Abstract
Objective: The aim of this survey conducted by 20 leading Spanish oncologists was to analyze the concurrence between Spanish clinical practice and the recently published definition of the optimal sequence for the systemic treatment of metastatic breast cancer (MBC) according to patient profiles.
Methods: A self-administered questionnaire was developed, divided into five sections comprising 34 specific questions related to sequential treatments, plus three additional general questions. Respondents were asked to justify negative answers. Participants were recruited randomly by invitation out of a total of 619 oncologists. The questionnaire was sent and collected via e-mail between October 2015 and May 2016. A total of 191 completed questionnaires were received.
Results: Overall, 70% of oncologists would keep the three patient profiles exactly as proposed (hormone receptor-positive and HER2-negative, HER2-positive, and triple negative breast cancer). Affirmative answers to questions regarding treatment sequences for these patient profiles (1–34) ranged from 77.8–99.5%, with an average of 90.9% of oncologists being in agreement with the recommended sequential treatments. The lowest degree of consensus was observed for endocrine treatments in pre-menopausal women and for chemotherapy options in hormone-resistant patients, whilst the highest degree of consensus was reached for targeted therapies in HER2-positive patients and for endocrine therapy in post-menopausal women. In their comments, participants revealed a number of economic constraints that prevented them from implementing some of the best treatment options.
Conclusions: In conclusion, despite the complexity of MBC treatment, there is general agreement on the optimal treatment sequences.
Introduction
The incidence of breast cancer in Spain is around 27,700 cases per yearCitation1. Metastatic breast cancer (MBC) may present in several forms associated with various prognosesCitation2, and the main goal of treatment is to increase survival while minimizing drug toxicity. The systemic treatment of MBC is a complex issue involving different factors related to patient and tumor characteristics. In addition to intrinsic tumor features (e.g. hormone receptor and human epidermal growth factor receptor 2 [HER2 receptor] status), the patient’s characteristics (e.g. age and menopausal status) need to be considered when choosing a therapy. Moreover, there are different patient profiles, some with their corresponding sub-types, together with an overwhelming plethora of therapeutic options. All these factors complicate decision-making and generate considerable variability in clinical practice.
Several clinical guidelines are available for MBCCitation3–9, but recommendations and optimal treatment sequences are still not clearly standardizedCitation10, and there is a pressing need for optimal and homogeneous treatment sequences. MBC treatment is an evolving field in which new drugs and therapies are emerging, and therefore oncologists usually combine solid evidence from clinical trials with their personal experience when choosing the best therapy.
To deal with this complexity, 20 Spanish breast cancer oncologists defined MBC patient profiles over the course of several meetings held in 2015, and reached a degree of consensus on the different sub-groups of patients with similar characteristics and specific treatment recommendations for each sub-group. A document describing their working criteria has been recently publishedCitation11. Four patient profiles were defined: the pre-menopausal hormone receptor-positive sub-type; the post-menopausal hormone receptor-positive sub-type; the triple-negative sub-type; and the HER2-positive sub-type. This consensus document detailed a treatment sequence for each profile. Pre- and post-menopausal patients with hormone receptor-positive tumors would mostly benefit from hormonal therapy with tamoxifen, aromatase inhibitors, and fulvestrant, including mTOR inhibitors for post-menopausal patients. Thus, all these patients could be grouped as a hormone receptor-positive and HER2-negative sub-type. In the case of triple negative patients, the recommended chemotherapy sequence would be first-line paclitaxel, with or without bevacizumab, or a combination based on platinum compounds or a combination of capecitabine and vinorelbine, followed by eribulin or nab-paclitaxel as second line, and liposomal anthracycline and carboplatin combined with gemcitabine as the third-line options. For patients with HER2-positive disease, the combination of taxane with dual anti-HER2 blockade with trastuzumab and pertuzumab would be the recommended option. In these patients, vinorelbine could be useful when taxanes are contraindicated. Finally, in patients with hormone receptors positives, the recommended chemotherapy sequence included first-line paclitaxel, with or without bevacizumab, or vinorelbine or capecitabine in the case of prior treatment with anthracyclines and taxanes. This should be followed by second-line oral cytotoxics if these have not already been administered, or nab-paclitaxel, eribulin, or liposomal anthracyclines. As third-line treatment, all these options, including metronomic schemes, were included.
