Abstract
Background: Patients with acute peripheral arterial occlusion (aPAO) are candidates for operative thrombectomy, bypass, or catheter-directed thrombolysis (CDT) using a plasminogen activator. Human plasma-derived plasmin may offer another CDT option.
Objectives: To evaluate the efficacy, safety, and tolerability of two intrathrombus delivery methods and two doses of plasmin compared with recombinant tissue plasminogen activator (rtPA) and placebo in patients with aPAO.
Patients/methods: This was a phase 2, randomized, open-label study of intra-arterial CDT of plasmin in patients with aPAO. The study used infusion catheters with or without balloon occlusion (BOC) to evaluate 150 mg plasmin (2 and 5 h post-infusion) and 250 mg plasmin (5 h post-infusion). The efficacy of plasmin, rtPA and placebo was assessed.
Results: One hundred and seventy-four subjects were enrolled. Overall, the thrombolytic efficacy (>50% thrombolysis) was 59% (58/99) for 150 mg plasmin without BOC, which is comparable to 89% (8/9) for rtPA without BOC (p = 0.149) and 40% (2/5) for placebo control (p = 0.648). The thrombolytic efficacy was 33% of the 250 mg plasmin group. There was no difference (p > 0.999) in thrombolytic efficacy with BOC (59%, 58/99) or without BOC (59%, 17/29). Plasmin-treated groups experienced treatment-emergent adverse events (TEAEs) at 71% (76/107) without BOC and 63% (24/38) with BOC; 78% (7/9) of the rtPA-treated group and 89% (8/9) of the placebo group had TEAEs. Serious AEs (SAEs) occurred in 29% (31/107) of the 150 mg plasmin group without BOC and 24% (9/38) with BOC. No SAEs occurred in the 250 mg plasmin group.
Conclusions: Plasmin demonstrated less bleeding during catheter-directed administration at 150 mg and 250 mg doses compared to rtPA. BOC utilization did not improve efficacy. CDT with plasmin has a potential thrombolytic benefit in patients presenting with aPAO.
ClinicalTrials.gov Identifier: NCT01222117
Trial registration: ClinicalTrials.gov identifier: NCT01222117.
Introduction
Acute limb ischemia resulting from acute peripheral arterial occlusion (aPAO) is a limb- and potentially life-threatening manifestation of peripheral arterial diseaseCitation1. Patients with aPAO are candidates for thrombolytic and/or mechanical removal of clot, and, at times, surgical bypass. Catheter-directed thrombolysis (CDT) with human plasma-derived plasmin is a potential new treatment option for aPAO.
Treatment of aPAO patients using recombinant tissue plasminogen activator (rtPA), is a 2-step process beginning with CDT followed by definitive correction of any underlying culprit lesion(s)Citation1. Thrombolysis is intended to eliminate arterial thrombus, re-establish blood flow, and visualize the underlying vascular lesions responsible for the acute arterial thrombosis. Subsequent corrective measures, such as angioplasty with or without stenting, localized minor open surgical procedures, or major open surgical procedures (e.g. bypass graft) are performed. Initial treatment with thrombolysis is intended to reduce the extent of these corrective measures such that minor procedures (e.g. angioplasty) can be performed more frequently and major open surgical procedures less frequently.
When plasmin is administered into a thrombus it is protected from neutralization by alpha-2-antiplasmin (A2AP), whereas circulating free plasmin is rapidly inactivated by A2APCitation2–4. Plasmin delivered directly into a thrombus via catheter should not reach remote, vulnerable hemostatic plugs, and should not induce the remote bleeding observed with PAsCitation5–7. CDT with PAs carry a high frequency of major bleeding complications, ranging from 5.6–11.8%Citation8–10. Thus, there is a need to study whether plasmin is a safer thrombolytic agent for aPAO. Pre-clinical data suggest that this may be the case.
In a rabbit model, plasmin did not produce bleeding at up to 6-times (6 mg/kg) the standard thrombolytic dose (1 mg/kg)Citation5. Plasmin-induced bleeding occurred at 8-times the standard thrombolytic dose, which then only correlated with depletion of fibrinogen and factor VIII. In contrast, rtPA produced bleeding in the same model at doses as low as 25% of the standard dose. Therefore, it is reasonable to conclude that plasmin has a ≥ 6-fold margin of safety relative to rtPA, an agent known to cause hemorrhage at therapeutic doses.
The purpose of this study was to evaluate the optimal catheter delivery technique of 150 mg and 250 mg doses of plasmin (human), TAL-05-00018 (Grifols Therapeutics, Inc., Research Triangle Park, NC) on the potential effectiveness of clot dissolution at 2 or 5 h post-treatment with varying rates of infusion (10–75 mL/h), with or without pulsing, with or without distal balloon occlusion, and infusion of plasmin more distally vs proximally along the clot length. The safety and tolerability of the delivery methods were performed through analyses of bleeding, death, adverse events (AEs), serious adverse events (SAEs), and abnormal laboratory values. The efficacy of different doses and methods of delivery was evaluated using the threshold of >50% thrombolysis.
