Abstract
Objective: Little evidence is available on the management of patients with metastatic and/or unresectable gastric cancer (mGC) after the failure of first-line treatment. This study presents real-world data on characteristics and treatment patterns of patients with mGC in Russia.
Methods: Eligible patients were ≥18 years old, diagnosed with mGC ≥ January 1, 2012, received first-line chemotherapy followed by second-line chemotherapy or best supportive care (BSC), had ≥3 months of follow-up after the start of second-line chemotherapy or BSC (except in cases of death), and had not participated in a clinical trial. Data were summarized using descriptive statistics.
Results: A total of 88 physicians provided data from 202 charts. Mean age at mGC diagnosis was 53.7 (standard deviation: 11.2) years; 70.8% of patients were male. Reasons for first-line treatment discontinuation included disease progression (50.5%) and adverse events/toxicity (39.1%). There were 52 unique treatment regimens prescribed in second-line; capecitabine (14.5%), paclitaxel (9.3%), and capecitabine + oxaliplatin (8.7%) were the most frequent. Reasons for second-line treatment discontinuation included disease progression (39.8%) and patient refusal to continue (37.5%). During 2nd-line treatment, the most common treatment-related symptoms were nausea/vomiting (75.0%), while pain (73.8%) was the most common disease-related symptom. Antiemetics (63.4%), chemotherapy (61.6%), non-narcotic analgesics (48.3%), endoscopy (45.9%), and nutritional support (35.5%) were most frequently used as supportive care.
Conclusions: Second-line treatment patterns for patients with mGC in Russia are heterogeneous. Results of this study indicate the need for more intensive implementation of the most active regimens in second-line treatment of mGC according to international and national guidelines.
Introduction
Gastric cancer, originating in the stomach, esophagus, or the esophagogastric junction, is one of the leading causes of cancer-related deaths, with an estimated 1.6 million new cases worldwide in 2018 [Citation1]. In Russia, the age-standardized incidence of gastric cancer in 2016 was 14.1 per 100,000 and the death rate from gastric cancer was 10.9 per 100,000 [Citation2,Citation3]. The one-year mortality rate from gastric cancer in Russia in 2016 was 48.5% [Citation3]. Risk factors for gastric cancer include male gender, Helicobacter pylori infection, tobacco use, atrophic gastritis, partial gastrectomy and Ménétrier's disease [Citation4,Citation5]. Distal or antral gastric cancers that are associated with H. pylori infection, alcohol use, high-salt diet, processed meat and low fruit and vegetable intake are more common in East Asia. Tumors of the proximal stomach (cardia) are associated with obesity, and tumors of the gastroesophageal junction are associated with reflux and Barrett's esophagus and are more common in non-Asian countries [Citation4,Citation6].
Complete surgical resection of cancer and extended lymph node dissection remain the only curative treatment options [Citation7]. However, up to 50% of patients present with advanced or metastatic disease at diagnosis, and 40–60% relapse after surgery with curative intent. Only 25% of patients with gastric cancer have resectable disease at presentation [Citation8]. In Russia, among patients first diagnosed with gastric cancer in 2016, 33.4% had tumor stage I-II, 23.6% had stage III, and 40.4% had stage IV [Citation3]. Advanced unresectable or metastatic gastric cancer (mGC) has a poor prognosis, with a median survival rarely exceeding 12 months, and is only amenable to palliative treatment [Citation7]. Chemotherapy is the standard treatment in these patients, but other treatments, such as radiotherapy, may be used [Citation9]. Best supportive care (BSC) is usually offered in advanced stages of cancer, and may be provided concomitantly with chemotherapy. This involves palliative interventions to manage symptoms and complications such as bleeding, gastric obstruction, pain, nausea/vomiting and ascites, which may arise from the tumor or as a consequence of therapy. In recent years, treatment of patients with advanced gastric cancer is increasingly expanded beyond first-line, but even the most effective regimens have failed to significantly improve overall survival [Citation8,Citation10,Citation11].