After these profiles and recommended treatments had been agreed as guidelines, this study was launched to determine to what extent they reflected clinical practice in the Spanish medical community. Accordingly, we report here the results of a questionnaire designed to determine the opinion and practices of Spanish oncologists with respect to MBC therapy.
Methods
A self-administered questionnaire was developed by a working group of 20 Spanish breast cancer oncologists on the basis of the previously published theoretical patient profiles and optimal sequencesCitation11. Three MBC profiles were outlined: patients with hormone receptor positive disease; patients with HER2-positive disease, and patients with triple-negative cancer. Treatment guidelines were defined for each profile. The aim and design of the questionnaire were discussed in person, medical databases were searched, and questionnaire items were drafted after reaching a consensus. The questionnaire included a total of 37 items, divided into five sections (Supplementary Appendix 1): endocrine therapy in patients with hormone receptor-positive tumors; chemotherapy in patients with hormone receptor-positive tumors; therapy in patients with HER2-positive disease; therapy in patients with triple-negative cancer; and additional questions. The questionnaire and a cover letter containing details of the study were sent to participants. For clarification, a summary of the treatment guidelines was provided along with the questionnaire, so that participants were aware of the available options at the time of the survey.
The sample size was estimated considering an acceptable confidence level of 95%, an accuracy of 0.06, and a ratio of p = 0.5 of professionals who used a specific treatment. The minimum sample size required was n = 187 respondents. Accepting an estimated loss of 10%, 207 respondents were needed. The sample was stratified by autonomous region. Among 619 oncologists initially considered, 191 were polled. Participants were recruited at random, by invitation only, and remained anonymous. They were asked to base their answers on the ideal treatment strategy they would use in their patients, regardless of practical limitations. Consent to participate was indicated by the completion and return of the questionnaire. All answers were entered into a computerized database, and data were analyzed by an independent investigator who was unrelated to the data collection. Any questions that were left blank or had two answers were considered invalid. Regarding the final three additional questions, failure to answer was taken to indicate no comment or agreement with the statement. The survey was conducted between October 2015 and May 2016. Descriptive statistics were used to test the variables.
Results
General characteristics of the participants
In total, 191 specialists responded to the questionnaire; 72 (37.7%) were male (). Out of 163 oncologists who provided that information, 84 and 79 were general oncologists and specialized oncologists in breast cancer, respectively. Twenty-eight oncologists did not specify their role within the unit and, consequently, were classified as “unknown”. Distribution by autonomous region was as follows: Andalusia 9; Aragón 9; Cantabria 2; Castile la Mancha 8; Castile and León 8; Catalonia 42; Valencia 32; Extremadura 3; Galicia 8; Balearics 3; Canary Islands 4; Madrid 49; Murcia 5; Navarra 2; and Basque Country 7. Out of 186 oncologists, 24 were resident doctors and 142 were specialists; nine respondents were unit heads and 11 were departmental heads. Overall, 162 participants answered all the questions, 19 left one question unanswered, and seven left between two and eight questions unanswered.
Table 1. Characteristics of participants (n = 191).