Methods
Study design
This was a phase 2, multi-center, randomized, open-label study of plasmin in subjects with angiographically confirmed acute lower extremity native artery or bypass graft occlusion (). The study was conducted from December 9, 2010 through September 3, 2014 in adult patients from Europe, South America, and North America. Key inclusion/exclusion criteria are in . Additional details of study drug administration are described in Supplementary Table S1. A subject withdrawal was defined as a discontinuation from the study for any reason.
Figure 1. Study schema. Abbreviations. BOC, balloon occlusion catheter; rtPA, recombinant tissue plasminogen activator. aThere was no 2-h arteriogram for blinded rtPA treatment group E or blinded placebo control group F. bThere was no 5-h arteriogram for plasmin groups D, H, or J. cPost-intervention assessments were required for all treatment groups. Post-intervention arteriograms were required post-angioplasty. Any additional post-intervention arteriograms, if performed, were collected. Treatment group A: 5-h infusion at 10 mL/h, 150 mg plasmin in 75 mL, pulse, possible repositioning after 2-h arteriogram without BOC. Treatment group B: 5-h infusion at 15 mL/h, 150 mg plasmin in 75 mL, pulse, possible repositioning after 2-h arteriogram without BOC. Treatment group C: 5-h infusion (30 mL/h), 150 mg plasmin in 150 mL, pulse, possible repositioning after 2-h arteriogram without BOC. Treatment group D: 2-h infusion at 35 mL/h, 150 mg plasmin in 75 mL, pulse, without BOC. Treatment group E: the rtPA dose, volume, and infusion rate were administered according to the clinical judgment of the investigator. Placebo control group F: the placebo volume matched the equivalent rtPA volume according to the clinical judgment of the investigator. Treatment group G: 5-h infusion at 60 mL/h, 150 mg plasmin in 300 mL, no pulse, no repositioning without BOC Treatment group H: 2-h infusion at 75 mL/h, 150 mg plasmin in 50 mL, no pulse, without BOC. Treatment group I: 5-h infusion at 30 mL/h, 150 mg plasmin in 150 mL, no pulse, with BOC. Treatment group J: 2-h infusion at 35 mL/h, 150 mg plasmin in 70 mL, no pulse, with BOC. Treatment group M: 5-h infusion at 30 mL/h, 250 mg plasmin in 150 mL, no pulse, with BOC. NOTE: Plasmin groups K (250 mg plasmin, 5-h infusion at 30 mL/h) and L (250 mg plasmin, 15-h infusion at 30 mL/h) were never implemented and, thus, not included in this study schema.
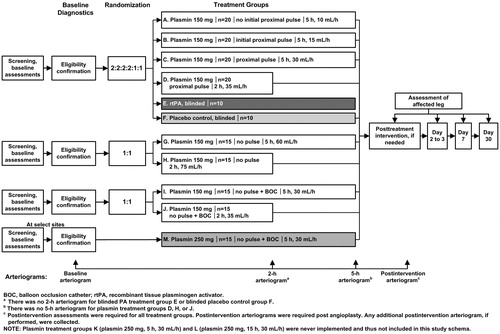
Table 1. Key inclusion and exclusion criteria.
The primary efficacy outcome was the percentage of subjects with > 50% thrombolysis at the end of treatment. The study also assessed the safety and tolerability of plasmin. The 150 mg dose was chosen for its safety profile and evidence of thrombolytic activity in a previous studyCitation11, which showed greater thrombolysis at higher doses of plasmin (125, 150, 175 mg). All plasmin doses (25–175 mg) were safe and well tolerated. The objective for groups A–H was to optimize plasmin delivery by comparing six different delivery regimens (treatment groups A–D, G, H). This portion of the study included blinded control groups (rtPA treatment group E and placebo group F). In treatment groups A–H, the study drug was delivered using a multi-side-hole infusion catheter with an occluding ball to block the distal end of the infusion catheter. The administration regimens for 150 mg plasmin delivery in treatment groups without BOC were designed to examine the effect of different infusion catheter positions (as catheters have fixed infusion lengths), different plasmin pulse regimens, and different infusion rates on efficacy and safety. There was no sample size calculation, as data addressing optimal treatment delivery were unknown. When a subject met all inclusion/exclusion criteria, treatment assignment was done based on a computer-generated randomization schedule. Approximately 100 subjects were planned for randomization into one of six treatment groups (A:B:C:D:E:F) in a 2:2:2:2:1:1 ratio. The randomization was stratified by vessel type (native artery or graft) in order to ensure approximately equal balance of each presenting vessel type across all treatment groups. Subjects were also stratified by age group (<65 years old vs ≥65 years old) to ensure approximately equal balance of each age group across all treatment groups. No more than 40 subjects with occluded native artery were to be included during the enrollment of the first 100 subjects in this study. For groups G and H, ∼30 subjects were planned for randomization in a 1:1 ratio. Again, the randomization was stratified by vessel type (native artery or graft) in order to ensure an approximately equal balance of each presenting vessel type across these two treatment groups (G:H). No additional restrictions were included in the randomization method.
