Abstract
Purpose: The objective of this pilot study was to explore the feasibility of conducting a drug utilization study of lipegfilgrastim in Europe using medical records and to examine the pattern of lipegfilgrastim on-label and off-label use.
Methods: Data on lipegfilgrastim use between September 2014 and April 2017 were abstracted from medical records by two independent medical abstractors. Lipegfilgrastim indication was categorized either as on-label or as one of four types of off-label (I–IV) according to pre-defined criteria. An inter-rater reliability analysis was conducted to measure the degree of abstractor agreement for on-label and off-label use.
Results: Information from 46 medical records was abstracted. Lipegfilgrastim use during the first chemotherapy treatment cycle was mostly indicated for prevention of neutropenia (82.6% of patients). On-label use was documented in 42 patients (91.3%), while off-label use was documented in two patients (4.3%); all events of off-label use were attributed to use with non-cytotoxic drugs. The remaining two patients (4.3%) had missing data. Overall agreement between the abstractors was high (91.6%). For three types (Types I–III) of off-label use, the kappa values suggested a perfect agreement (κ = 1). For Type IV off-label use (use in patients treated with non-cytotoxic drugs), κ = 0, suggesting a poor agreement.
Conclusions: While recruitment was challenging, the results of this pilot study confirm the feasibility and availability of medical records and the use of pharmacists as abstractors to assess on- and off-label use of lipegfilgrastim. Lipegfilgrastim was mainly prescribed according to the approved indications.
Findings from this pilot study confirm the feasibility and availability of medical records and the use of pharmacists as abstractors to assess on-label and off-label use of lipegfilgrastim in routine clinical practice.
Lipegfilgrastim was mainly prescribed according to the approved indications, and the proportion of off-label use was low.
The high inter-rater agreement between the two abstractors suggests that one abstractor is sufficient for conducting chart abstraction of on- and off-label use.
Additional data abstraction sources other than pharmacists will need to be identified to improve response rate and center recruitment.
Findings from this pilot study are important for the successful planning and execution of subsequent drug utilization studies.
Key points
Introduction
Chemotherapy-induced neutropenia (CIN) is a common and serious complication of cancer myelosuppressive chemotherapy treatmentCitation1,Citation2. Life-threatening CIN complications, such as febrile neutropenia and infections, may limit the use of optimal chemotherapy dosing or treatment schedules. Yet, over the years, several granulocyte-colony stimulating factors (G-CSFs) have been introduced and used for reducing the duration and severity of neutropenia, as well as the incidence of febrile neutropenia (FN)Citation3–6. In Europe, several G-CSFs were approved, including filgrastim and pegfilgrastimCitation4,Citation6. In 2013, lipegfilgrastim1, a glycopegylated long-acting G-CSF, was approved for reduction in the duration of neutropenia and the incidence of FN in adult patients treated with cytotoxic chemotherapy for malignancy (with the exception of chronic myeloid leukemia and myelodysplastic syndromes [MDS])Citation7,Citation8.
While lipegfilgrastim and pegfilgrastim, both long-acting G-CSFs, are used in the management of CIN in cancer patients, filgrastim, a short-acting G-CSF, is approved for additional indications. These indications include reduction in the duration of neutropenia in patients undergoing myeloablative therapy followed by bone marrow transplantation; mobilization of peripheral blood progenitor cells; treatment of children or adult patients with severe congenital, cyclic, or idiopathic neutropenia; and treatment of persistent neutropenia in patients with advanced human immunodeficiency virus (HIV) infectionCitation8–10. Since all G-CSFs have a common mechanism of actionCitation11–14, there is the potential for off-label use of lipegfilgrastim for indications approved for other G-CSFs (e.g. filgrastim).
In the context of the regulatory approval for market authorization of lipegfilgrastim in the European Union (EU), the European Medicines Agency (EMA) requested a drug utilization study (DUS) as part of the pharmacovigilance plan to characterize the extent of off-label use of lipegfilgrastimCitation15. To address the challenges of implementing a study of off-label use in multiple countries with diverse healthcare systems and clinical practices, a pilot study was designed. The objective of this pilot study was to explore the feasibility of conducting a DUS of lipegfilgrastim using medical record review. In addition, this study examined the use of lipegfilgrastim in the EU after product approval and estimated the rate of off-label use. This investigation offered the opportunity to assess access to medical records by pharmacists, data availability in various centers in the EU, and reliability of the data abstractor and the questionnaire as instruments for ascertaining off-label use.
