Abstract
Objective: The purpose of this study was to determine the efficacy of Extracorporeal Shock Wave Therapy (ESWT) in combination with the dietary supplement Tendisulfur Forte in the treatment of shoulder tendinopathy, lateral epicondylitis, and Achilles tendinopathy.
Methods: Patients were sub-divided for each pathology into two equal sized groups of 15: one treated with ESWT supplemented with Tendisulfur Forte, and the other treated with ESWT only. Shoulder functionality was measured through the UCLA shoulder score. Treatment of epicondylitis was assessed with the Mayo elbow score. Achilles tendinopathy was measured with the VISA-A score. Pain through the various groups of the study was measures with the Visual Analog Scale (VAS).
Results: Patients in the Tendisulfur Forte group had overall better functional and VAS scale scores for shoulder tendinopathy, elbow epicondylitis, and Achilles tendinopathy. UCLA scores for shoulder tendinopathy showed significant results at 60 days in the Tendisulfur Forte group (p = 0.0002). Mayo scores in the treatment of lateral epicondylitis was significant at 60 days in the study group (p < 0.0001). Achilles tendinopathy was improved in the study group at 30 days (p < 0.0001). VAS scales were significant for each pathology at 60 days (p < 0.0001). In addition, NSAIDs consumption was greatly reduced and, in most cases, stopped in the Tendisulfur Forte Groups.
Conclusion: Concerning the results obtained, this paper underlines the effectiveness of combined treatment of ESWT plus Tendisulfur Forte, in the absence of side-effects. Indeed, oral supplementation lead to a faster recovery and better outcomes with a significant reduction in NSAIDs consumption.
Introduction
Tendinopathies are common disorders representing ∼30% of all complaints for pain and functional limitation of the musculoskeletal system in the general populationCitation1. They affect both sedentary individuals, as well as professional and recreational athletes. Inflammation and chronic degeneration are the key conditions in the pathogenesis, and their natural history is influenced by the quality of the tendon tissue, along with mechanical overuse or misuse. Rotator cuff tendons, forearm extensor tendons, and the Achilles tendon are the most commonly afflicted sitesCitation1.
Rotator cuff tendinopathy (RCT) is the most common source of shoulder pain, and its prevalence is estimated to be 2–3.8% in the general populationCitation2.
Lateral epicondylitis is a common chronic inflammatory degeneration of the wrist extensor tendons at their insertion to the lateral epicondyle of the humerus. It affects 1–3% of the general population, especially between the ages of 30 and 70, without gender predispositionCitation3.
Achilles tendinopathy is the most prevalent tendon disorder among athletes, especially runners, track and field, volleyball, tennis, and soccer playersCitation4. Acute and chronic Achilles tendinopathies are estimated to be almost 50% of all sports-related injuries, and they are generally activity-associated, leading to pain and functional limitation, with diminished athletic performance. Asymptomatic degeneration of the Achilles tendon may also occur in 4% of active adults. The male gender is mostly involvedCitation5.
Conservative treatment of tendinopathies includes rest, cryotherapy, non-steroidal anti-inflammatory drugs (NSAIDs), orthotic devices, braces/slings, physiotherapy, and corticosteroid injections. Recently physical therapies have obtained a large consensus in the literature for their effectiveness in the treatment of tendinopathies. The most employed conservative treatments are ultrasound (US), laser therapy, TECAR (Transfer of Energy Capacitive and Resistive) therapy, and extracorporeal shock wave therapy (ESWT). Ten per cent of patients do not respond to conservative management and, for this group of patients, surgery represents the best optionCitation6.
Extracorporeal Shock Wave Therapy has shown positive and consistent results in the treatment of musculoskeletal diseasesCitation7,Citation8.
It has been demonstrated that ESWT is effective for tendinopathy, leading to a success rate ranging from 55–90%. In particular, recovery is achieved in 83% of calcific tendonitis of the shoulder, 55% of sub-acromial impingements, 76% of epicondylitis, 74% of plantar fasciitis, 90% of pertrochanteric bursitis, 82% of Achilles tendinopathy, and 86% of patellar tendinopathyCitation8.
ESWT stimulates soft-tissue healing through inhibition of afferent pain-receptor function, downregulation of expression of inflammatory cytokines, improvement of cellular proliferation, synthesis of extracellular matrix, and enhanced angiogenesis.
Recently several clinical studies have demonstrated that exogenous supplementation with micronutrients can facilitate recovery of tendinopathiesCitation9–11. The main substances essential for tendon healing include arginine, polyphenols (Vinitrox™ Bio Serae Laboratories SAS, Bram, France), collagen, methyl-sulfonyl-methane (MSM), vitamin C, and bromelainCitation6.
Few studies have verified the effectiveness of co-treatment of tendinopathy based on a combination of ESWT and nutraceutical supplementation.