Plasmin groups I and J evaluated the effects of 150 mg plasmin delivery using the infusion catheter with a BOC to facilitate the retention of plasmin in the treatment zone. Approximately 30 additional subjects were planned for randomization in a 1:1 ratio, following a similar strategy for groups G and H.
Plasmin group M was added to evaluate a higher dose of 250 mg plasmin administered over 5 h with BOC; this arm of the study was implemented only in the European Union, where BOC had received Conformité Européene Marking. Treatment group M was exploratory; therefore, its six subjects were not randomized. BOC utilization was intended to retain plasmin within the clot by restricting blood flow to maximize the contact of plasmin with the thrombus and to minimize the risk of inactivation by circulating plasmin inhibitors, thus improving thrombolysis.
If complete thrombolysis was not achieved while BOC was utilized, release of thrombus fragments upon deflation of the balloon was a potential risk. If distal embolization was suspected, the investigators were to use their clinical judgment regarding release to standard of care.
An independent Data Monitoring Committee reviewed safety data throughout the study.
The Central Reading Facility comprised three radiologists, who performed blinded, retrospective evaluations of arteriogram imaging quality, degree of thrombolysis, evidence of thrombosis, or rethrombosis, and restoration of patency of the native artery or bypass graft. The readers evaluated success of thrombolysis and flow based on arteriography at 2 h (when available) and at the end of treatment (EOT), relative to baseline. Readers used categories of: ≤50%, 51–75%, 76–90%, and >90% thrombolysis. The readers also assessed the absence of flow at baseline in the affected leg by duplex ultrasound, and restoration of flow at EOT or post-intervention, day 7, and day 30 ± 3. Each patient’s arteriograms were reviewed by two radiologists. If the readings agreed, there was no further review. If the reading of the initial two radiologists did not agree, the interpretation of the third radiologist served as the adjudicator.
The intent-to-treat (ITT) population included all enrolled subjects. The number of subjects in each treatment group reflected the planned 2:2:2:2:1:1 randomization ratio: A (n = 20), B (n = 20), C (n = 22), D (n = 20), E (n = 9), and F (n = 10). Although we were not able to enroll 30 patients for groups G and H as planned, the ratio for G (n = 13) and H (n = 12) did reflect the planned 1:1 randomization ratio. Groups I (n = 23) and J (n = 23), with more than 30 planned subjects, also fulfilled the planned 1:1 randomization ratio. Given that the number of subjects in each treatment group of the ITT population and the treated population were similar, except for groups G (n = 12) and H (n = 10) in the treated group, one can assume that the demographic and baseline characteristics of both populations were similar as well.
The evaluable population included all subjects receiving ≥ 90% of their assigned dose of study drug and having both baseline and EOT arteriograms. Subjects receiving plasmin treatment with BOC must have had confirmed cannulation of the target vessel by post-baseline arteriography and successful BOC inflation.
The safety population included all subjects receiving any dose of study drug. An AE was defined as any untoward medical occurrence in a subject or clinical investigation subject administered a study drug that did not necessarily have a causal relationship with this study drug. A TEAE (treatment-emergent AE) was an AE that occurred either during drug administration or after drug administration for the entire study period, which was 30 days long after the drug was infused in this trial. An SAE (serious AE) was defined as any untoward medical occurrence that at any dose resulted in death, was life-threatening, required in-patient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, was a congenital anomaly or birth defect, or was an important medical event.
The protocol and informed consent of this study were approved by the Institutional Review Board and the ethics committees of all participating centers. All patients or their legally authorized representatives provided written informed consent. ClinicalTrials.gov identifier: NCT01222117.
All safety and efficacy analyses provided descriptive statistics. Post-hoc analyses (i.e. Fisher Exact test) were performed to calculate p-values for the most salient data. Statistical analyses and data calculations were performed using SAS version 8.2.
Results
Two hundred and thirty-five patients with aPAO were screened (). Sixty-seven subjects were screen failures; 174 patients were randomized into the study from 43 study centers in 12 countries (Belgium, n = 15; Bulgaria, n = 5; Czech Republic, n = 45; Germany, n = 3; India, n = 4; Peru, n = 4; Poland, n = 5; Romania, n = 3; Serbia, n = 43; Slovakia, n = 40; Ukraine, n = 2; US, n = 5). Study completion rates (85–100%) varied among all groups. The subjects evaluable for efficacy comprised 151 of 174 (86%), and the safety population included 169 of 174 (97%). Demographic and baseline characteristics of the ITT population are summarized in .