Methods
This pilot study of retrospective data abstraction from patient medical records was conducted from December 2016 through April 2017 in European countries. A list of medical centers in which lipegfilgrastim might have been prescribed or administered was identified. Pharmacists in these medical centers, hospitals, and outpatient clinical settings were contacted and invited to take a survey to assess their interest in participating in the study and the medical center’s eligibility (i.e. availability of the required patient information and accessibility to medical records). Several recruitment strategies were used in surveying the pharmacists, including initially a passive outreach via email and later a more active outreach via both email and telephone reminders to non-responders. Responding pharmacists who were interested and whose medical centers were confirmed eligible were sent an additional site recruitment questionnaire (SRQ) to re-confirm access to medical records and availability of two independent pharmacist abstractors to review the patients’ records. The study was conducted in medical centers according to regulatory requirements and local independent ethics committees in participating countries. Ethics committee approval was required and obtained in all participating countries, including Germany, Belgium and Croatia, and Hospital Board of Director approval was obtained in the Netherlands. A waiver for informed consent was obtained in all participating countries.
Medical records of patients administered lipegfilgrastim from September 2014 to April 2017 were identified. Fifty patients’ records (∼10% of the targeted investigation sample) were planned to be abstractedCitation16. Information on demographic characteristics, previous use of G-CSF, type of cancer, cancer treatment (e.g. cytotoxic or non-cytotoxic), indication for lipegfilgrastim use, and lipegfilgrastim dose, frequency and route of administration were captured in a questionnaire designed as an electronic case report form (CRF). Data were abstracted by two independent medical pharmacists in each medical center. Pharmacists were selected as the abstractors since they had access to the patient’s medical records and, thus, were well-positioned to identify off-label use. Wherever a discrepancy was identified between the two data abstractors that was related to whether the indication met the criteria for on-label or off-label use, an independent hematologist/oncologist adjudicator reviewed the abstractors’ responses and provided a final decision. Data were anonymized and captured in a secure study database using electronic data capture (EDC).
Utilization of lipegfilgrastim was examined for each treatment cycle. Lipegfilgrastim indication was categorized by each of the medical abstractors as either on-label or off-label use. The on-label indication was determined based on the approved indication, as specified in the Summary of Product Characteristics (SmPC) of lipegfilgrastim, for reduction in the duration of neutropenia and the incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancy, with the exception of chronic myeloid leukemia and MDSCitation8. Off-label use was determined and categorized according to the following four types: (1) use in children ≤ 17 years old (Type I); (2) use in patients with chronic myeloid leukemia and MDS (Type II); (3) use in non-cancer patients, e.g. healthy blood donors (for harvesting of peripheral blood), or patients with HIV, congenital, cyclic, or idiopathic neutropenia (Type III); and (4) use in cancer patients treated with non-cytotoxic drugs (Type IV). In cases of missing information to determine on- or off-label use, the record was noted as missing.
The primary analysis of on- and off-label use was carried out for the first chemotherapy treatment cycle documented in the medical records. The inter-rater agreement for each of the four types of off-label use was also measured for the first chemotherapy treatment cycle for a sub-set of patients who had complete medical records. To assess this agreement between abstractors reviewing the same medical record, the percentage agreement was calculated for all assessable medical records and specifically for the four types of off-label use. In addition, Cohen’s kappa (κ) statistic was calculatedCitation17, where κ = 1 indicates perfect agreement and κ = 0 suggests that there is no more agreement among the abstractors than would be expected by chanceCitation17–19. A κ-value of 0.80 was considered the minimum acceptable inter-rater agreementCitation18. All analyses were performed using SAS version 9.4.