Responses by patient profile
Hormonal therapy in patients with hormone receptor-positive and HER2-negative tumors
As shown in , for question 1, in the case of pre-menopausal patients, 89.9% of respondents agreed with the use of ovarian ablation along with either tamoxifen or an aromatase inhibitor as first-line therapy for patients with de novo metastasis or with disease-free survival (DFS) of 12 months or more. Of those who considered other options, 10 (5.3%) said they would use only ovarian ablation with tamoxifen, and 3 (1.6%) would use ovarian ablation with an aromatase inhibitor. As second-line hormonal treatment in pre-menopausal patients, or for patients with DFS of less than 12 months after treatment, 77.8% said they would use ovarian ablation combined with either an aromatase inhibitor, fulvestrant, or tamoxifen. Those who disagreed preferred the following therapeutic alternatives: ovarian ablation with fulvestrant or an aromatase inhibitor, but not with tamoxifen (3.2%), only ovarian ablation combined with an aromatase inhibitor (4.9%), only ovarian ablation together with fulvestrant (1.6%), ovarian ablation together with exemestane plus everolimus (1.6%), or chemotherapy (2.7%). As third-line treatment in pre-menopausal patients, 87.3% agreed with using ovarian ablation and fulvestrant. For those disagreeing, eight (4.2%) would use only fulvestrant, and four (2.1%) would use exemestane combined with everolimus.
Figure 1. Percentage of affirmative answers to questions 1–34 of the questionnaire. The red dotted line indicates the lowest percentage of affirmative answers in the questionnaire as a whole. The number of total answers is indicated for each question.
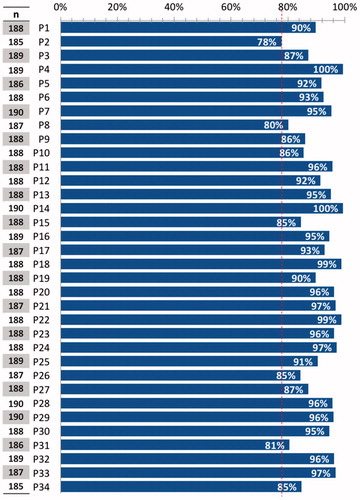
In the case of first-line hormonal therapy in post-menopausal patients, 99.5% of oncologists would use aromatase inhibitors (). In patients diagnosed with recurrence during adjuvant treatment with an aromatase inhibitor, or with DFS of less than 12 months, treatment with fulvestrant was recommended as first line by 91.9% of specialists. The five remaining experts (2.7%) would use tamoxifen. If the post-menopausal patient responded to first-line treatment with an aromatase inhibitor, 92.6% of specialists would use either fulvestrant or the combination of exemestane and everolimus as second-line therapy. Of those who gave other answers, nine oncologists (4.8%) would use only fulvestrant. In third-line endocrine therapy in post-menopausal patients, 95.3% of respondents agreed with using exemestane combined with everolimus, if not used previously.
Table 2. Absolute frequencies and percentages of affirmative and negative answers.
Chemotherapy in patients with hormone receptor-positive and HER2-negative tumors
In the case of resistance to first-line hormonal therapy, 86.2% of oncologists would use either weekly paclitaxel plus bevacizumab, vinorelbine alone, capecitabine alone, or weekly paclitaxel plus sequential anthracyclines. Of respondents who used other options, six (3.2%) would use only weekly paclitaxel plus bevacizumab.
In the case of visceral crisis and resistance to first-line hormonal therapy, 80.2% of oncologists would use combined chemotherapy, such as weekly paclitaxel plus bevacizumab, or anthracyclines and taxanes (either sequentially or in combination), or paclitaxel plus gemcitabine. Of those with a different approach, four (2.1%) coincided in using weekly paclitaxel plus bevacizumab, or anthracyclines and taxanes but not paclitaxel plus gemcitabine, 11 (5.9%) would use only weekly paclitaxel plus bevacizumab, and six (3.2%) would use only anthracyclines and taxanes.
In the case of elderly patients or those in special situations and with resistance to hormonal therapy, 85.6% of oncologists would use metronomic regimens. Of those disagreeing, 10 (5.3%) stated that they would administer either capecitabine or vinorelbine.
As second-line therapy, 95.7% agreed with using either vinorelbine, capecitabine, nab-paclitaxel, eribulin, weekly paclitaxel, or liposomal anthracyclines. Furthermore, 91.5% of oncologists agreed with using metronomic regimens as second-line therapy in elderly patients or those with special situations.