Figure 2. Subject disposition. Abbreviations. BOC, balloon occlusion catheter; rtPA, recombinant tissue plasminogen activator. Study was conducted from December 9, 2010 through September 3, 2014 in 43 study centers located in 12 countries (Belgium, Bulgaria, Czech Republic, Germany, India, Peru, Poland, Romania, Serbia, Slovakia, Ukraine, and the US). A total of 174 subjects were randomized into the study; 67 subjects were screen failures. There were 107 subjects who received 150 mg plasmin without BOC (treatment groups A–D, G, and H), 42 subjects received 150 mg plasmin with BOC (treatment groups I and J), six subjects received 250 mg plasmin with BOC (treatment group M), nine subjects in the rtPA treatment group E, and 10 subjects in the placebo control treatment group F. aOne subject in placebo control group F was not dosed.
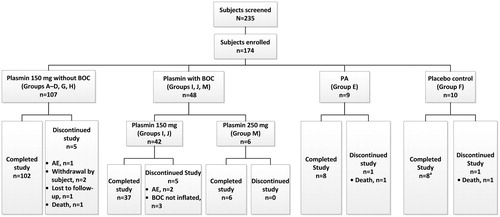
Table 2. Demographics of intent-to-treat population.
Efficacy
Overall, 59% treated with 150 mg plasmin achieved the primary efficacy endpoint (>50% thrombolysis at end of treatment) without BOC (58 of 99) and with BOC (17 of 29), in contrast to 89% (8 of 9) receiving rtPA (p = 0.149) and 40% (2 of 5) receiving placebo control (p = 0.648) (, ). Thirty-three per cent (2 of 6) of the group treated with 250 mg plasmin with BOC achieved >50% thrombolysis. BOC did not improve efficacy (59% [58 of 99] without BOC vs 59% [17 of 29] with BOC, p > 0.999). Of note, 19% (4 of 21) of plasmin group C (5-h infusion at 30 mL/h without BOC) and 13% (2 of 15) of plasmin group I (5-h infusion at 35 mL/h with BOC) demonstrated >90% thrombolysis. Eighty-one per cent (17 of 21) of plasmin group C achieved >50% thrombolysis, in contrast to 40% (2 of 5) in the placebo control group. The results support an infusion rate of 30 mL/h and longer infusion duration (5 h) in administering plasmin for the treatment of aPAO.
Figure 3. Percentage of subjects who achieved greater than 50% thrombolysis. p-values indicate comparisons of the combined ABCDGH group (plasmin-treated) vs group E (rtPA-treated) and the combined ABCDGH group (plasmin-treated) vs group F (placebo control).
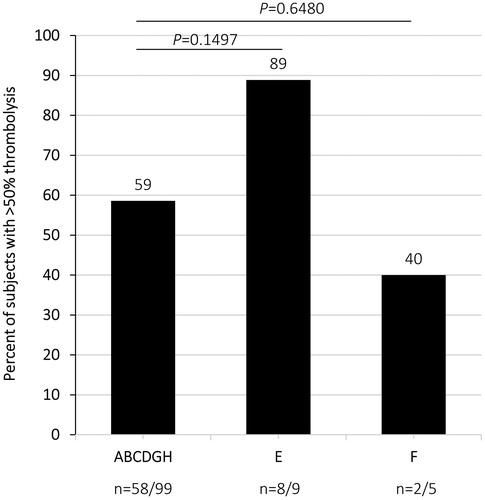
Table 3. Thrombolysis (primary efficacy outcome) at end of treatment compared to baseline (evaluable population).
The rtPA dose administered to group E was based on the clinical discretion of the investigators. Analysis of rtPA dose data indicated that four subjects in group E received almost the full physician-prescribed dose within 5 h, which was a much shorter duration than the anticipated 24- to 48-h infusionCitation12. Sixty-seven per cent (6 of 9) of rtPA-treated subjects achieved >90% thrombolysis after 5 h of infusion ().
An ad hoc analysis correlating vessel patency and thrombolysis at EOT was performed in the 150 mg plasmin groups, using thrombolysis and patency assessments of the investigators. The frequency of vessel patency was similar in groups without and with BOC (41%, 41 of 100 vs 42%, 16 of 38, respectively), suggesting that BOC does not provide additional benefit. In the 250 mg plasmin group with BOC, 33% (2 of 6) achieved patency. Overall, 93% (38 of 41) of subjects who had patency restored achieved >50% thrombolysis. In the 150 mg plasmin groups without BOC, 66% (66 of 100) achieved >50% thrombolysis. Of those 66 subjects achieving >50% thrombolysis, 58% (38) achieved patency. In comparison, 34% (34 of 100) achieved ≤50% thrombolysis, and only 9% (3 of 34) regained patency. Results in rtPA treatment group E were consistent with observations in the plasmin groups. All six subjects (100%) in rtPA group E, who regained patency, had >50% thrombolysis. In the placebo group F, one subject had a patent vessel at EOT but did not achieve >50% thrombolysis. These results suggest that the subjects who achieved >50% thrombolysis at EOT were more likely to regain patency.