In light of the introduction of newer treatment options for advanced unresectable or mGC, there is a need to investigate the current treatment landscape in this patient population, particularly beyond first-line treatment. There are no studies on treatment patterns and associated healthcare resource use (HRU) among patients with mGC in Russia. Furthermore, while clinical trials have shown clinical efficacy of some of these treatment options, data on the real-world effectiveness and resource utilization of these treatment options are limited. The availability of such real-world data would enable clinicians and their patients to make informed treatment decisions regarding their care, and also inform payers in regard to the resources needed to manage patients with mGC.
The objective of this study was to describe real-world treatment patterns and cancer-related HRU among patients with mGC in Russia.
Methods
Data source and study design
A retrospective chart review study was conducted. Data were collected from a convenience sample of physicians practicing in the field of oncology in Russia recruited from market research panels of physicians who agreed to be contacted about studies. An online chart extraction tool was designed and made available to participating physicians via an electronic portal. Each physician was asked to review patient charts and provide de-identified information for up to three eligible patients each.
The study index date was defined as the date of mGC diagnosis. The baseline period was defined as any time prior to the index date (i.e. all medical history available prior to mGC diagnosis). Patients were observed until death, loss to follow-up, or study cut-off date, whichever occurred first. Chart abstraction took place between September 30, 2016 and November 2, 2016. Data collected from the physicians did not include any patient-identifying information, and the study received IRB exemption from the Independent Interdisciplinary Ethics Committee on Ethical Review for Clinical Studies (http://ethicuni.ru/).
Inclusion criteria
Physicians who participated in the study had oncology or chemotherapy specialty and treated at least one patient meeting the eligibility criteria for the study. Patients were included if they (1) had a diagnosis of mGC (including adenocarcinoma of the stomach or gastroesophageal junction with adenocarcinoma histology), on or after January 1, 2012 (patients could have been diagnosed with an earlier stage gastric cancer before January 1, 2012); (2) had completed first-line treatment with chemotherapy that included a platinum analogue (cisplatin, carboplatin, oxaliplatin) and/or a fluoropyrimidine (5-FU, capecitabine, TS-1, UFT) with or without any other medication (biologic or cytotoxic agent), and had continued with either second-line treatment or BSC; (3) had at least three months of follow-up after the first administration of a second-line therapy regimen or the initiation of BSC following the end of first-line therapy (except for patients with a documented death after mGC diagnosis); (4) were at least 18 years of age at the time of mGC diagnosis; (5) had no other primary malignant tumors; and (6) had no prior participation in clinical trials following mGC diagnosis.
Data collection
Physicians reported information on their medical specialty, whether they were affiliated with a teaching hospital, years in practice, and the number of eligible patients treated. For each patient, physicians reviewed medical records to provide detailed patient demographic and clinical characteristics. Patient baseline characteristics included age as of the index date, sex, ethnicity, body mass index (BMI), smoking history, alcohol consumption, history of H. pylori infection, family history of gastric cancer, the Charlson Comorbidity Index (CCI) (excluding malignancy, adaptation from Romano et al.[Citation12]), location of the primary tumor, location(s) of metastasis, HER2/neu gene expression test results, time between gastric cancer diagnosis and mGC diagnosis, and prior therapies.
Information on treatment patterns by line of therapy and cancer-related HRU following mGC diagnosis was collected during the follow-up period. This included patients' Eastern Cooperative Oncology Group (ECOG) score [Citation13] at the start of first-line treatment, first-line treatment regimens, duration of first-line treatment period, and reason(s) for ending first-line treatment. Among patients who received second-line treatment, ECOG score at the start of second-line, regimens, treatment duration, and reason for ending the treatment were also recorded. Information regarding the nature of BSC received (pharmaceuticals, procedures [e.g. endoscopy], and nutritional support) as well as HRU was collected following the index date. Common symptoms experienced by mGC patients were reported during patients' first, second and third lines of therapy (if applicable).
Statistical analyses
All variables were analyzed using descriptive statistics. Means and standard deviations (SDs) were reported for continuous variables and frequencies and percentages were used for categorical variables.