As third line, 95.2% of oncologists would administer either vinorelbine, capecitabine, nab-paclitaxel, eribulin, weekly paclitaxel, liposomal anthracyclines, metronomic vinorelbine, or metronomic cyclophosphamide, according to previous treatments.
Patients with HER2-positive disease
Nearly all (99.5%) oncologists agreed with the recommendation to use taxanes with trastuzumab and pertuzumab as first-line treatment of de novo metastasis or relapse after the first year following the end of adjuvant treatment with trastuzumab.
If taxanes are contraindicated, or in elderly patients with a risk of taxane toxicity, 84.6% of respondents would use vinorelbine with trastuzumab and pertuzumab in first-line therapy. Among those disagreeing, 11 oncologists (5.9%) would use vinorelbine plus trastuzumab. In the case of relapse within 6 months of completing trastuzumab adjuvant treatment, 94.7% of respondents would use trastuzumab emtansine (T-DM1); six oncologists (3.2%) would use taxanes with trastuzumab plus pertuzumab instead.
In hormone receptor-positive patients who were not candidates for chemotherapy, the administration of endocrine therapy combined with trastuzumab or lapatinib as first line was approved by 93.0% of oncologists, and as second line by 96.3%. Of those considering other options as first line, five (2.7%) would use endocrine therapy plus trastuzumab, and of those considering other options as second line, three (1.6%) would use T-DM1.
As second-line therapy, 98.9% of oncologists would use T-DM1, and, in the case of patients with brain metastases, 89.9% would be in favor of administering T-DM1—as supported by the EMILIA studyCitation12—or the combination of capecitabine and lapatinib (both considered as optimal treatments). For this sub-group of patients, other experts would use T-DM1 (n = 10; 5.3%) or capecitabine plus trastuzumab (n = 8; 4.3%).
In patients previously receiving pertuzumab with trastuzumab and T-DM1, 96.8% of respondents would choose either the combination of trastuzumab with vinorelbine, capecitabine with lapatinib, or capecitabine with trastuzumab, and in the case of patients with hormone receptor-negative tumors, trastuzumab plus lapatinib as third line. Three of the remaining doctors (1.6%) would choose only capecitabine plus lapatinib, or trastuzumab plus lapatinib.
If the patient had not received T-DM1 previously, 98.9% of experts would administer it as third line. In hormone receptor-positive patients who were not candidates for chemotherapy, endocrine therapy plus lapatinib or trastuzumab as third line would be the treatment of choice for 96.3% of experts.
Patients with triple-negative breast cancer
For patients with de novo metastasis or relapse after more than 12 months of DFS, 97.3% of respondents agreed with using first-line weekly paclitaxel, either alone or combined with bevacizumab. Some who disagreed would include the use of platinum-based combinations, such as paclitaxel-carboplatin for patients with BRCA wild-type breast cancer (1%), chemotherapy alone (0.5%), or paclitaxel-gemcitabine (0.5%). When a switch is needed due to early toxicity or intolerance to paclitaxel, 90.5% of oncologists would use first-line capecitabine either alone or combined with bevacizumab. Those who disagreed would use adriamycin (0.5%), anthracycline (1.6%), carboplatin (1%), eribulin (1%), nab-paclitaxel (2.6%), or capecitabine in other combinations (1%), or would not use bevacizumab (0.5%).
To treat frail patients, or in patients wishing to avoid alopecia or who cannot or do not wish to receive intravenous chemotherapy, 84.5% of respondents would use first-line oral capecitabine and vinorelbine separately or in combination. Among those who disagreed, 19 (10.2%) would use capecitabine or vinorelbine in monotherapy. BRCA-positive patients or those with hereditary syndrome may receive a platinum salt in combination with gemcitabine or a taxane, or a taxane combined with bevacizumab, in the view of 87.2% of respondents. Six oncologists (3.2%) would use a platinum salt in combination with either gemcitabine or a taxane, and five (2.7%) would use only a platinum salt with a taxane. In the case of visceral crisis, 95.8% of respondents would administer concomitant or sequential polychemotherapy, with or without biological agents (defined as substances made from living organisms or their products and used in the prevention, diagnosis, or treatment of cancer and other diseases, including antibodies, interleukins, and vaccines), in first-line therapy; three experts (1.6%) would use monochemotherapy with a biological agent instead. In the second line, 91.1% of oncologists would use either capecitabine or vinorelbine, a combination of both, eribulin, or nab-paclitaxel. Six experts (3.2%) would not use vinorelbine in combination with capecitabine.