Safety
One hundred and forty-five subjects were treated with 150 mg plasmin; six subjects were treated with 250 mg plasmin. Safety was evaluated through analyses of major and minor bleeding events and deaths. AEs and SAEs are listed in . Overall, plasmin demonstrated less bleeding during catheter-directed administration at 150 mg and 250 mg doses compared to rtPA. Major bleeding occurred in seven patients receiving plasmin (4.6%, 7 of 151), one in the rtPA group (11.1%, 1 of 9, p = 0.377 compared to the plasmin-treated group) and one in the control group (11.1%, 1 of 9, p = 0.377 compared to the plasmin-treated group). There was one death in the plasmin group (0.7%, 1 of 151), one death in the rtPA group (11.1%, 1 of 9, p = 0.110 compared to the plasmin-treated group) and one death in the control group (11.1%, 1/9, p = 0.110 compared to the plasmin-treated group). Minor bleeding occurred in 18.5% (28/151) of the plasmin group, 33% of the rtPA group (3/9, p = 0.3776 compared to the plasmin-treated group) and 33% of the control group (3/9, p = 0.3776 compared to the plasmin-treated group). There were no unexpected safety concerns. Ninety-four per cent received ≥ 90% of the planned plasmin dose. All 38 subjects who received 150 mg plasmin with BOC and six subjects who received 250 mg plasmin with BOC received their entire dose. The TEAE and SAE profiles were similar across all plasmin groups (, Supplementary Tables S2 and S3). There was no difference in TEAE profiles based on plasmin groups or doses, without or with BOC (Supplementary Table S2). The proportion of subjects with adverse drug reactions (ADR, potentially drug-related AEs) in the 150 mg plasmin group without BOC (14%, 15 of 107) compared favorably to those in the rtPA treatment group E (22%, 2 of 9) (). The pattern of TEAEs was diverse and most occurred in no more than one subject. Most of the TEAEs were of mild or moderate severity. Several TEAEs observed in 150 mg plasmin-treated subjects with vascular disorders (with or without BOC) were severe, but were not unexpected due to the underlying disease. There were no severe TEAEs in the 250 mg plasmin group with BOC. The overall SAE frequencies by subject in the 150 mg plasmin groups were similar at 29% (31 of 107) without BOC and at 24% (9 of 38) with BOC, compared to 22% (2 of 9) in the rtPA group E. (). The placebo control group F had a high frequency of SAEs (56%, 5 of 9), while no SAEs occurred in the 250 mg plasmin group with BOC. Furthermore, there was no unexpected SAE (Supplementary Table S3) in the 150 mg plasmin groups. According to the investigators, TEAEs and SAEs in the plasmin groups were mainly attributed to interventions for ischemia or existing comorbidities.
Table 4. Overall summary of treatment-emergent adverse events (safety population).
The death rate was lower in the 150 mg plasmin groups without BOC (0.93%, 1 of 107) than in the rtPA group E (11%, 1 of 9) and placebo control group F (11%, 1 of 9) (). The one death reported from the plasmin groups (acute respiratory distress syndrome) was considered not related to plasmin. None in the plasmin groups with BOC died.
Fifty-six per cent (5 of 9) of the placebo group F discontinued the study drug prematurely (); 80% of those discontinuations were due to ongoing ischemia during the infusion. In contrast, 6% (6 of 107) of subjects receiving plasmin without BOC, including three peripheral embolism, one peripheral ischemia, one anastomotic hemorrhage, one infusion-related reaction (pain), and none with BOC discontinued study drug. The rate of discontinuation in rtPA-treated subjects was 11% (1 of 9) due to a hematoma.
Plasmin appeared to have a better safety profile than rtPA with respect to bleeding (). The rtPA group E had a higher rate (44%, 4 of 9) of bleeding events relative to the 150 mg plasmin groups without BOC (21%, 22 of 107) or with BOC (21%, 8 of 38) and the 250 mg plasmin group with BOC (33%, 2 of 6). Of note, there was no major difference in bleeding events across the plasmin groups. In the 150 mg plasmin groups without BOC, one of the five major bleeding events and one of the 21 minor bleeding events were attributed by the investigator as related to plasmin. Ten bleeding events (two major and eight minor events) occurred in the 150 mg plasmin groups with BOC, and two minor bleeding events occurred in the 250 mg plasmin group with BOC. In the rtPA group E, there were three minor bleeding events and one major bleeding event resulting in death.
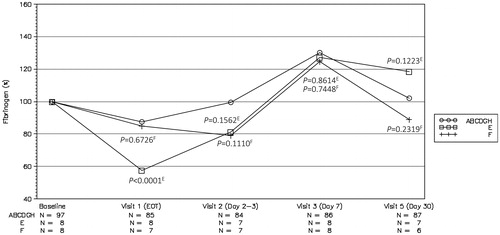
Treatment with rtPA led to a major drop in A2AP and fibrinogen () levels at EOT, to 22% and 13% of baseline, respectively. These changes likely contributed to the bleeding events in the rtPA treatment group. The drops in A2AP (43%) and fibrinogen (57%) by plasmin were less than rtPA.