Results
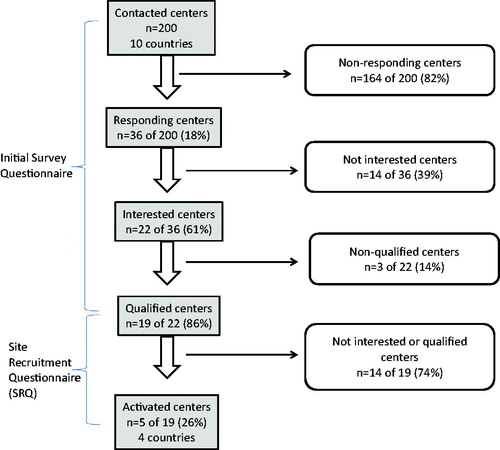
A total of 200 potential medical centers in 10 countries (Austria, Belgium, Croatia, Finland, Germany, Hungary, Ireland, Luxemburg, the Netherlands, and Poland) were contacted for initial outreach and asked to complete a survey. Of these 200 medical centers, only 36 centers responded to the initial questionnaire (18% response rate); 14 were not interested in participating, and 22 (61% of responding centers) in seven countries were interested in participating in the study (). Among the 22 interested centers, two centers in Belgium and one center in the Netherlands did not qualify for the study (due to no/limited access to patient data or no lipegfilgrastim being prescribed), so 19 centers qualified and were sent the SRQ.
Of these 19 centers receiving the SRQ, pharmacists in seven centers re-confirmed their interest and their centers were qualified to participate in the study. However, two of these seven centers either were unresponsive or lacked the resources to support the study within the requested timeframe. Thus, finally, five centers in Belgium, Croatia, Germany, and the Netherlands were activated for the study.
Demographic and baseline characteristics
In these five centers in four countries, a total of 54 medical records were screened. After excluding eight medical records (14.8%) due to patient participation in clinical studies, 46 medical records were included. Overall, the abstractors reviewed 111 distinct chemotherapy cycles where lipegfilgrastim was administered. Of these, 109 were classified as on-label use and two as off-label Type IV use (i.e. use in cancer patients treated with non-cytotoxic drugs). Lipegfilgrastim was not used for any of the following conditions that had been categorized as off-label: harvesting of peripheral blood progenitor cells; treatment of patients undergoing myeloablative therapy followed by bone marrow transplantation; treatment of patients with chronic myeloid leukemia and/or MDS; treatment of patients with severe congenital, cyclic, or idiopathic neutropenia; and treatment of persistent neutropenia in patients with advanced HIV infection.
Demographic and baseline characteristics of the study population in the pilot study are shown in . The on-label use group contained the majority of patient medical records (n = 42, 91.3%). The mean age in the on-label use group was 55 years (standard deviation [SD] = ±12.4), with ages ranging from 27–75 years. Most of the patients in this group were female (n = 36, 85.7%). The off-label use group only contained two patients (4.3% of the total records) and they were female and aged 32 and 64 years from the Netherlands. Two additional patient records (4.3%), both from the Netherlands, were missing age information and, thus, could not be categorized.
Table 1. Demographic and baseline characteristics of patients administered lipegfilgrastim between September 2014 to April 2017.
Most of the medical records were derived from Germany, followed by the Netherlands, Belgium, and Croatia (63.0%, 21.7%, 13.0%, and 2.2%, respectively). The two off-label and two missing medical records were from the Netherlands. Overall, breast cancer was the most common (73.9%) type of cancer. Other cancers included Hodgkin and non-Hodgkin lymphoma (13.0%), lung (4.3%), gynecological (except breast) (4.3%), and other (4.3%). The mean number of lipegfilgrastim treatment cycles per patient was 2.5 (SD = ±1.88) in the on-label use group and 1.0 in both the off-label use and missing groups.
Further assessment was performed of lipegfilgrastim use for the first chemotherapy treatment cycle. Fifty percent of the patients were not previously treated with G-CSFs, 43.5% of patients did not have this information available, and only three patients (6.5%) had used other G-CSFs prior to the first treatment cycle of lipegfilgrastim. All patients received lipegfilgrastim subcutaneously via pre-filled syringes with doses of 6 mg according to the dosage in the SmPC. The most common frequency of administration was once within 24 hours after chemotherapy (84.8%). During the first treatment cycle, lipegfilgrastim was most often indicated for neutropenia (82.6% of patients), and specifically for prevention of neutropenia (97.4% of the neutropenia indications). Lipegfilgrastim was seldom indicated for prevention of febrile neutropenia (2.2% of patients).
The most common physician specialty prescribing lipegfilgrastim was oncology (82.6%), followed by internal medicine (41.3%), and hematology (32.6%). Other physician specialties included other (10.9%), and pediatrics (2.2%). The settings in which the lipegfilgrastim was administered were outpatient clinics (89.1%) and/or inpatient clinics (67.4%).