For the third line, 94.7% of experts would administer either capecitabine, vinorelbine, vinorelbine plus capecitabine, eribulin, nab-paclitaxel, liposomal anthracycline, or carboplatin with or without gemcitabine. Seven experts (3.7%) would only use these drugs in monotherapy. For patients with metastasis due to disease recurrence during the first 12 months after adjuvant treatment with taxanes and anthracyclines, 80.6% of experts would administer either capecitabine with or without bevacizumab, vinorelbine, or vinorelbine plus capecitabine. Seven (3.9%) would consider using carboplatin either alone or as doublet chemotherapy. Four others (2.2%) would only use capecitabine plus bevacizumab, and four more (2.2%) would administer paclitaxel plus bevacizumab. Four experts (2.2%) stated that they would not use vinorelbine in monotherapy, and six others (3.3%) said they would choose this option only.
Six respondents (3.3%) would not use the combination of vinorelbine plus capecitabine, but another three (1.7%) would prefer this option.
In the case of visceral crisis, 96.3% of respondents would administer concomitant or sequential polychemotherapy, with or without biological agents, as first-line therapy.
As second line, 96.8% of respondents would use eribulin or nab-paclitaxel. Those who disagreed would use adriamycin-docetaxel (1%), paclitaxel-bevacizumab (1%), or monotherapy alone (1%).
As third line, 84.9% of experts would use capecitabine with or without bevacizumab, vinorelbine, vinorelbine plus capecitabine, eribulin, nab-paclitaxel, liposomal anthracycline, or carboplatin with or without gemcitabine. Twelve other experts (6.5%) would not use bevacizumab, four others (2.2%) would not use combined chemotherapy, and a further three (1.6%) would not use bevacizumab or combined chemotherapy.
Additional questions
It is important to note that the inclusion of additional questions allowed us to collect comprehensive data on the protocols and therapies used in different hospitals. Only 60 experts declared they were free to prescribe any of the drugs referred to in the questionnaire; 33 oncologists said they could administer any drug as long as it was designated for on-label use. Seventy-one oncologists said they needed specific approval for off-label use of drugs, and for some of the most expensive therapies. In the case of private hospitals, six experts said that oral therapies were not covered by private insurance and, thus, could not be prescribed.
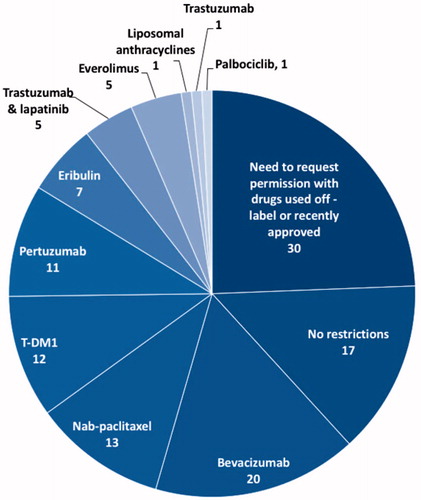
Participants mentioned as the most restricted drugs, in descending order, bevacizumab, nab-paclitaxel, T-DM1, pertuzumab, eribulin, everolimus, the combination of trastuzumab and lapatinib, liposomal anthracyclines, and drugs not approved at the time of this survey, such as palbociclib ().
Figure 2. Results of question 35. The number of participants stating that the use of some drugs was restricted in their hospital (n = 76), number with no restriction at all or need to request permission in particular cases (i.e. off-label use or recently approved drugs) (n = 115).