Figure 4. Plasma fibrinogen concentrations normalized to baseline value across all visits in plasmin-treated groups without balloon occlusion catheter. p-values indicate comparisons of the combined ABCDGH group (plasmin-treated, circle symbol) vs group E (rtPA-treated, square symbol) and the combined ABCDGH group (plasmin-treated, circle symbol) vs group F (placebo control, cross symbol). The superscript letter on each p-value indicates the group to which that p-value belongs.
Review of the laboratory values raised no significant safety concerns. Immunogenicity testing at baseline and at day 30 were done in 141 plasmin-treated subjects. All but one subject in treatment group J (with BOC) were negative for plasmin antibody. For the subject who tested positive, additional testing revealed a low plasmin antibody titer with no measurable neutralizing antibody activity against plasmin. Plasmin-treated subjects were also monitored for potential transmission of HAV, HBV, HCV, HIV, or B19V. No treatment-emergent viral seroconversion was detected by viral nucleic acid testing or viral serology testing.
Discussion
The pursuit of safer and potentially more effective thrombolytic agents for use in patients with aPAO is a worthy goal. Randomized trial data have shown that catheter-based delivery of thrombolytic agents results in improved amputation-free survival in patients presenting with non-embolic aPAOCitation8,Citation11. The concern has always been the risk of major and distant bleeding, which appears to be associated with a systemic coagulopathy induced by PAs.
Safety results revealed plasmin was safe and well tolerated at the 150 and 250 mg doses, with an improved clinical safety profile compared to rtPA. There were no unexpected safety concerns.
Hematologic parameters, such as A2AP and fibrinogen, were less affected by plasmin than rtPA, which are likely related to its safety profile.
The delivery of plasmin using a BOC did not appear to affect outcome. Since plasmin has great avidity for binding to fibrin with intrathrombus delivery, the presence or absence of distal balloon occlusion of the infusion catheter seems inconsequential. It was possible that some leakage may have occurred around the balloon and that optimal positioning of the BOC may not have been achieved consistently due to variations in the locations of the thrombi. Depending on where a particular thrombus was located, the study drug may have been drawn away by collateral vessels. It became apparent during this study that the fixed infusion lengths of available drug delivery catheters were a major disadvantage, as their delivery segments were either too short or too long to treat existing thrombi. If drug delivery was more precise, one might expect more effective lysis over a shorter period of time with less drug, since all of the drug would be delivered to the thrombus, and the entire length of the thrombus would be treated from the onset of infusion.
Results from this study suggest that plasmin has a lower risk of bleeding events relative to rtPA treatment. Although the overall occurrences of TEAEs and SAEs in the rtPA group were comparable to the plasmin groups, subjects receiving rtPA had a higher incidence of bleeding events, with a rate that is consistent with published data for alfimeprase (78%)Citation13. Treatment with rtPA led to decreases in A2AP and fibrinogen levels at EOT, which likely predisposed to the bleeding events observed in the rtPA treatment group. Circulating plasmin inhibitors A2AP and alpha-2-macroglobulin are present in sufficient quantity to neutralize plasmin which escapes into the bloodstream. Unbound plasmin entering the systemic circulation during IA infusion is rapidly inhibitedCitation5,Citation6,Citation14. This prevents plasmin from lysing hemostatic thrombi elsewhere in the body. Previous phase 1 and phase 2 studies indicated that plasmin has an acceptable safety profile, particularly pertaining to bleedingCitation11,Citation15,Citation16. In contrast, rtPA acts indirectly by activation of fibrin bound plasminogen, which generates plasmin, the active enzyme that dissolves the thrombus. Plasminogen activators that escape from the clot during IA infusion can be systemically active and are known to dissolve pathologic and hemostatic thrombi in remote locations, causing remote site bleedingCitation5,Citation9,Citation13,Citation17–19. Intracranial hemorrhage is a major concern with rtPA, occurring in 1–2% of aPAO patientsCitation17,Citation20.
The catheter-directed TOPAS trial, examining recombinant urokinase (rUK) at three different doses, reported that complete lysis after 23 h of infusion was achieved in 67% (2000 IU/min), 71% (4000 IU/min), and 60% (6000 IU/min) of subjects, respectivelyCitation18. After 4 h of rUK infusion, complete clot lysis occurred in 11% (2000 IU/min), 22% (4000 IU/min), and 20% (6000 IU/min) of subjects, respectively. In the current study, the percentage of subjects achieving clinically relevant levels of thrombolysis with plasmin was comparable to the TOPAS study. Specifically, 19% of subjects in plasmin group C (5-h infusion at 30 mL/h without BOC) and 13% of subjects in plasmin group I (5-h infusion at 35 mL/h with BOC) demonstrated > 90% thrombolysis.