Inter-rater agreement
Of the 46 medical records reviewed, 10 patient records could not be assessed for inter-rater agreement and were excluded from this analysis: nine of these records had inconsistencies between the abstractors in the count of treatment cycles, and one record was reviewed by only one abstractor. Thus, a total of 36 patient medical records with non-missing data for the first lipegfilgrastim cycle were used for evaluating the inter-rater agreement between the two abstractors regarding the indication of lipegfilgrastim use (i.e. on-label or four types of off-label use).
The abstractors disagreed in three cases where the first abstractor classified lipegfilgrastim use as off-label Type IV (i.e. use in cancer patients treated with non-cytotoxic drugs), but the second abstractor classified them as on-label (). Two cases were resolved as Type IV off-label use. However, one case was not considered off-label use per the adjudicator’s decision and was classified as on-label.
Table 2. Abstractor assessments for on-label and off-label use during first treatment cycle for the 36 assessable medical records.
Based on these three cases of disagreement, the overall inter-rater agreement for all 36 assessable medical records was 91.6%. Inter-rater agreement for the four types of off-label use (n = 144 assessments) was 97.9%. Thus, for Type I, II, and III off-label use, the abstractors were in perfect agreement (κ = 1) in classifying use. However, for Type IV off-label use, the agreement between the abstractors was the same as expected by chance (κ = 0).
Discussion
Based on the study results, the data collection and screening tools (initial survey questionnaire, and SRQ) used in this study were found to be useful and informative about the availability of the relevant medical data and the use of two independent abstractors within a center. Overall, lipegfilgrastim was prescribed according to the indications in the SmPC. It was prescribed to adult patients treated with cytotoxic chemotherapy for malignancy, the majority of whom had breast cancer. In most first treatment cycles, lipegfilgrastim was indicated for the prevention of neutropenia. During this cycle, lipegfilgrastim was administered according to the indicated age, dose, and route of administration; in a small proportion of records (15.2%), drug administration was not consistent with the dosing instructions of the SmPC. On-label use was documented in the majority of records and Type IV off-label use (use in patients treated with non-cytotoxic drugs) was estimated at 4.3% (two patients from a single center in the Netherlands). The high overall agreement between the abstractors suggests that the CRF was robust and reliable for collecting the necessary data on lipegfilgrastim use.
To the best of our knowledge, there is no evidence in the literature regarding off-label use of lipegfilgrastim. Compared to the rates of off-label use reported for other medicinal products (e.g. 20% for outpatient prescriptions and 30–64% for biologic chemotherapeutic agents), the rate of off-label use with lipegfilgrastim was relatively lowCitation20–22. Other than the limited sample size in this study, there could be other reasons for the low off-label use rate of lipegfilgrastim. First, according to the Nordic MDS Group’s guidelines for the diagnosis and treatment of MDS, long-acting G-CSFs have not been studied in MDS and thus cannot be recommendedCitation23. Second, potential off-label use (e.g. for peripheral stem cell mobilization or HIV, and for use in pediatric patients) may be less likely with lipegfilgrastim, since practitioners have a long-standing experience with filgrastim which is approved for these indications. Third, it is reasonable that the use pattern of lipegfilgrastim would follow that of pegfilgrastim (another long-acting G-CSF) in current practice, since lipegfilgrastim is a close analog of pegfilgrastim. Fourth, off-label use of long-acting G-CSF formulations is expected to be low, due to their long duration of action (10 days) and the inconvenience of using them for pediatric use because of their presentation as a fixed dose of 6 mg/0.6 mL in a prefilled syringe.
A major strength of this study design is the retrospective evaluation of data captured in the medical records. Even though information is only available to the extent that it is recorded by the physicians, medical records are considered the gold standard in many patient safety studies due to the availability of clinical details and their completenessCitation24. Furthermore, this study design reduces the potential of under-reporting off-label use and, thus, decreases misclassification bias by having the medical record abstraction performed by two independent abstractors, specifically pharmacists. Pharmacists rather than prescribers were selected as the investigators, since they potentially have access to medical records, and because they could reduce the bias that might be introduced when the prescriber reports data for potential off-label use.