Patients were stratified into two cohorts: patients whose first-line therapy was followed by BSC only (BSC cohort), and patients whose first-line therapy was followed by second-line therapy (2nd-line cohort). Baseline patient characteristics, duration of the follow-up period, and first-line chemotherapy treatment patterns (i.e. ECOG score at the start of first-line treatment, first-line treatment regimens, duration of first-line treatment period, and reason[s] for ending first-line treatment) were compared between the 2nd-line and the BSC cohorts. Chi-squared tests were used for comparisons of proportions, and Wilcoxon rank-sum tests were used for comparisons of continuous variables. p-values <.05 were considered statistically significant. All analyses were performed using SAS 9.3 or more recent (SAS Institute, Cary, NC).
Results
Physician and patient characteristics
A total of 88 physicians participated in the study and provided de-identified data based on 202 patient charts. Most physicians (53, 60.2%) specialized in oncology with an average of 11.2 years (SD = 6.1) in practice; the other 35 physicians (39.8%) reported chemotherapy as their specialty. A total of 35 (39.8%) physicians reported affiliation with a teaching hospital. Physicians reported an average of 25 eligible patients under their care since January 1, 2012.
Patients were diagnosed with mGC between February 1, 2012, and October 12, 2016. Mean age at mGC diagnosis was 53.7 years (SD = 11.2), and 70.8% were male. Patients with no history of smoking comprised 42.1% of the study sample, while 31.2% and 21.3% were current or former smokers, respectively. A majority of patients had a history of light to moderate alcohol use (55.0%) and 6.4% of patients had heavy alcohol consumption. A history of H. pylori infection or a family history of gastric cancer was observed in 26.2% and 16.8% of patients, respectively. On average, mGC diagnosis occurred 3.2 months (SD = 7.2) after initial gastric cancer diagnosis. Almost half of patients (49.5%) had metastatic gastric cancer (Stage IV) when first diagnosed. In 32.7% of patients, the fundus and corpus were the primary tumor locations. Other common primary tumor locations included the whole stomach (21.8%), antrum and pylorus (19.8%), and gastric cardia (18.3%). A total of 92 patients were tested for HER2/neu gene expression, among whom 30 (32.6%) were positive. Among patients with at least one metastasis, (overall n = 181; BSC cohort: n = 26, 2nd-line cohort: n = 155, p = 0.58), the most frequently reported metastatic sites were the liver (53.6%), lymph nodes (53.0%), and peritoneum (44.8%).
Patients in the BSC cohort had lower BMI and faster progression between gastric cancer diagnosis and mGC diagnosis than patients in the 2nd-line cohort (1.5 months [SD = 5.4] vs 3.5 months [SD = 7.4], p = .03). Patients in the BSC cohort had a higher likelihood of having a metastasis in the peritoneum compared to the 2nd-line cohort (76.9% vs. 39.4%, p < .01). A higher proportion of patients in the BSC cohort had received adjuvant chemotherapy prior to mGC diagnosis compared to the 2nd-line cohort. A similar proportion of patients had a gastric cancer-related surgery prior to the index date (BSC cohort = 10.0%, 2nd-line cohort = 26.7%, p = .13) ().
Table 1. Patient baseline characteristics.
Treatment regimens by line of therapy
The majority of patients in this study were diagnosed with mCG in 2015 (36.6%) or 2016 (42.1%). The mean follow-up from mGC diagnosis to the end of observation was 11.2 (median = 8.6, SD = 8.4) months. Per eligibility criteria, all patients in the study had fluoropyrimidine/platinum as first-line therapy for mGC, with or without other agents. At first-line therapy initiation, 58.3% of patients were symptomatic but completely ambulatory (ECOG = 1), while some remained asymptomatic (ECOG = 0, 15.5%). For first-line therapy, the most frequently reported regimens were fluoropyrimidine and platinum agent combinations (44.1%), which consisted mostly of 5-FU and cisplatin (18.3%) and capecitabine and oxaliplatin (5.9%) followed by fluoropyrimidine monotherapy (17.3%), and platinum monotherapy (9.9%). Physicians reported selecting first-line treatment based on own experience (76.2%) and national guidelines (63.4%). Mean duration of first-line treatment was 139.2 days (median = 122, inter-quartile range [IQR]: 71–173). In 50.5% of patients, first-line therapy was discontinued because of disease progression, and in 39.1% of patients first-line therapy ended due to adverse events (AEs) or toxicity. Patients in the BSC cohort discontinued first-line therapy because of disease progression less often than patients in the 2nd-line cohort (33.3% vs. 53.5%, p = .04). They also discontinued because of reaching the end of their treatment protocol more often than patients in the 2nd-line cohort (16.7% vs. 5.2%, p = .04).