The goal of this study was to assess the effects of our synergistic therapy using clinical and functional recovery indexes to assess three groups of patients affected by shoulder tendinopathy, lateral elbow tendinopathy, and insertional Achilles tendinopathy.
Materials and methods
In the period between January 2017 and September 2018 in our orthopedic outpatient clinic, we recruited 90 patients, divided into groups of 30 patients, for each of the following conditions: Achilles tendinopathy, rotator cuff tendinopathy of the shoulder, and lateral epicondylitis of the elbow. The diagnoses were confirmed through clinical evaluation (pain and functional limitation of the affected joint) as well as radiographic evidence. In particular, Achilles tendinitis and lateral epicondylitis were confirmed by ultrasonography, and shoulder tendinopathy was confirmed by MRI.
For each condition, the patients were further sub-divided into a control group, treated with ESWT, and an experimental group, treated with a combination of ESWT and the dietary supplement (DS) Tendisulfur Forte. Tendisulfur Forte is a commercially available Dietary Supplement (DS) containing methylsulfonylmethane (MSM), hydrolyzed swine collagen (Type I and Type II), l-arginine and l-lysine, vitamin C, Condroitin Sulfate, Glucosamine, Curcuma longa extracted to obtain curcuminoids, dry Boswellia serrata extracted to obtain acetyl-11-keto-β-boswellic acid (AKBA), and Myrrh. The dietary supplement was consumed twice a day for 1 month, then once a day for an additional month. We advised patients to take the dietary supplement at roughly the same time every day on an empty stomach in order to increase its bioavailability. VAS was selected to measure the perceived pain intensity, by asking the patient to report a value between 0–10 cm along a horizontal line (10 = severe pain; 0 = no pain). A two-centimeter change was considered clinically relevantCitation6.
In addition, clinical functional evaluation was performed using different scores.
For patients with Achilles tendinopathy, VISA-A score was chosen. VISA-A is a questionnaire that contains eight questions, and gives information about: pain, functional status, and activity (= three significant domains of dysfunction). The final score has a range from 0–100 pointsCitation6 ().
Table 1. Achilles tendinopathy group patient characteristics.
Patients with shoulder tendinopathy were evaluated with the UCLA (University of California Los Angeles) shoulder score, a 4-item combined patient–clinician survey that reports pain, function, range of motion (ROM), and strength. Each item has a score ranging from 0–10 (a perfect score = 30) ().
Table 2. Shoulder tendinopathy group patient characteristics.
Patients with lateral elbow tendinopathy were assessed with Mayo Elbow Performance Score (MEPS). This score is a widely used performance index for evaluation of clinical outcomes for a variety of elbow disorders. It consists of an evaluation of pain, arc of motion, stability, and patient rating of daily function, ranging from 0–100 points. A higher score indicates a better prognosisCitation12 ().
Table 3. Elbow (epicondylitis) tendinopathy group patient characteristics.
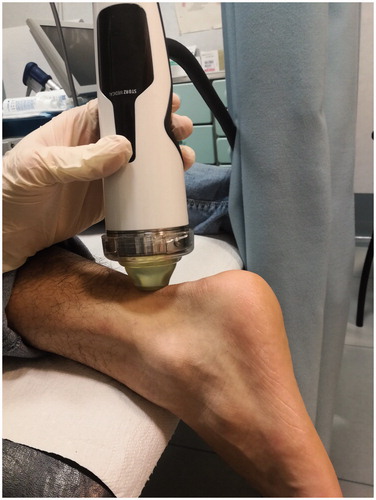
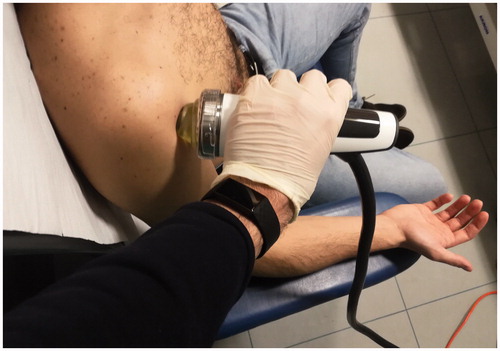
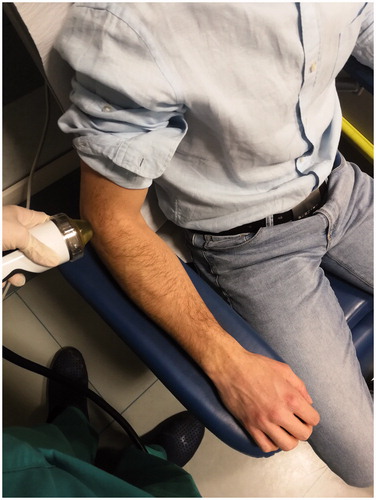
All 90 patients were subjected to one cycle of three high-energy Extracorporeal Shock Wave Therapy sessions, performed once a week for three consecutive weeks, with 1,700–2,000 shocks applied with a flux energy density of 0.12–0.35- mJ/mm2 using a Storz Medical Duolith SD1a machine. Patients affected by Achilles tendinitis were treated in a prone decubitus with the foot off the edge of the visitation table, allowing the ankle to be in its neutral position (). Otherwise, patients affected by shoulder tendinopathy were subjected to ESWT in a sitting position with the superior limb positioned at 90° of elbow flexion, as well as patients affected by lateral epicondylitis. In the first case, a supine position of the palm was requested, aiming for a better exposure of the long head of the biceps (). Instead, in the second case the palm was positioned in prone position (). The trigger points were identified by palpation of the painful areas during the first physical examination and later were balanced through shock waves. Energy density was set up at 0.12 mJ/mm2 for all the patients and progressively raised up to 0.35 mJ/mm2 if the pain was bearable. For patients who didn’t bear the pain the energy density was individually adapted observing the aforementioned range, in order to perform ESWT without any interruption.