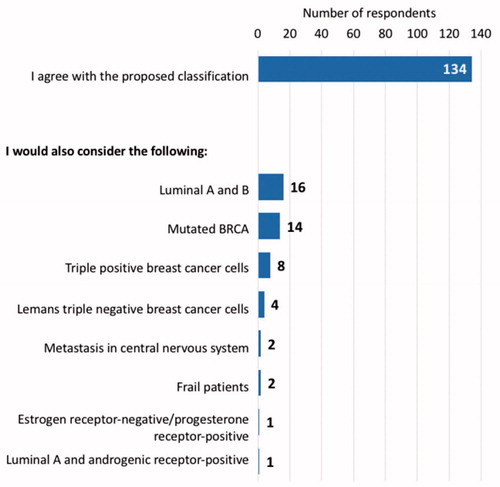
In general, 134 respondents agreed with the patient profiles as proposed in the questionnaire; 16 oncologists would split the hormone receptor-positive patient profile into two sub-groups: luminal A and luminal B; 14 others would include a sub-group of mutated BRCA patients; eight clinicians would consider a new category of triple-positive breast cancer patients (hormone receptors and HER2-positive); four others would classify triple-negative breast cancer following Leman’s classification; two experts would detail sub-groups of patients with metastasis to distant organs in the central nervous system within the three profiles; two experts would include a sub-group of frail patients; one respondent would include a sub-group of patients with estrogen hormone receptor-negative cells and progesterone hormone receptor-positive cells; and another would include a subgroup of luminal A plus androgenic receptor-positive cells ().
Figure 3. Results of question 36. The number of participants who agree with the present classification or who would modify or add new subgroups to the three patient profiles proposed.
Overall, 153 experts agreed with the questionnaire as it was, without further additions; 12 oncologists considered that it lacked reference to the use of anti-resorptive therapies; six others would have liked to find information on bone metastasis; four clinicians would have liked to see questions on maintenance therapy; four others thought it was also necessary to consider the positioning of new drugs, such as cyclin inhibitors; and two participants thought it should also have addressed pregnant patients, male patients, or further treatment after the third line.
Likewise, other respondents would have liked the questionnaire to properly address treatment selection according to patient preferences, quality-of-life, comorbidities and frailty, or novel pharmacoeconomic approaches.
Discussion
This is one of the first studies to assess the opinion of Spanish oncologists on MBC treatment and the strategies used in clinical practice. Despite the number of cytotoxic agents available to clinicians and the variety of patient- and disease-related parameters, the results of this survey show that data from clinical trials, scientific research, and international and national guidelines can be used to define optimal treatment sequences for MBC, and that those proposals are followed by most specialists (more than 90% on average).
Likewise, greater understanding of this type of cancer has identified distinctive sub-types based on tumor genomic signaturesCitation13 that may define several patient profiles. The three patient profiles proposed in the questionnaire were generally accepted, since 70% of respondents would not change the classification, and the remaining specialists would not reject that division, but would refine it further.
Second-line treatment for pre-menopausal patients and for those with DFS less than 12 months in the first-line setting showed the lowest degree of consensus (77.8%) in the whole questionnaire. Answers revealed that many oncologists would not use ovarian ablation with tamoxifen as second-line treatment because they would administer it as first line, as previously publishedCitation14,Citation15; nor would they use it as first line for those patients with DFS less than 12 months. These were considered by many of the respondents as hormonal therapy-resistant and should receive chemotherapy. However, a high degree of consensus was reached for first-line hormonal therapy in post-menopausal patients, in which nearly all experts would use aromatase inhibitors, in line with the literatureCitation16. However, many patients develop resistance to aromatase inhibitors. It has been suggested that the cyclin-D-cyclin-dependent kinase 4/6 (CDK4/6)–Rb pathway plays a role in resistance, and recent clinical trials are exploring CDK4/6 inhibitors as alternative therapies for these patients. In the MONALEESA study, the administration of ribociclib plus letrozole increased the duration of progression-free survival and the rate of myelosuppressionCitation17. In the FALCON trial that examined the effect of fulvestrant, a selective inhibitor that promotes degradation of the estrogen receptor, fulvestrant showed superior efficacy when compared to aromatase inhibitors, and may be advantageous in terms of resistance, as it does not directly affect estrogen levelsCitation18. The PALOMA 3 trialCitation19 showed that CDK4/6 inhibitor palbociclib treatment is associated with a median progression-free survival of 24.8 months, with a favorable safety profile. This is the first treatment to extend beyond the 2-year threshold in these patients. Consequently, these new treatments will likely change the recommendations in patients with hormone resistance.