Although there was a perfect agreement between the abstractors for on-label use and Types I–III of off-label use, the results of the inter-rater agreement analysis should be interpreted with caution. The underlying assumptions for the κ statistic calculation were not fully met. Specifically, it was not feasible to ensure that the same two abstractors would be used to abstract all the records. In addition, the poor inter-rater agreement for Type IV off-label use suggests that the fairly small sample size used for the inter-rater agreement estimation could make the κ estimation and interpretation unreliable. Nonetheless, the relatively small proportion of disagreement between the abstractors with regard to ascertaining off-label use indicates the robustness of the CRF and could justify using a single independent abstractor. This route could be enhanced by requiring more rigorous abstractor training with an emphasis on drug class or by utilizing an automated classification process through the EDC system design that would minimize variations in the selection of drug therapy class categories (cytotoxic vs non-cytotoxic).
The low response rate and participation of medical centers in the study are noteworthy, even though the study was intended as a pilot and the recruitment goal for the pilot was met. Observational post-authorization safety studies involving primary data collection may suffer from insufficient recruitment and participationCitation25,Citation26. In order to increase participation, several recruitment strategies were used, including passive outreach (e.g. email) followed by active follow-up (e.g. phone call to non-responders). The active outreach was found to improve the response rate. Yet, the majority of pharmacists contacted in the centers were unresponsive. Of the 200 potential centers in 10 countries targeted for participation, only 36 centers responded, resulting in a low response rate of 18%. Among the responding centers that did not participate, the main reason for non-participation was a lack of interest in the study. Since the abstraction of patient records depends on the willingness of abstractors at the medical centers to participate in the study, the potential for selection bias due to a self-selected group of participants should be taken into account. Despite the small sample size and challenges in recruitment, diversity in the sample was achieved as lipegfilgrastim utilization was captured in five medical centers representing both outpatient and inpatient clinical settings within four countries in the EU.
This pilot study is the first to offer insight on lipegfilgrastim use in the real world and to assess potential off-label use. Although the study was small and was undertaken in only a few centers, the results have highlighted the general compliance of prescribers with the approved indications of lipegfilgrastim. The study has illustrated the reliability of pharmacists as data abstractors (evidenced by the high inter-rater agreement), and the complexities of recruitment. Importantly, it has also revealed a wide range of aspects that will need to be addressed in designing a full-scale study of off-label use in the future. In particular, this study has shown that considerations will need to be made to use a single questionnaire for recruitment, to simplify data abstraction using one abstractor, and to extend recruitment and approach various types of HCPs and medical record administrators. While chemotherapy schedules were not collected in this pilot study, this information could be further explored in future research.
Thus, several changes have been implemented in the design of the subsequent main DUS. The recruitment process will be enhanced by using a single questionnaire for recruitment (SRQ) to reduce the burden for potential investigators of completing two questionnaires. In addition, data collection will be simplified by: (1) using a single abstractor at each medical center, based on the high agreement of data abstraction, to increase participation; (2) using any medical staff as abstractors, not just pharmacists, to facilitate data abstraction and recruitment; and (3) assessing off-label use of the most recent lipegfilgrastim administration to decrease the possibility of unidentified treatment cycles. Furthermore, extensive outreach to professional groups and training of abstractors in participating centers will be conducted to increase awareness of the study and to facilitate the conduct of the study. It is noteworthy that the study CRF was found to be a robust and reliable data collection tool and does not require further modification.
Conclusions
Despite the low response rate, this pilot study confirmed the feasibility of conducting a DUS of lipegfilgrastim to evaluate on-label and off-label use utilizing retrospective medical record review. Based on this limited pilot study, lipegfilgrastim is used according to its labeled indications, and the off-label use rate is low. The high inter-rater agreement suggests that one abstractor is sufficient for conducting chart abstraction of off-label use. Moreover, pharmacists were found to be a reliable source for medical data abstraction to capture on- and off-label use. Yet, additional data abstraction sources other than pharmacists will be identified to improve response rate and center recruitment. Findings from this pilot study are important for the successful planning and execution of the subsequent DUS.
Transparency
Declaration of funding
This study was funded by Teva Pharmaceutical Industries Ltd.
Declaration of financial/other relationships
SK and CR are employees of Teva Pharmaceutical Industries Ltd (and/or its affiliates). NL and MG were employees of Teva Pharmaceutical Industries Ltd (and/or its affiliates) at the time of study conduct. GH and EB are employees of PRA, which received financial support from Teva to conduct this research. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
The authors would like to acknowledge Jackie Raskind, PharmD, for editing the manuscript and Mahir Al-Banna, MSc, PhD, for providing statistical support.