The investigators of this study prescribed rtPA dose for subjects in group E based on their clinical discretion; 67% of rtPA subjects achieved > 90% thrombolysis after 5 h of infusion. These results are comparable to the TOPAS study, where 60–71% of subjects achieved > 90% thrombolysis after 24–48 h of infusionCitation18.
An issue that may require further consideration is plasmin pH at the site of the thrombus. A neutral pH is required for plasmin activation; however, the enzyme is acidified to a pH of 3–4 during the manufacturing stages to prevent autodegradation. It is possible that the buffering capacity was not consistently sufficient in the microenvironment where plasmin was delivered. It would have been informative to test the delivery of plasmin in a neutralization buffer to determine whether a higher pH could improve thrombolytic activity. Without these data, we can only speculate that plasmin was at an effective pH for the cases that achieved thrombolysis.
Limitations
This study had several limitations. Although p-values from post hoc analyses were provided for some of the data, it should be noted that the sample size in each treatment arm was too small to determine whether any of the treatment and/or delivery methods substantially improved thrombolysis. For the placebo group, the Steering Committee of this study felt the small size of this group was justified on the basis that an invasive procedure was being performed with infusion of a placebo solution in patients at high risk of ongoing severe morbidity. Within each treatment arm, native vessels and grafts were allowed, which meant that the volume and position of the clot within the vessel might have varied from subject to subject. Even with the inclusion criteria that all patients entered were required to have aPAO symptoms for ≤ 14 days, non-occlusive thrombus often exists prior to vessel occlusion (and onset of symptoms). Since non-occlusive thrombus is frequently asymptomatic, it could have been a confounder. In addition, it is also unknown to what extent residual clot vs atherosclerotic plaque in native arteries contribute to vessel obstruction or re-occlusion.
The imprecise delivery of plasmin along the length of the clot may have compromised its efficacy (e.g. escape of plasmin outside of the clot or untreated portions of the clot). Precise delivery of plasmin into the entire thrombus, perhaps aided by pulsatile infusion, likely would improve results of plasmin while taking advantage of its safety profile. Atherosclerotic plaque cannot be dissolved by thrombolytic agents; therefore, the proportion of thrombolysis may be under-estimated by the presence of such plaque. The rtPA comparator group was confounded by the differences in treatment practices (e.g. dose and duration) across multinational study centers.
Conclusion
The results of this study indicate a favorable safety profile of plasmin at 150 and 250 mg doses relative to rtPA treatment, suggesting a potential benefit for plasmin in aPAO. Plasmin appeared to have a lower risk of bleeding events compared to rtPA and potentially a lower frequency of potentially drug-related AEs. Although the optimal delivery method for plasmin has not been determined, the best result (81% in plasmin group C) was observed with an infusion rate of 30 mL/h over 5 h. Adding BOC did not improve thrombolytic efficacy of plasmin, and increasing the dose from 150 to 250 mg did not improve outcomes. Because of the thrombolytic potential of plasmin and its promising safety profile, further optimization of plasmin treatment is warranted.
Transparency
Declaration of funding
This study was funded by Grifols, the sponsor of this clinical trial. The sponsor (Grifols) participated in study design, data collection, analysis, interpretation, and manuscript preparation.
Declaration of financial/other interests
Anthony J. Comerota has been paid a consulting fee for responsibility as the principal investigator and has received funding as a study site (Jobst Vascular Institute). Lazar Davidovic has received funding as a study site (University of Belgrade, Clinic for Vascular and Endovascular Surgery, Serbian Clinical Center). Richard Shlansky-Goldberg has been paid a consulting fee for responsibility as a steering committee member. Kim Hanna and Kecia L. Courtney are employees of Grifols, which is a manufacturer of plasmin. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (55.2 KB)Acknowledgments
