of sepsis, shock, diabetes, and uremia (). Sepsis is defined as a WBC count >12,000/μL or <4000/μL with immature form above 10%.
Table 1. Comparison between survival and mortality cases in PUBS.
In relative risk (RR) analysis, uremia (17.8), shock (14.4), diabetes (4.8), leukocytosis (1.1), and female sex (1.1) were all significant risk factors for mortality after PUBS (). We listed the pathogens of all PUBS cases and those from the mortality group in . Among these, eight died of sepsis (mortality rate =7%).
Table 2. Relative risk of sex, white blood cell count, presentation of shock, diabetes, and uremia for mortality of PUBS patients.
Table 3. List of pathogens in all PUBS cases and fatal PUBS cases.
Discussion
UTI is one of the most common infectious diseases in adults, and 40–50% of women experience >1 UTI in their lifetimeCitation15. In urological or emergency daily practice, many different stages of UTI might be seen. It could be asymptomatic UTI (bacteriuria), pyelonephritis, cystitis in female patients, prostatitis in male patients, or a catheterized febrile patient. PUBS may present with one of the clinical aspects of UTI’s as above.
Age and gender
PUBS is a warning sign of UTICitation2. It is more commonly seen among women. In a Mexican study, 73% of PUBS cases occurred among womenCitation10. In our study, 60% of PUBS cases occurred among women, and the mean age was 75.5 years, indicating that PUBS is most often seen in elderly women.
Laboratory tests
PUBS is a sign of UTI, and the mean white blood cell (WBC) count in our study reached 12,695/µL. Red and white blood cells lyse when the urine pH is >6, and a urine pH > 7.5 indicates a UTICitation15. So, PUBS is easily overlooked in clinical practice when the red and white blood cells are lysed. In our study, the mean urine pH in PUBS cases was 8, consistent with most articles reporting that PUBS cases are associated with alkaline urine. However, there have been cases with acidic urineCitation16. There was no difference in urine pH between the surviving PUBS cases and the mortality group in our study (8 ± 0.9 vs 8 ± 0.7, p = 0.67). Presentation with fever, an indwelling urinary catheter, constipation, and living in a long-term care unit are all predisposing factors not leading to mortality in cases of PUBS.
Constipation
Around 50% of older people living in nursing homes experience chronic constipationCitation17. Chronic constipation is thought to be one of the factors leading to PUBS. It slows gut motility and changes intestinal bacterial flora. Bowel bacterial flora containing tryptophanase convert dietary tryptophan to indole, pyruvic acid, and ammoniaCitation3–5,Citation8,Citation10. Indole is absorbed and enters the liver via the portal circulation. Indole is converted to indoxyl sulfate with both nephrotoxicity and cardiotoxicityCitation18, and it is then catalyzed by bacterial phosphatases or sulfatases to indoxyl. In the urine, indoxyl is metabolized into two pigments, indigo (blue) and indirubin (red). Mixing of indigo and indirubin leads to purplish discoloration of the urineCitation3,Citation11.
The reason why the majority of cases were not constipated may be that this information was not always recorded. There may also be other mechanisms responsible for this syndrome.
Pathogens
In cases of UTIs, women infected with Escherichia coli (E. coli) are often seen clinicallyCitation19. The most commonly seen UTI pathogens are E. coli and Klebsiella pneumoniae. E. coli is the most commonly seen pathogen in UTI cases and in cases of fatality associated with UTIsCitation20.
In our study, the pathogens involved in PUBS were E. coli (28%), mixed microorganisms (18%), Enterococcus faecalis (13%), Proteus spp. (9%), Morganella morganaii (9%), Klebsiella spp. (9%), Providencia rettgeri (8%), Pseudomonas aeruginosa (6%), Streptococcus spp. (2%), and Staphylococcus spp. (1%). In our mortality group, the ranking of pathogens was similar to all cases: E. coli (27%), mixed microorganisms (13%), E. faecalis (13%), Proteus spp. (13%), M. morganaii (13%), P. rettgeri (13%), and P. aeruginosa (7%). These findings suggest that the pathogen in PUBS is not one of the factors influencing mortality associated with PUBS.
The most common microorganism involved in PUBS is E. coli, mixed, Enterococci, and Proteus spp. Enterococci is highly associated with catheter-related UTIs, and Proteus Spp. is associated with patients who live in long-term care facilities. It is estimated that 63.6% of cases of enterococcal bacteremia occur after urinary catheter infectionCitation21. K. pneumoniae is commonly seen in catheterized patients or presents as a long-term hospitalized opportunistic infectionCitation22. Presence of an indwelling catheter and living in long-term care facilities are both predisposing factors for PUBSCitation21,Citation23. M. morganii is an opportunistic infection often seen in post-operative wound and urinary tract infectionsCitation24.
Prognosis
Several studies describe PUBS as a benign processCitation3,Citation7,Citation13. In assessing the prognosis of catheterized urinary tract infections (CAUTIs), several risk factors, including female sex, emergency admission, and transfer from another healthcare facility are relevantCitation25. In another study from China, DM, prolonged catheter placement, and longer hospital and intensive care unit (ICU) stay were all high risk factors for mortality in CAUTIsCitation26. Patients with dementia or chronic kidney diseases may develop a bloodstream infection after urinary catheter placementCitation27. In our study, the RR of mortality in PUBS patients was significantly higher in cases presenting with shock (14.4), uremia (17.8), diabetes (4.8), leukocytosis (1.1), and of female sex (1.1). We should pay special attention to female patients with PUBS who are hypotensive, uremic, diabetic, and have leukocytosis. Age, urine pH, presence of fever, constipation, and nursing home residency were not associated with increased mortality.