All the patients were evaluated using VAS and the specific functional scores at the following stages: pre-treatment (t0), at 7 days (t1), at 30 days (t2), and after 60 days (t3).
This study was conducted following the principles of the Declaration of Helsinki and with the patients’ permission expressed through written consent.
Inclusion/exclusion criteria
We recruited patients between 18–70 years of age (mean age = 49 years) presenting with complaints for at least 1 month and with clinical (functional tests and VAS) and imaging studies (Xray, Ultrasound, MRI) confirming tendinopathy.
Exclusion criteria included: prior tendon rupture or surgery, systemic inflammatory diseases, diabetes, irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), Gastroesophageal Reflux Disease (GERD), and intolerance or allergies to any single ingredient of Tendisulfur Forte. Moreover, we excluded all the patients who showed contraindications to ESWT (Pacemaker, pregnancy, systemic or in-situ neoplasia, local infection, history of epilepsy, severe coagulopathies)Citation6.
Statistical analysis
For each studied pathology, the mean average of both and respective functional scores was calculated at each time interval during the course of the study (t0 = pre-treatment, t1 = 7 days, t2 = 30 days, t3 = 60 days). Values were compared between each group for each pathology at corresponding follow-ups. p-values were calculated at each time interval. The Student T-test and Z-score were used to calculate differences between values. Intra-group trajectories and inter-group difference at each time interval were calculated using the Bonferroni correction. Statistical significance was set at p < 0.05.
Results
All study groups, sub-divided by pathology, were similar and uniform in terms of distribution of age, sex, and affected side.
In the treatment of shoulder tendinopathy, the Tendisulfur Forte group had significant results compared to the control group for both UCLA (p-value = 0.0002) and VAS scores (p-value < 0.0001) at 60 days follow-up ( and ). NSAIDs consumption was significantly decreased in the Tendisulfur Forte group at 30 (p-value = 0.0308) and 60 days (p-value = 0.0061) ().
Figure 4. Within-group trajectories and between-group within-time difference for shoulder VAS scores.
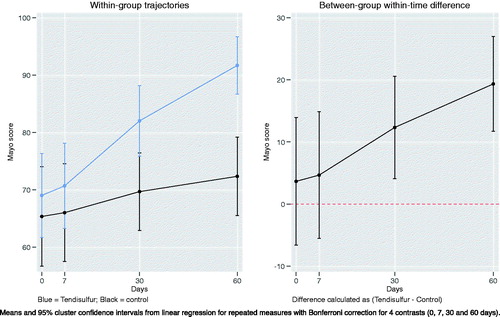
Figure 5. Within-group trajectories and between-group within-time difference for shoulder UCLA scores.
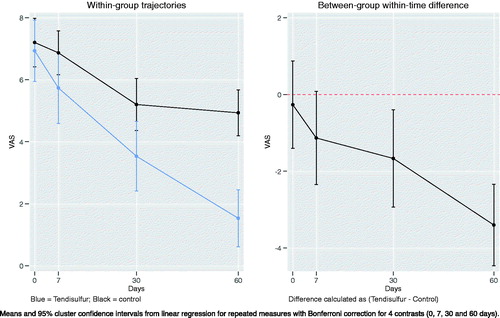
Table 4. Shoulder tendinopathy results.