At second-line therapy initiation, 46.5% of patients were symptomatic but completely ambulatory (ECOG = 1), while 29.2% were symptomatic and spend <50% of the day in bed (ECOG = 2). The most frequent regimen categories used in second-line therapy were fluoropyrimidine monotherapy (18.6%), fluoropyrimidine and platinum agent combinations (18.6%), taxane monotherapy (14.0%), and irinotecan and platinum agent and/or fluoropyrimidine (8.7%). The remaining patients (40.1%) were given other various types of therapy, including irinotecan monotherapy (6.4%), capecitabine + cisplatin + trastuzumab combination therapy (2.9%), and cisplatin monotherapy (2.3%). The most frequent regimens were capecitabine (14.5%), paclitaxel (9.3%), and capecitabine + oxaliplatin (8.7%). For 70.9% of patients, physicians reported selecting therapy based on national guidelines; for 68.6%, choice of therapy was guided at least in part by own experience. Median duration of second-line treatment was 201 days [IQR: 71-Not reached]. The most frequent best response to therapy was stable disease (39.5%). Disease progression was the leading cause of discontinuing second-line therapy (39.8% of patients), followed by patient refusal to continue (37.5%) (). Approximately 10% (n = 20) of patients in the study received third-line chemotherapy.
Table 2. Patient status and treatment regimens by line of therapy.
To gain insight into the potential reasons why some patients received second-line therapy with fluoropyrimidine monotherapy vs. fluoropyrimidine and platinum agent combinations, select patient characteristics such as first-line regimen category, reason for discontinuation of first-line therapy, and ECOG status were described separately for patients with different second-line regimens. No statistical comparisons were conducted due to the small sample sizes. Among patients who were treated with fluoropyrimidine monotherapy in second-line relative to those treated with fluoropyrimidine and platinum agent combinations, a numerically higher proportion (31.3% vs. 15.6%, respectively) had other regimens in first-line (i.e. these patients had first-line therapy other than fluoropyrimidine monotherapy, platinum monotherapy, fluoropyrimidine and platinum agent combinations, or triple therapy with fluoropyrimidine, platinum agent and taxane). In addition, a numerically higher proportion of patients treated with fluoropyrimidine monotherapy in second-line had first-line therapy with fluoropyrimidine and platinum agent combinations (43.8% vs. 34.4%), suggesting that these patients may have received fluoropyrimidine monotherapy in second-line due to toxicity with a platinum combination in first-line therapy. The distributions of ECOG scores at the start of second-line therapy suggested worse performance status among patients who were treated with fluoropyrimidine monotherapy in second-line relative to those treated with fluoropyrimidine and platinum agent combinations (no statistical comparisons were conducted).
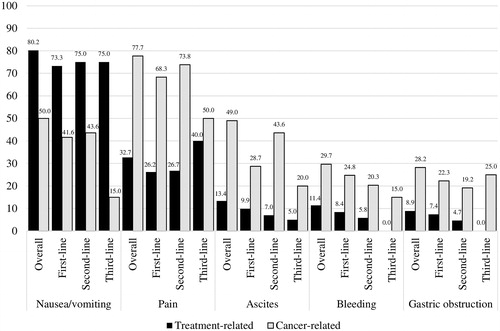
Physician-reported patient symptoms
The most commonly experienced treatment-related symptoms throughout the study period were nausea/vomiting (80.2%) and pain (32.7%). Treatment-related nausea/vomiting occurred in 73.3% of patients during first-line therapy, 75.0% during second-line therapy, and 75.0% during third-line therapy. Pain and nausea/vomiting related to cancer were reported in 77.7% and 50.0% of patients, respectively ().