In the case of patients with hormonal therapy resistance and visceral crisis, fewer respondents (80.2%) agreed on the choice of the best chemotherapy treatment in the first line, since some would avoid the use of combined chemotherapy with paclitaxel plus gemcitabine. However, in patients with hormonal therapy resistance without visceral crisis, it is interesting to note that 86.2% of oncologists would use either weekly paclitaxel plus bevacizumab or another of the drugs proposed in the survey. Additionally, six (3.2%) of the oncologists who disagreed with these options said that they would only use weekly paclitaxel plus bevacizumab. This is particularly important when considering that combination treatment is no longer recommended by the FDA (November 2011), as it has not been shown to provide any benefit in terms of delaying tumor growth that would justify its serious and potentially life-threatening risks, although the EMA still recommends this approach.
The questionnaire showed that, as previously publishedCitation20–22, the use of metronomic regimens in elderly patients or those in special situations or for further lines is a well-established practice among Spanish oncologists. The highest-level agreement regarding the optimal treatment sequence among oncologists was obtained in the case of HER2-positive MBC. The use of pertuzumab in combination with chemotherapy and trastuzumab as first-line treatment, and the use of T-DM1 for the second and third lines were unanimously acceptedCitation23–26.
Regarding triple-negative MBC, treatment options for patients with recurrence during the first 12 months after adjuvant treatment with taxanes and anthracyclines showed the greatest discrepancies among respondents. Some experts remarked that carboplatin was lacking among the options, as combination platinum regimens are often used in these patients, based on a higher rate of complete response that is also associated with greater myelosuppressionCitation27. Although these patients respond to taxanes and anthracyclines better than other sub-types of MBC, their prognosis remains poorCitation28,Citation29. Several ongoing clinical trials are exploring new therapeutic strategies including targeted therapy and immunotherapyCitation30.
Despite the advantages of oral therapy and its well-established indication, it has become apparent that coverage restrictions implemented by insurance companies can limit their use, as some participants pointed out. Additionally, Pharmacoeconomics is emerging as a relevant factor to consider in the systemic treatment of MBC, since healthcare budgetary restrictions may make the best therapy options unavailable in some cases.
This consensus study has the limitations inherent to questionnaires, namely, the bias introduced by the selection of a panel of experts and limiting the methodology to focus on particular expert evaluations. Another potential limitation involves seeking consensus in the Spanish Public Health system, which may prevent extrapolation of our findings to other settings. Due to the promising advances in therapeutic options explored in recent clinical trials, the proposed patient profiles and their associated treatment sequences should be regularly reviewed and updated as new data become available.
In conclusion, using a systematic approach in which 20 leading Spanish oncologists met on several occasions to define molecular phenotypes and specific therapies for MBC, we have been able to highlight and bring order to a complex field of medicine that needed to be defined. Contrary to expectations, a high degree of consensus on patient profiles and treatment sequences in clinical practice was achieved among Spanish oncologists. This reinforces the notion that the guidelines published by Mestres et al.Citation11 reflect the current approach to MBC treatment in clinical practice.
Transparency
Declaration of funding
This paper was not funded.
Declaration of financial/other relationships
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (38 KB)Acknowledgements
The authors would like to thank Francisco Javier García Verdejo and Natalia Luque Caro for their valuable comments while drafting this manuscript. The authors thank Almudena Fuster of Medical Statistics Consulting (Valencia, Spain), who provided editorial support.