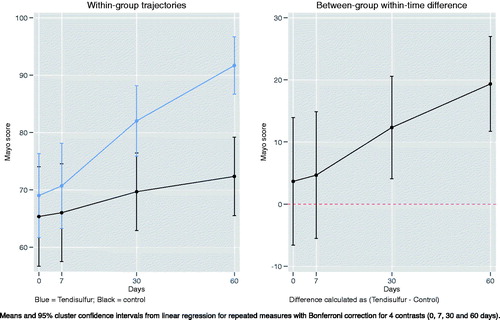
When treating epicondylitis with ESWT + Tendisulfur Forte, we saw an early significant improvement in pain reduction at the 7th day follow-up (p-value = 0.0024), with subsequent follow-up showing increased improvement (p-value < 0.00001) (). Mayo scores showed significant results in the Tendisulfur Forte group as compared to the Control group at 30 days and 60 days from the start of the treatment (p-value = 0.0004 and p-value < 0.00001, respectively) (). Patients in the study group consumed significantly less NSAIDs than in the control group at the 30 and 60 day follow-ups (p-value = 0.0001 and p-value = 0.0053, respectively) ().
Table 5. Elbow tendinopathy (epicondylitis) results.
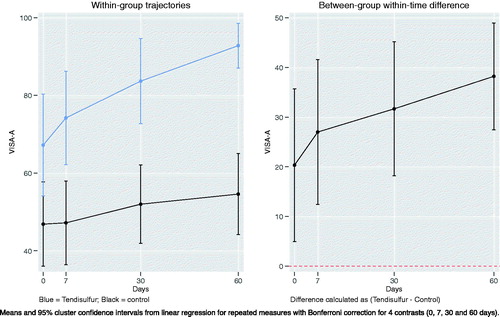
VISA-A scores in Achilles tendinopathy were significant in the Tendisulfur group at each follow-up, with the greatest significance observed at t2 (p-value < 0.00001) (). Mean VISA-A scores had a greater improvement in the Tendisulfur Forte group, +27 (+39%) as compared to the control group, +7 (17%) (). VAS saw a significant reduction at day 7 in patients consuming Tendisulfur Forte (p-value = 0.0179) with subsequent follow-up showing a further decrease in VAS values (). Reduction of NSAID consumption was significant at 30 days (p-value = 0.0143) and at 60 days (p-value = 0.0143) ().
Figure 8. Within-group trajectories and between-group within-time difference for Achilles tendinopathy VAS scores.
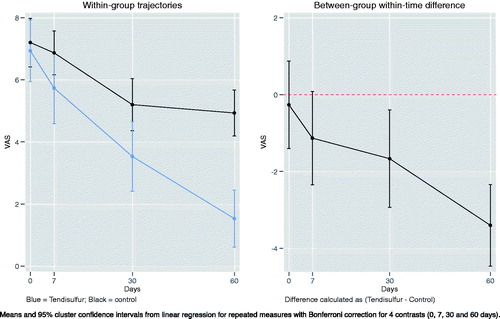
Figure 9. Within-group trajectories and between-group within-time difference for Achilles VISA-A scores.
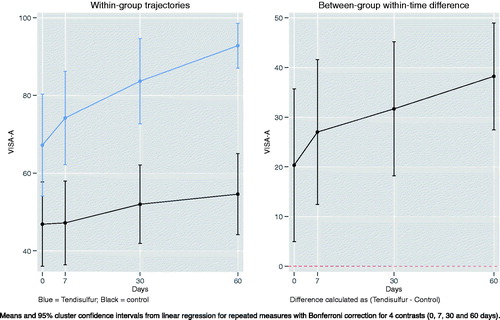
Table 6. Achilles tendinopathy results.
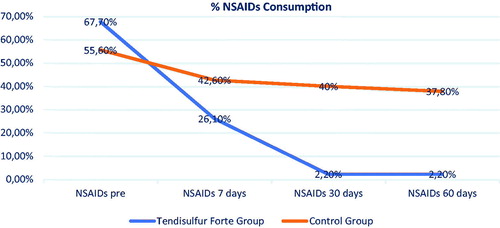
We note that nearly all patients (44/45) in the Tendisulfur Forte groups were NSAID-free at 60 days follow-up. In addition, we observed that, during the course of the study, and the subsequent follow-up period (up to 2 months) there was a significant reduction of NSAIDs consumption in the Tendisulfur Forte group ().
Figure 10. Graph Showing overall NSAIDs consumption, taking into consideration all participants from the Tendisulfur Forte group and the control groups.
We report no adverse effects from the dietary supplement with no discontinuation and good overall tolerability during ESWT session in both groups.
Discussion
Tendinopathies affect a relevant part of the population. In particular, they affect young and active people due to stress and overuse, as well as the elderly because of tendon degeneration. Among the most frequent tendinopathies, we can distinguish: Rotator cuff tendinopathy, lateral epicondylitis, and insertional Achilles tendinopathyCitation13. According to different studies, lower limb tendinopathies are more related to overuse, as in the case of sports and physical recreational activities, while rotator cuff tendinopathy is most likely linked to degenerationCitation14.
The risk of recurrence is very high, and a definite therapy is still not availableCitation6.