Supportive care and healthcare resource use
Overall, supportive care most frequently consisted of antiemetics, chemotherapeutic medication (i.e. treatments that do not serve a curative purpose), and non-narcotic analgesics. Antiemetics were administered in 66.8%, 23.3%, 63.4%, and 55.0% of patients during first-line, BSC, second-line, and third-line therapy respectively. Non-narcotic analgesics in 53.5%, 40.0%, 48.3%, and 20.0% of patients during first-line, BSC, second-line, and third-line treatment, respectively.
Endoscopies were conducted in 52.5% of patients during first-line treatment: 6.7% of patients in BSC, and 45.9% of patients during second-line treatment. Among patients with known inpatient hospitalization information, inpatient hospitalizations for regularly-scheduled cancer drug administration were reported for 88.7% of all patients during first-line treatment (mean total days during first-line 26.1 [SD = 20.6]), 33.3% of patients during BSC among patients in the BSC cohort (mean total days during BSC 6.0 [SD = 5.7]), and 74.5% of patients during second-line treatment among patients in the 2nd-line cohort (mean total days in the hospital during second-line 16.3 [SD = 13.5]).
Inpatient hospitalizations for other cancer-related care were reported for 38.1% (mean total days 24.2 [SD = 16.4]) of patients during first-line, 20.0% (mean total days 8.8 [SD = 3.1]) during BSC among patients in the BSC cohort, and 24.4% (mean total days 15.8 [SD = 12.8]) of patients during second-line treatment (data not shown). Among patients with known outpatient hospital visit information, outpatient hospital visits for regularly-scheduled cancer drug administration were reported in 62.8% of patients during first-line treatment, 45.5% of patients in BSC, and 56.0% of patients during second-line treatment. Similarly, outpatient hospital visits for other cancer-related care, which were most frequent for disease symptom management or an AE/toxicity, occurred in 43.9%, 50.0%, and 48.1% of patients during first-line, BSC, and second-line treatment, respectively ().
Table 3. Supportive care and healthcare resource use.
Discussion
In this study of 202 mGC patients who completed first-line treatment with chemotherapy with a platinum analogue (cisplatin, carboplatin, oxaliplatin) or a fluoropyrimidine regimen, and continued with either second-line therapy or BSC, the majority of patients (85%) received second-line chemotherapy. Half of the patients discontinued first-line therapy due to disease progression and among those who received second-line therapy, 39.8% discontinued treatment due to disease progression. Second-line regimens were highly variable; there were 52 unique treatment regimens prescribed in second-line. Supportive care was commonly used to treat both disease- and treatment-related symptoms.
HRU was driven both by chemotherapy administration and disease symptom management. The most commonly reported cancer treatment-related symptoms experienced by patients were nausea/vomiting and pain, and the most commonly used supportive care agents were antiemetics and chemotherapeutic medication. In addition to inpatient visits for chemotherapy administration, patients were often seen in outpatient hospitals and oncology clinics for the management of disease symptoms and AEs/toxicities.
In this study sample, 70.8% of patients were male, which is consistent with the higher incidence of gastric cancer in men [Citation14]. Half of the patients in the present study were current or former smokers; 55% had light or moderate, and 6.4% had heavy alcohol consumption. The association between smoking and heavy alcohol consumption in the etiology of gastric cancer is well-established.[Citation15,Citation16] These observations are consistent with findings of a previous study based on the Russian population, that reported an association between smoking and heavy alcohol consumption with increased risk of gastric cancer [Citation17].