The authors thank Tam M. Nguyen-Cao, PhD, CMPP of Grifols for her expert manuscript review and medical writing services under the direction of the authors. We acknowledge Dr Victor Marder (deceased) of University of California, Los Angeles for his contributions to the design of this study protocol; he was a steering committee member. We acknowledge Junliang Chen, PhD of Grifols for his assistance with the post hoc statistical analyses in this study. We also thank the following investigators for their participation in this trial: Juan F. Bautista of Hospital Nacional Guillermo Almenara Irigoyen-EsSalud, Peru; Sumit Bhatla of Remington Davis, Inc., USA; Miroslav Bulvas of University Hospital Kralovske Vinohrady, Czech Republic; John Hoch of University of Wisconsin, USA; Adrian Iancu of Institutul Inimii de Urgenta Pentru Boli Cardiovasculare, Romania; Dusan Kucera of Vaskularni centrum Vitkovicka nemocnice, Czech Republic; Patrick Lauwers of Universitair Ziekenhuis Antwerpen, Belgium; Lubomir Spak of East Slovakian Institute of Cardiovascular Diseases, Slovakia; Geert Maleux of Universitair Ziekenhuis Leuven, Belgium; Dragoslav Nenezic of Institut za kardiovaskularne bolesti Dedinje, Serbia; Przemyslaw Nowakowski of KS American Heart of Poland Sp. z o.o w Chrzanowie; Rajiv Parakha of Medanta – The Medicity, India; Vladan Popovic of Klinički Centar Vojvodine, Serbia; Dierk Scheinert of University Hospital Leipzig, Germany; Ivan Vulev of The National Institute of Cardiovascular Diseases, Slovakia; Jean-Claude Wautrecht of Hopital Erasme, Belgium; Fred Weaver of University of Southern California, USA; Norbert Weiss of Universitätsklinikum Carl Gustav Carus Dresden, Germany. The authors would like to acknowledge the participation of the following institutions: Amrita Institute of Medical Science & Research, India; Boston Medical Center, USA; Fakultná nemocnica Trenčín, Slovakia; Greenville Memorial Hospital- Vascular Health Alliance, USA, Hospital Nacional Edgardo Rebagliati Martins, Peru; Institute of General and Emergency Surgery NAMS of Ukraine, Ukraine; Institutul de Boli Cardiovasculare “Prof. Dr. George I. M. Georgescu,” Romania; Institutul de Urgenta pentru Boli Cardiovasculare “Prof. Dr. C.C. Iliescu,” Romania; Klinički Centar Niš - Klinika za vaskularnu hirurgiju, Serbia; Life Care Institute of Medical Sciences and Research, India; Samodzielny Publiczny Szpital Kliniczny Nr 2 PUM, Poland; МБаи National Cardiology Hospital, Bulgaria; Sterling Hospital, India; Wojewodzki Szpital Specjalistyczny nr 4 SUM, Poland.
References
- Working Party on Thrombolysis in the Management of Limb I. Thrombolysis in the management of lower limb peripheral arterial occlusion–a consensus document. J Vasc Interv Radiol. 2003;14:S337–S349.
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980;43:77–89.
- Wiman B, Lijnen HR, Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979;579:142–154.
- Cederholm-Williams SA, De Cock F, Lijnen HR, et al. Kinetics of the reactions between streptokinase, plasmin and alpha 2-antiplasmin. Eur J Biochem. 1979;100:125–132.
- Marder VJ, Landskroner K, Novokhatny V, et al. Plasmin induces local thrombolysis without causing hemorrhage: a comparison with tissue plasminogen activator in the rabbit. Thromb Haemost. 2001;86:739–745.
- Stewart D, Kong M, Novokhatny V, et al. Distinct dose-dependent effects of plasmin and TPA on coagulation and hemorrhage. Blood. 2003;101:3002–3007.
- Marder VJ, Novokhatny V. Direct fibrinolytic agents: biochemical attributes, preclinical foundation and clinical potential. J Thromb Haemost. 2010;8:433–444.
- The STILE Investigators. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. Ann Surg. 1994;220(3):251–266.
- Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–1111.
- Berridge DC, Kessel DO, Robertson I. Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database of Systematic Reviews. 2013, 6. Art. No.: CD002784. DOI: 10.1002/14651858.CD002784.pub2
- Marder VJ, Comerota AJ, Shlansky-Goldberg RD, et al. Safety of catheter-delivered plasmin in patients with acute lower extremity arterial or bypass graft occlusion: phase I results. J Thromb Haemost. 2012;10:985–991.
- Patel NH, Krishnamurthy VN, Kim S, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24:3–15.
- Han SM, Weaver FA, Comerota AJ, et al. Efficacy and safety of alfimeprase in patients with acute peripheral arterial occlusion (PAO). J Vasc Surg. 2010;51:600–609.
- Novokhatny V, Taylor K, Zimmerman TP. Thrombolytic potency of acid-stabilized plasmin: superiority over tissue-type plasminogen activator in an in vitro model of catheter-assisted thrombolysis. J Thromb Haemost. 2003;1:1034–1041.
- Shlansky-Goldberg RD, Matsumoto AH, Baumbach GA, et al. A first-in-human phase I trial of locally delivered human plasmin for hemodialysis graft occlusion. J Thromb Haemost. 2008;6:944–950.
- Shlansky-Goldberg R. Phase 1 study of human plasma-derived plasmin (TAL-05-00018) in hemodialysis graft occlusion. Thromb Res. 2008;122:S16–S19.
- Ouriel K, Shortell CK, DeWeese JA, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–1030.
- Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23:64–73.
- Ouriel K, Kandarpa K. Safety of thrombolytic therapy with urokinase or recombinant tissue plasminogen activator for peripheral arterial occlusion: a comprehensive compilation of published work. J Endovasc Ther. 2004;11:436–446.
- Ouriel K, Gray B, Clair DG, et al. Complications associated with the use of urokinase and recombinant tissue plasminogen activator for catheter-directed peripheral arterial and venous thrombolysis. J Vasc Interv Radiol. 2000;11:295–298.