References
- Galceran J, Ameijide A, Carulla M, et al. Cancer incidence in Spain, 2015. Clin Transl Oncol. 2017;19:799–825.
- Puig-Vives M, Sanchez MJ, Sanchez-Cantalejo J, et al. Distribution and prognosis of molecular breast cancer subtypes defined by immunohistochemical biomarkers in a Spanish population-based study. Gynecol Oncol. 2013;130:609–614.
- Alvarez Lopez I, de la Haba Rodriguez J, Ruiz Simon A, et al. SEOM clinical guidelines for the treatment of metastatic breast cancer. Clin Transl Oncol. 2010;12:719–723.
- Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. JCO. 2014;32:2255–2269.
- Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21:242–252.
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)dagger. Ann Oncol. 2014;25:1871–1888.
- Gavila J, Lopez-Tarruella S, Saura C, et al. SEOM clinical guidelines in metastatic breast cancer 2015. Clin Transl Oncol. 2015;17:946–955.
- GEICAM. Guía GEICAM de Práctica Clínica para el Diagnóstico y tratamiento del cáncer de mama metastásico Madrid2015 [updated 14/09/2016]. Available from: www.guiasalud.es/GPC/GPC_538_AF%20GUIA%20GEICAM_resumida.pdf
- Lin NU, Thomssen C, Cardoso F, et al. International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)–MBC Task Force: surveillance, staging, and evaluation of patients with early-stage and metastatic breast cancer. Breast. 2013;22:203–210.
- Zagouri F, Liakou P, Bartsch R, et al. Discrepancies between ESMO and NCCN breast cancer guidelines: an appraisal. Breast. 2015;24:513–523.
- Mestres JA, iMolins AB, Martinez LC, et al. Defining the optimal sequence for the systemic treatment of metastatic breast cancer. Clin Transl Oncol. 2017;19:149–161.
- Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26:113–119.
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752.
- Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641.
- Partridge AH, Pagani O, Abulkhair O, et al. First international consensus guidelines for breast cancer in young women (BCY1). Breast. 2014;23:209–220.
- Sini V, Cinieri S, Conte P, et al. Endocrine therapy in post-menopausal women with metastatic breast cancer: from literature and guidelines to clinical practice. Crit Rev Oncol Hematol. 2016;100:57–68.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;3;375:1738–1748.
- Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet (London, England). 2016;388:2997–3005.
- Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21:1165–1175.
- Addeo R, Sgambato A, Cennamo G, et al. Low-dose metronomic oral administration of vinorelbine in the first-line treatment of elderly patients with metastatic breast cancer. Clin Breast Cancer. 2010;10:301–306.
- Biganzoli L, Lichtman S, Michel JP, et al. Oral single-agent chemotherapy in older patients with solid tumours: a position paper from the International Society of Geriatric Oncology (SIOG). Eur J Cancer. 2015;51:2491–2500.
- Gebbia V, Boussen H, Valerio MR. Oral metronomic cyclophosphamide with and without methotrexate as palliative treatment for patients with metastatic breast carcinoma. Anticancer Res. 2012;32:529–536.
- Hernández-Blanquisett A, Touya D, Strasser-Weippl K, et al. Current and emerging therapies of HER2-positive metastatic breast cancer. Breast. 2016;29:170–177.
- Isakoff SJ, Baselga J. Trastuzumab-DM1: building a chemotherapy-free road in the treatment of human epidermal growth factor receptor 2-positive breast cancer. JCO. 2011;29:351–354.
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734.
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791.
- Byrski TG, Huzarski T, Marczyk E, et al. Annual conference on hereditary cancers 2011 szczecin, poland. 17-18 november 2011. Hereditary Cancer Clin Pract. 2012;10(Suppl 3):A1–A23.
- Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9:S73–S81.
- Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. JCO. 2010;28:3271–3277.
- Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer Basic Clin Res. 2016;10:25–36.