The vast majority of patients with mGC in the present study received chemotherapy which is consistent with prior reports. In the Registry of gastric cancer treatment evaluation (REGATE) II study [Citation18], overall, 90% of patients received chemotherapy, mostly fluoropyrimidine/platinum combinations, which was also the most common first-line regimen physicians recommended in the present study (single agent fluoropyrimidine or fluoropyrimidine/platinum combination). Several evidence-based guidelines for the management of gastric cancer have been developed [Citation4,Citation14,Citation19]. European (ESMO), North American guidelines (NCCN) and Asian guidelines all recommend primary palliative chemotherapy with doublet or triplet fluoropyrimidine/platinum combinations for management of patients with mGC [Citation4,Citation14,Citation19]. However, these guidelines may not be implemented consistently across these various geographies, due to important differences in mGC epidemiology, socioeconomic environment, resources, and healthcare policy. Significant geographical differences exist in Asian countries in the use of second-line chemotherapy for patients with advanced or metastatic gastric cancer, which is partly attributable to the absence of strong evidence to support survival benefit of second-line treatment regimens [Citation14]. The heterogeneity in second-line treatment regimens observed in the present study is in line with these findings.
In the present study, approximately 15% of patients received BSC only after first-line therapy. This is slightly lower than what was observed in a 2017 real-world study on the treatment patterns of mGC in South Korea, which found that 19.7% of patients received BSC only after first-line therapy, compared to 80.3% of patients who pursued to second-line therapy [Citation20]. A similar 2017 study conducted in Taiwan found that 35.2% of patients received BSC only whereas 64.8% of patients received second-line therapy [Citation21]. It is interesting to note that, when asked a general question about the approximate percentage of patients who received BSC only after first-line therapy among patients treated with first-line therapy as part of this chart review, physicians estimated that, on average, 41% received BSC only. It is possible that physicians had follow-up information only for patients under active treatment, and thus may have had more patients initiating second-line therapy for whom they had at least 3 months of follow-up. Therefore, while the proportion of patients who received BSC only compared to second-line therapy appears to be lower in Russia than in other countries, caution is warranted when interpreting this finding, as the inclusion criteria may have underestimated the true proportion of BSC only patients.
Approximately 10% of the patient received third-line therapy. However, the percentage of patients receiving third-line therapy in this study is likely an underestimation of the true proportion of patients with mGC who receive third-line therapy, because the median follow-up from mGC diagnosis to last contact was 8.6 months and data were censored at the time of chart abstraction.
Future research should examine the implications of testing for the genomic subtype of gastric cancer on real-world treatment patterns (Epstein–Barr virus [EBV]-infected tumors, microsatellite instability [MSI] tumors, genomically stable tumors, and chromosomally unstable tumors) [Citation22]. Recent data have demonstrated the potential benefit of targeted therapy, including among patients with EBV-infected tumors and MSI tumors which may respond to PD-1 inhibition [Citation23,Citation24]. Thus, it is likely that the tumor genomic subtype will have a substantial impact on the real-world treatment patterns observed among patients with mGC in the years to come. The novelty of genetic testing may also explain the low frequency of HER2/neu gene expression test observed in the results. At the time of data collection, HER2/neu gene expression was not routinely performed among patients with mGC because treatments for HER2-positive cancers (i.e. trastuzumab) were not commonly available. This testifies of the rapidly-changing treatment landscape for the treatment of patients with mGC in Russia.
Limitations
This study is subject to some limitations common to retrospective studies using data collected through patient chart reviews. First, the completeness and accuracy of collected patient information depended on the physician recording of medical history information in patient charts. Second, although automated quality control checks for the chart abstraction form helped minimize possible inconsistencies in the recording of information from medical charts, patient-reported information documented in medical records and abstracted in this study (e.g. smoking and alcohol use) may be subject to self-report bias. Third, physicians may not have had full access to records documenting all medical care administered to patients over the course of mGC treatment. For example, some HRU such as emergency room visits, gastrointestinal clinic visits, hospice and home visits could have been unknown to treating physicians. Finally, despite the inclusion of physicians from practices across a number of geographic regions in Russia, treatment patterns reported in this study may not be representative of the treatment practice of all physicians or the treatments for all mGC patients in Russia, and global generalizability of the study results may be limited. Indeed, previous studies have noted differences between gastric cancer patients in eastern and western countries [Citation25–27].