Note
Notes
1 Marketed as Lonquex (Teva B.V.).
References
- Weycker D, Barron R, Kartashov A, et al. Incidence, treatment, and consequences of chemotherapy-induced febrile neutropenia in the inpatient and outpatient settings. J Oncol Pharm Pract. 2014;20:190–198.
- Lalami Y, Klastersky J. Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: an overview about well-established and recently emerging clinical data. Crit Rev Oncol Hematol. 2017;120:163–179.
- Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32.
- Guariglia R, Martorelli MC, Lerose R, et al. Lipegfilgrastim in the management of chemotherapy-induced neutropenia of cancer patients. Biologics. 2016;10:1–8.
- Koinis F, Nintos G, Georgoulias V, et al. Therapeutic strategies for chemotherapy-induced neutropenia in patients with solid tumors. Expert Opin Pharmacother. 2015;16:1505–1519.
- Pfeil AM, Allcott K, Pettengell R, et al. Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Support Care Cancer. 2015;23:525–545.
- European Medicine Agency (EMA). European public assessment report: Lonquex 2013 [cited 2018 July 18]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002556/WC500148383.pdf
- Summary of Product Characteristics: Lonquex: Teva Pharma B.V.; [cited 2018 July 18]. Available from: https://www.medicines.org.uk/emc/product/5422/smpc#INDICATIONS
- Summary of Product Characteristics: Neulasta: Amgen Ltd; [cited 2018 July 18]. Available from: https://www.medicines.org.uk/emc/product/6770
- Summary of Product Characteristics: Neupogen: Amgen Ltd; [cited 2018 July 18]. Available from: https://www.medicines.org.uk/emc/product/608/smpc#INDICATIONS
- Panopoulos AD, Watowich SS. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and 'emergency' hematopoiesis. Cytokine. 2008;42:277–288.
- Saloustros E, Tryfonidis K, Georgoulias V. Prophylactic and therapeutic strategies in chemotherapy-induced neutropenia. Expert Opin Pharmacother. 2011;12:851–863.
- Cortes de Miguel S, Calleja-Hernandez MA, Menjon-Beltran S, et al. Granulocyte colony-stimulating factors as prophylaxis against febrile neutropenia. Support Care Cancer. 2015;23:547–559.
- Silvestris N, Del Re M, Azzariti A, et al. Optimized granulocyte colony-stimulating factor prophylaxis in adult cancer patients: from biological principles to clinical guidelines. Expert Opin Ther Targets. 2012;16:S111–S117.
- European Medicine Agency (EMA). Committee for Medicinal Products for Human Use (CHMP) Assessment Report: Lonquex. 30 May 2013 [cited 2018 July 18]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002556/WC500148382.pdf
- Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12.
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276–282.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- Conti RM, Bernstein AC, Villaflor VM, et al. Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. JCO. 2013;31:1134–1139.
- Eaton AA, Sima CS, Panageas KS. Prevalence and safety of off-label use of chemotherapeutic agents in older patients with breast cancer: estimates from SEER-medicare data. J Natl Compr Canc Netw. 2016;14:57–65.
- Morris J. The use of observational health-care data to identify and report on off-label use of biopharmaceutical products. Clin Pharmacol Ther. 2012;91:937–942.
- Nordic MDS Group. Guidelines for the diagnosis and treatment of Myelodysplastic Syndrome and Chronic Myelomonocytic Leukemia, 8th update, May 2017 2017 [cited 2018 September 4]. Available from: https://www.nmds.org/attachments/article/92/Guidelines%20for%20the%20diagnosis%20and%20treatment%20of%20MDS%20and%20CMML_17.pdf
- Murff HJ, Patel VL, Hripcsak G, et al. Detecting adverse events for patient safety research: a review of current methodologies. J Biomed Informatics. 2003;36:131–143.
- Gavrielov-Yusim N, Bidollari I, Kaplan S, et al. Challenges of post-authorization safety studies: lessons learned and results of a French study of fentanyl buccal tablet. Pharmacoepidemiol Drug Saf. 2018;27:457–463.
- Waller PC, Wood SM, Langman MJ, et al. Review of company postmarketing surveillance studies. BMJ. 1992;304:1470–1472.