Standard treatment regimens of these diseases include rest, cryotherapy, activity modification, stretching exercises, NSAIDs, and eccentric loading.
Several physical therapies include extracorporeal shock waves, Tecar therapy, cryo-us, and physiotherapyCitation15.
In regards to Tecar therapy, it is a type of “thermotherapy”, specifically based on deep heat production in body tissues. It is indicated to treat joint and muscular pathologies, traumas, tendinopathies, neuropathies, and acute or chronic painCitation16,Citation17.
Evidence on the efficacy of the use of exercise as a conservative therapy to treat pain and functional disability are reported in the literatureCitation18,Citation19.
ESWT, initially introduced for the treatment of nephrolithiasis, has become a viable option for the treatment of different soft-tissue disordersCitation8 such as calcific tendinopathy of the rotator cuff, lateral elbow tendinopathy, and insertional Achilles tendinopathy. ESWT modifies cell membrane and cytoplasm organelles, resulting in nucleus stimulation. All of these changes lead to synthesis of proteins, nitric oxide (NO), and specific growth factors (GF) which activate several biological processesCitation7. Erroi et al.Citation20 demonstrated that both ESWT and platelet-rich plasma (PRP) injections are effective in the treatment of insertional Achilles tendinopathy in people who participate in sports. PRP is a concentrate of platelet-rich plasma protein derived from the patient’s own blood containing growth factors, with healing, regeneration, and anti-inflammatory effectsCitation21–23. Lee et al.Citation24 have reported optimal outcomes after ESWT in the treatment of chronic refractory Achilles tendinopathy.
Frassanito et al.Citation25 stated that a treatment of kinesio taping, followed by ESWT, can be an effective treatment in patients affected by calcific shoulder tendinopathy. Kinesio taping indeed can be considered an adjuvant therapy, which improves the regenerative effects of ESWT.
Li et al.Citation26 have demonstrated the effectiveness of ESWT in shoulder pain relief in patients affected by chronic rotator cuff tendinopathy.
Moreover, Vulpiani et al.Citation27 showed that ESWT had better clinical outcomes at 6 and 12 months follow-up when compared to Cryo-US in the treatment of symptomatic chronic lateral epicondylitis.
A more recent retrospective study, conducted by Chou et al.Citation28, underlined the effectiveness of performing ESWT in the management of chronic rotator cuff tendinitis or partial tears of the rotator cuff before undergoing surgery. They compared two groups of patients, an athletic group and a non-athletic group, and observed a faster recovery with a rate of satisfaction greater than 50% in both groups.
In recent years, there has been an introduction of dietary supplements tailored for degenerative and/or inflammatory joint pathologies. Nutraceuticals are not pharmacologic in their nature, and contain compounds that have been scientifically proven to have modulatory effects on inflammation. In light of these considerations, nutraceutical supplements are widely adopted in the general and athletic population, aimed at the reduction of symptoms, and prevention of tendon injuriesCitation1. One of these products is Tendisulfur Forte. It contains methylsulfonylmethane (MSM), hydrolyzed swine collagen (Type I and Type II), l-arginine and l-lysine, vitamin C, Condroitin Sulfate, Glucosamine, Curcuma longa extracted to obtain curcuminoids, dry Boswellia serrata extracted to obtain acetyl-11-keto-β-boswellic acid (AKBA), and Myrrh.
It is widely accepted in the clinical practice that nutritional components could play a significant role in tendon healing, as shown by several other studiesCitation1.
Methylsulfonylmethane (MSM) is a sulfur-containing compound. It is a natural component of vegetables, grains, fruits, and beverages. Through oral administration it is rapidly absorbed, well distributed, and completely excreted from the body. Fusini et al.Citation1 state that it can be an efficacious analgesic and anti-inflammatory supplement. Its employment in the management of osteoarthritis showed a rapid reduction of pain and swelling while improving functional ability. In the literature, it is reported that MSM may have clinical applications for arthritis and other inflammatory disorders such as interstitial cystitis, allergic rhinitis, and acute exercise-induced inflammation. The mechanism of action includes the prevention of degradation of the NF-kB inhibitor, blocking of phosphorylation of the p65 subunit at Serine-536, the downregulation of the expression of inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) through suppression of NF-kBCitation29.
Vitamin C is a co-enzyme of proline hydroxylase, involved in the synthesis of procollagen chainsCitation1,Citation30. For this reason, an appropriate concentration of vitamin C is indispensable for normal development of tendons. Nevertheless, vitamin C is not able to increase collagen secretion; however, it is an anti-oxidative agent, that enhances the mechanical and thermal stability of rat tail tendons, as stated in a review of Fusini et al.Citation1.