Conclusions
Treatment patterns for patients with mGC in Russia are highly heterogeneous. The results of this study indicate the need for more intensive implementation of the most active regimens in second-line treatment of mGC according to international and national guidelines. This study also provides stakeholders with information on the current treatment landscape and related HRU among patients with advanced and metastatic gastric cancer in Russia. Further studies will be needed to understand the impact of future novel agents on treatment patterns and healthcare use in this population.
Transparency
Declaration of funding
This study was funded by Eli Lilly and Company.
Declaration of financial/other relationships
ES and DN are employees of Eli Lilly and Company and may own stock/stock options. WYC and PT-L are employees of Analysis Group Inc., which has received research funding from Eli Lilly and Company to conduct analyses for the current study. JII and LMS were employees of Analysis Group, Inc. at the time of the study. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed to the study design, interpretation of the results, and manuscript development. LMS and PT-L performed data analysis. All authors agreed to submit the manuscript for publication.
Prior presentations
Parts of the material in this manuscript have been presented as a poster at the International Society for Pharmacoeconomics and Outcomes Research 20th Annual European Congress (Glasgow, Scotland 2017).
Acknowledgements
The authors would like to thank Sara Kaffashian for editorial assistance.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Kaprin A, Starinsky V, Petrov G. Malignant neoplasms in Russia in 2016 (Morbidity and Mortality). Moscow, Russia: P. A. Hertsen Moscow Oncology Research Center - Branch of FSBI NMRRC of the Ministry of Health of Russia; 2018.
- Kaprin A, Starinsky V, Petrov G. Status of oncological assistance to the population of Russia in 2016. Moscow, Russia: P. A. Hertsen Moscow Oncology Research Center - Branch of FSBI NMRRC of the Ministry of Health of Russia; 2017.
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl_5):v38–v49.
- Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20(4):633–649.
- World Cancer Research Fund International. Continuous Update Project: Diet, nutrition, physical activity and stomach cancer 2016. [cited 2018 26 Mar]. Available from: https://wcrf.org/sites/default/files/Stomach-Cancer-2016-Report.pdf
- Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388(10060):2654–2664.
- Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Db Syst Rev. 2017;8:Cd004064.
- Bilici A. Treatment options in patients with metastatic gastric cancer: current status and future perspectives. WJG.. 2014;20(14):3905–3915.
- Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC–A Randomized Phase III Trial. JCO. 2016;34(5):443–451.
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14(6):490–499.
- Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079. discussion 1081-90.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
- Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14(12):e535–47.
- Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19(7):689–701.
- Rota M, Pelucchi C, Bertuccio P, et al. Alcohol consumption and gastric cancer risk-A pooled analysis within the StoP project consortium. Int J Cancer. 2017;141(10):1950–1962.
- Zaridze D, Borisova E, Maximovitch D, et al. Alcohol consumption, smoking and risk of gastric cancer: case-control study from Moscow, Russia. Cancer Causes Control. 2000;11(4):363–371.
- Ter-Ovanesov M, Yalcin S, Zalcberg J, et al. Registry of gastric cancer treatment evaluation (REGATE): II treatment practice. Asia-Pac J Clin Oncol. 2013;9(4):373–380.
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Ne. 2016;14(10):1286–1312.
- Carter GC, Kaltenboeck A, Ivanova J, et al. Real-world treatment patterns among patients with advanced gastric cancer in South Korea. Cancer Res Treat. 2017;49(3):578–587.
- Cuyun Carter G, Kaltenboeck A, Ivanova J, et al. Treatment patterns in patients with advanced gastric cancer in Taiwan. Asia-Pac J Clin Oncol. 2017;13(3):185–194.
- Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209.
- Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458.
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413.
- Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15(5):489–538.
- Levi F, Lucchini F, Gonzalez JR, et al. Monitoring falls in gastric cancer mortality in Europe. Ann Onco. 2004;15(2):338–345. Feb
- Primic-Zakelj M, Zadnik V, Zagar T. Is cancer epidemiology different in Western Europe to that in Eastern Europe? Ann Onco. 2005;16(Suppl 2):ii27–9.