Although most articles report PUBS as a relatively benign process, its mortality rate was 7% in our study, slightly higher than that of older people who develop a UTI (5%)Citation28. We conclude that not all PUBS cases are harmless in their clinical course, and we should pay more attention to patients who are female, have leukocytosis, and are uremic, diabetic, and hypotensive.
Our study has limitations. We collected all cases from PubMed. There is the possibility that PUBS cases are under-reported worldwide.
PUBS is a warning sign of UTI, and, although it often has a benign clinical course, we have found that women with leukocytosis who present with shock and have diabetes and uremia are at risk of mortality from PUBS. Prescribing antibiotic therapy after culture of urine or blood is mandatory to treat PUBS.
Transparency
Declaration of funding
There is no funding to declare for this research.
Declaration of financial/other relationships
The authors have no financial or other relationships to disclose. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Buist NR. Purple urine bags. Lancet. 1978;1:883–884.
- Su YJ. A warning sign of infection. J Emerg Med. 2012; 42:183–184.
- Hadano Y, Shimizu T, Takada S, et al. An update on purple urine bag syndrome. Int J Gen Med. 2012;5:707–710.
- Pillai BP, Chong VH, Yong AM. Purple urine bag syndrome. Singapore Med J. 2009;50:e193–e194.
- Harun NS, Nainar SK, Chong VH. Purple urine bag syndrome: a rare and interesting phenomenon. South Med J. 2007;100:1048–1050.
- Ihama Y, Hokama A. Purple urine bag syndrome. Urology. 2002;60:910
- Wang IK, Ho DR, Chang HY, et al. Purple urine bag syndrome in a hemodialysis patient. Intern Med. 2005;44:859–861.
- Faridi MS, Rahman MJ, Mibang N, et al. Purple urine bag syndrome- an alarming situation. J Clin Diagn Res. 2016;10:PD05–PD06.
- Sriramnaveen P, Reddy YS, Sridhar A, et al. Purple urine bag syndrome in chronic kidney disease. Indian J Nephrol. 2016;26:67–68.
- Delgado G, Martínez-Reséndez M, Camacho-Ortiz A. Purple urine bag syndrome in end-stage chronic kidney disease. J Bras Nefrol. 2014;36:542–544.
- Duff ML. Case report: purple urine bag syndrome. J Emerg Med. 2013;44:e335–e336.
- Tasi YM, Huang MS, Yang CJ, et al. Purple urine bag syndrome, not always a benign process. Am J Emerg Med. 2009;27:895–897.
- Johnson JR. Purple urine bag syndrome. JAMA. 2012;307:1913.
- Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63.
- Franz M, Hörl WH. Common errors in diagnosis and management of urinary tract infection. I: pathophysiology and diagnostic techniques. Nephrol Dial Transplant. 1999;14:2746–2753.
- Restuccia MR, Blasi M. A PUBS Case in a Palliative Care Unit Experience. Case Rep Oncol Med. 2014;2014:169782.
- Mounsey A, Raleigh M, Wilson A. Management of Constipation in Older Adults. Am Fam Physician. 2015;92:500–504.
- Lekawanvijit S. Cardiotoxicity of uremic toxins: a driver of cardiorenal syndrome. Toxins (Basel). 2018;10(9). pii: E352.
- Behzadi P, Behzadi E, Yazdanbod H, et al. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica (Buchar). 2010;5:111–115.
- Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J. 2016;57:485–490.
- Ceci M, Delpech G, Sparo M, et al. Clinical and microbiological features of bacteremia caused by Enterococcus faecalis. J Infect Dev Ctries. 2015;9:1195–1203.
- Clegg S. Murphy CN. Epidemiology and Virulence of Klebsiella pneumoniae. Microbiol Spectr. 2016;4(1): UTI-0005-2012.
- Schaffer JN, Pearson MM. Proteus mirabilis and Urinary Tract Infections. Microbiol Spectr. 2015;3(5):UTI-0017-2013
- Liu H, Zhu J, Hu Q, et al. Morganella morganii, a non-negligent opportunistic pathogen. Int J Infect Dis. 2016;50:10–17.
- Daniels KR, Lee GC, Frei CR. Trends in catheter-associated urinary tract infections among a national cohort of hospitalized adults, 2001-2010. Am J Infect Control. 2014;42:17–22.
- Li F, Song M, Xu L, et al. Risk factors for catheter-associated urinary tract infection among hospitalized patients: a systematic review and meta-analysis of observational studies. J Adv Nurs. 2018; Sep 26. doi: 10.1111/jan.13863 [Epub ahead of print]
- Bursle EC, Dyer J, Looke DF, et al. Risk factors for urinary catheter associated bloodstream infection. J Infect. 2015;70:585–591.
- Beveridge LA, Davey PG, Phillips G, et al. Optimal management of urinary tract infections in older people. Clin Interv Aging. 2011; 6:173–180.