Another experimental study, performed by Omeroglu et al.Citation31, demonstrated that parenteral supplementation of high-dose vitamin C (once daily, every 2 days) accelerates the Achilles tendon healing in a healthy rat model. The mechanism of action seems to depend on improvement of angiogenesis in the early stage, and of type I collagen synthesis during healingCitation31. Nevertheless, in a more recent paper, Gemalmaz et al.Citation32 investigated the effects of a dietary supplement containing mucopolysaccharides, vitamin C, and collagen on Achilles tendon’s healing in rats, showing no advantages in collagen synthesis after 3 weeks. However, it must be noted that the rats which received the supplement showed an increase in TGF-ß and proliferating cell nuclear antigen (PCNA) in the endotenon fibroblasts of the healing site, suggesting a possible role of tendon healing.
Boswellia species have been demonstrated to have anti-inflammatory effects through the inhibition of cytokine production in human monocytes in vitro, as well as cytotoxic activity. This is likely due to inhibition of nuclear factor kappa B (NF-kB) signaling by neutrophilic granulocytes, resulting in downregulation of pro-inflammatory cytokines (such as TNF-α, IL-1, IL-2, IL-4, IL-6, and IFN-γ) as well as reduction of NF-kB-dependent anti-apoptotic gene expressionCitation1,Citation33. Other possible mechanisms are the inhibition of 5-lipoxygenase and leukotriene biosynthesis in neutrophilic granulocytes, along with the depression of the elastase enzyme activity.
Additionally, Boswellia prevents the TNF α-induced expression of matrix metalloproteinases MMP-3, MMP-10, and MMP-1237. It can suppress the conversion of C3 into C3a and C3b in the classic pathway of the complement systemCitation1.
Curcumin is a natural polyphenol with antioxidant properties derived from Curcuma LongaCitation1. Its mechanisms of action are based on modulation of transcription factors such as NFkB, AP-1, b-catenin peroxisome proliferator-activated receptor-c, enzymes (COX-2 and 5-LOX), inducible NOS, pro-inflammatory cytokines (TNF-a, IL-1b, and IL-6), and cell surface adhesion moleculesCitation33.
L-arginine and l-lysine are essential amino acids involved in the synthesis of elastase.
Moreover, l-arginine is involved in the production of NO by the constitutive enzyme endothelial NO oxide synthase (eNOS). NO leads to the vasodilation of blood vessels resulting in increased blood flowCitation34. During the healing process, the formation of NO is an indispensable step because its inhibition increases the level of TGF-β, therefore leading to fibrosis and chronic inflammationCitation1.
There is much evidence of a possible role of NO in the management of pain and range of motion in tendinopathiesCitation35–37.
Myrrh is a natural anti-inflammatory compound. Morikawa et al.Citation38 showed that myrrh contains several anti-inflammatory constituents, called terpenoids, which are able to inhibit nitric oxide production in lipopolysaccharide (LPS)-activated mouse peritoneal macrophages.
Gumina et al.Citation34 demonstrated that an oral supplement containing methylsulfonylmethane, arginine L-alpha-ketoglutarate, hydrolyzed type I collagen bromelain, and grape juice (Tenosan), could reduce shoulder pain and improve healing of large rotator cuff tears after arthroscopic repair.
Merolla et al.Citation33 used a dietary supplement containing Boswellia serrata and Curcumalonga (Tendisulfur, LaborestSpA, Nerviano, Italy), which showed short-term effectiveness in post-operatively reducing pain after rotator cuff tear repair surgery.
Notarnicola et al.Citation6 evaluated the efficacy of combined treatment with ESWT and arginine supplementation in patients with Achilles tendinopathy, observing an improvement of the therapeutic response.
Another interesting study was conducted by Balius et al.Citation39, who evaluated the effects of an oral supplement composed by mucopolysaccharides, type I collagen, and vitamin C in the treatment of Achilles tendinopathy. Their results show that the combination of the aforementioned supplement and either eccentric training or passive stretching provides further benefits compared to physical therapy alone.
Considering the wide consensus of ESWT in the literature, we believe that ESWT represents the best physical therapy to provide an effective recovery for tendinopathies, and Tendisulfur Forte may be useful in reducing inflammation and the possibility of recurrence because of its key features. Indeed, shock wave therapy increases bioavailability of the supplement to the tendon tissueCitation6, due to the neo-angiogenic properties of ESWTCitation40,Citation41. To confirm the positive effect of the association of Tendisulfur Forte and ESWT, our study showed that patients in the Tendisulfur Forte group reduced their use of NSAIDs during the 2 months of treatment when compared to the control group. Moreover, we observed a greater decrease of pain in the Tendisulfur Forte group and an improvement of tendon healing after 30 days of treatment.
We report no side-ffects in all patients treated with Tendisulfur Forte.
Limits
The limits of our study include the small number of patients for each group and lack of randomization in group allocation. The reason for the lack of randomization was in order to take into consideration regimen compliance and allergies patients might have to any of the components of Tendisulfur Forte. Moreover, we do not have functional scores for the patients after 2 months follow-up, as well as an instrumental monitoring with US or MRI after the therapy.
Conclusions
Our results show that the combination of ESWT and Tendisulfur Forte provides a greater and faster pain relief, with a significant reduction of NSAIDs consumption and without side-effects. Therefore, we believe that the combination of the aforementioned treatments could be considered as a valid first-line treatment for the most frequent tendinopathies. As a future prospective it could be interesting to evaluate the effects of a two-stage treatment based on ESWT and nutraceutical supplementation performed separately. Indeed, this different approach could clarify the role of both the therapies on the reduction of inflammation and the improvement of functionality in order to optimize their combination.
Transparency
Declaration of funding
This paper was not funded.
Declaration of financial/other interests
The dietary supplement TENDISULFUR FORTE used throughout the study was provided by Laborest S.r.l. Italia (Uriach Group). The authors on this manuscript have no relevant financial or other relationships to disclose. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledegments
Suppliers
a. Storz Medical AG. STORZ MEDICAL AG – Lohstampfestrasse 8, 8274 Tägerwilen, Switzerland.
b. Laborest S.r.l. Italia (Uriach Group). Via Vicinale per Parabiago, 22, 20014 Nerviano MI, Italy.
The authors would like to thank the nursing and orderly staff in the outpatient clinic at San Raffaele Hospital (Milan, Italy): Angela Marras, Nadia Spinelli, Graziella Saccà, Gianfranca Matera, Alessandra Izzo, Oronzo Vesmile, Fernando Prontera, and Ugo Angeli.
References
- Fusini F, Bisicchia S, Bottegoni C, et al. Nutraceutical supplement in the management of tendinopathies: a systematic review. Muscles Ligaments Tendons J. 2016;6(1):48–57.
- Hopkins C, Fu SC, Chua E, et al. Critical review on the socio-economic impact of tendinopathy. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2016;4:9–20.
- Vaquero-Picado A, Barco R, Antuña RBS. Lateral epicondylitis of the elbow. Efort Open Rev. 2016;1:391–397.
- Kozlovskaia M, Vlahovich N, Ashton KJ, et al. Biomedical risk factors of achilles tendinopathy in physically active people: a systematic review. Sports Med Open. 2017;3(1):20.
- Freedman BR, Gordon JA, Castro LJ. The Achilles tendon: fundamental properties and mechanisms governing healing. Muscle Ligaments Tendons J. 2019;04:245–255.
- Notarnicola A, Pesce V, Vicenti G, et al. SWAAT study: extracorporeal shock wave therapy and arginine supplementation and other nutraceuticals for insertional achilles tendinopathy. Adv Ther. 2012;29:799–814.
- Romeo P, Lavanga V, Pagani D, et al. Extracorporeal shock wave therapy in musculoskeletal disorders a review. Med Princ Pract. 2013;23:7–13.
- Vitali M, Peretti G, Mangiavini L, et al. The treatment with extracorpereal shock wave therapy in some of most frequently musculoskeletal pathologies. J Bone Surg Brit. 2006;8-B:423.
- Aiyegbusi AI, Duru FI, Awelimobor D, et al. The role of aqueous extract of pineapple fruit parts on the healing of acute crush tendon injury. Nig Q J Hosp Med. 2010;20:223–227.
- Bokhari AR, Murrell GA. The role of nitric oxide in tendon healing. J Shoulder Elbow Surg. 2012;21:238–244.
- Shakibaei M, Buhrmann C, Mobasheri A. Anti-inflammatory and anti-catabolic effects of TENDOACTIVE® on human tenocytes in vitro. Histol Histopathol. 2011;26:1173–1185.
- Cusick MC, Nicolas SB, Frederick MA, et al. Accuracy and reliability of the mayo elbow performance score. J Hand Surg Am. 2014;39:1146–1150.
- Abate M, Silbernagel KG, Siljeholm C, et al. Pathogenesis of tendinopathies: inflammation or degeneration?. Arthritis Res Ther. 2009;11(3):235.
- Benjamin JFD, Stephanie GD, Neal LM, et al. Review: emerging concepts in the pathogenesis of tendinopathy. Surgeon. 2017;15:349–354.
- Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536–544.
- Hawamdeh MM. The effectiveness of Capacitive Resistive Diathermy (Tecartherapy®) in acute and chronic musculoskeletal lesions and pathologies. Eur J Sci Res. 2014;18:336–340.
- McGorm H, Roberts LA, Coombes JS, et al. Turning up the heat: an evaluation of the evidence for heating to promote exercise recovery, muscle rehabilitation and adaptation. Sports Med. 2018;48(6):1311–1328.
- Littlewood C, Malliaras P, Chance-Larsen K. Therapeutic exercise for rotator cuff tendinopathy: a systematic review of contextual factors and prescription parameters. Int J Rehabil Res. 2015;38:95–106.
- Littlewood C, Ashton J, Chance-Larsen K, et al. Exercise for rotator cuff tendinopathy: a systematic review. Physiotherapy. 2012; 98:101–109.
- Erroi D, Sigona M, Suarez T, et al. Conservative treatment for insertional achilles tendinopathy: platelet-rich plasma and focused shock waves. A retrospective study. MLTJ. 2017;7:98–106.
- Andia I, Martin JI, Maffulli N. Advances with platelet rich plasma therapies for tendon regeneration. Expert Opin Biol Ther. 2018;18:389–398.
- Chen X, Jones IA, Park C, et al. The efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2018;46(8):2020–2032
- Nourissat G, Ornetti P, Berenbaum F, et al. Does platelet-rich plasma deserve a role in the treatment of tendinopathy?. Joint Bone Spine. 2015;82:230–234.
- Lee JY, Yoon K, Yi Y, et al. Long-term outcome and factors affecting prognosis of extracorporeal shockwave therapy for chronic refractory achilles tendinopathy. Ann Rehabil Med.. 2017;41:42–50.
- Frassanito P, Cavalieri C, Maestri R, et al. Effectiveness of extracorporeal shock wave therapy and kinesio taping in calcific tendinopathy of the shoulder: a randomized controlled trial. Eur J Phys Rehabil Med. 2017;54(3):333–340
- Li W, Zhang S-X, Yang Q, et al. Effect of extracorporeal shock-wave therapy for treating patients with chronic rotator cuff tendonitis. Medicine (Baltimore). 2017;96:e7940
- Vulpiani MC, Nusca SM, Vetrano M, et al. Extracorporeal shock wave therapy vs cryoultrasound therapy in the treatment of chronic lateral epicondylitis. One year follow up study. Muscle Ligaments and Tendons J. 2019;05: 167–174.
- Chou WY, Wang CJ, Wu KT, et al. Comparative outcomes of extracorporeal shockwave therapy for shoulder tendinitis or partial tears of the rotator cuff in athletes and non-athletes: Retrospective study. Int J Surg. 2018;51:184–190.
- Butawan M, Benjamin RL, Bloomer RJ. Methylsulfonylmethane: applications and safety of a novel dietary supplement. Nutrients. 2017;9:290.
- Curtis L. Nutritional research may be useful in treating tendon injuries. Nutrition. 2016;32:617–619.
- Omeroğlu S, Peker T, Türközkan N, et al. High-dose vitamin C supplementation accelerates the Achilles tendon healing in healthy rats. Arch Orthop Trauma Surg. 2009;129:281–286.
- Gemalmaz HC, Sarıyılmaz K, Ozkunt O, et al. Role of a combination dietary supplement containing mucopolysaccharides, vitamin C, and collagen on tendon healing in rats. Acta Orthop Traumatol Turc. 2018;52:452–458.
- Merolla G, Dellabiancia F, Ingardia A, et al. Co-analgesic therapy for arthroscopic supraspinatus tendon repair pain using a dietary supplement containing Boswellia serrata and Curcuma longa: a prospective randomized placebo-controlled study. Musculoskelet Surg. 2015;99(1):S43–S52
- Gumina S, Passaretti D, Gurzì MD, et al. Arginine L-alpha-ketoglutarate, methylsulfonylmethane, hydrolyzed type I collagen and bromelain in rotator cuff tear repair: a prospective randomized study. Curr Med Res Opin. 2012;28:1767–1774.
- Paoloni JA, Appleyard RC, Nelson J, et al. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915–920.
- Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806–813.
- Paoloni JA, Appleyard RC, Nelson J, et al. Topical glyceryl trinitrate treatment of chronic noninsertional achilles tendinopathy. A randomized, double-blind, placebo-controlled trial. J Joint Bone Surg Am. 2004;86:1916–922.
- Morikawa T, Matsuda H, Yoshikawa M. A review of anti-inflammatory terpenoids from the incense gum resins frankincense and myrrh. J Oleo Sci. 2017;66:805–814.
- Balius R, Álvarez G, Baró F, et al. A 3-arm randomized trial for achilles tendinopathy: eccentric training, eccentric training plus a dietary supplement containing mucopolysaccharides, or passive stretching plus a dietary supplement containing mucopolysaccharides. Curr Ther Res Clin Exp. 2016;78:1–7.
- Kisch T, Wuerfel W, Forstmeier V, et al. Repetitive shock wave therapy improves muscular microcirculation. J Surg Res. 2016;201:440–445.
- Hatanaka K, Ito K, Shindo T, et al. Molecular mechanisms of the angiogenic effects of low-energy shock wave therapy: roles of mechanotransduction. Am J Physiol Cell Physiol. 2016;311:C378–C385.