Abstract
Objective: Whether reducing low density lipoprotein cholesterol (LDL-C) is associated with cardiovascular benefits in low risk normocholesterolaemic subjects is unknown. The INTENSITY LOW [Investigating the lowest threshold of vascular benefits from LDL-cholesterol lowering with a PCSK9 mAb inhibitor (alirocumab) in healthy volunteers] study aims to assess whether lowering LDL-C by alirocumab monotherapy can improve endothelial-dependent vascular function compared with placebo (primary objective) in low-risk normocholesterolaemic healthy individuals. Changes in endothelial-dependent or endothelial-independent vascular function, arterial stiffness and biomarkers of systemic inflammation by alirocumab, atorvastatin or their combination are secondary objectives.
Study design and methods: This is a single-center, randomized, two-period, single-blind, placebo-controlled clinical trial. The study was registered on clinicaltrials.gov (N03273972).
It will include 30 healthy low-risk subjects with LDL-C < 4.1 mmol/l. After passing the screening visit (Visit 1), eligible participants will be randomized 1:1 to either subcutaneous alirocumab 150 mg or placebo. These will be administered as single doses in 2 visits 14 days apart (Visits 2 and 3). Atorvastatin 20 mg once nightly will be prescribed for 14 days at Visit 3 in both groups through to Visit 4.
At baseline (Visit 2) and during all post-dose visits (Visits 3–4), endothelial function will be assessed using venous occlusion plethysmography. Specifically, changes in forearm blood flow responses to intra-arterial infusions of acetylcholine, sodium nitroprusside and L-NG-monomethyl-arginine acetate will be assessed as surrogates of endothelial-dependent and -independent vasodilatation. Additionally, arterial stiffness and carotid intima-media thickness will be evaluated at the same timepoints. The above-mentioned changes will be correlated with changes in lipid and systemic inflammation biomarkers.
Introduction – rationale of the study
Cardiovascular (CV) disease remains the commonest cause of mortality worldwide. Hypercholesterolemia, particularly elevated low-density lipoprotein cholesterol (LDL-C), is considered an independent CV risk factorCitation1. Statins are first-line evidence-based medications to significantly lower LDL-C levels and CV events in both the primary and secondary prevention settingCitation2. Despite effective LDL-C lowering, a considerable residual CV risk still exists.
Endothelial dysfunction presents early in the atherosclerotic process and contributes to this residual riskCitation3. There is a cross-talk between hypercholesterolemia, low-grade vascular inflammation, oxidative stress and endothelial dysfunctionCitation4,Citation5. Namely, the accumulation of LDL particles in the subendothelial space and their oxidative modification (oxLDL) alongside the oxidation of the lipoprotein-associated phospholipids results in vascular inflammation. It also results in impaired endothelial-dependent vasodilatation through reduced nitric oxide (NO) bioavailabilityCitation6.
The gold standard for assessing endothelial function is measuring forearm blood flow using venous occlusion plethysmographyCitation7. This is a well described method of assessing and determining vascular function, which can interrogate endothelial NO dependent and independent mechanismsCitation8. It can also predict the risk of future CV eventsCitation9. Arterial stiffness is now rapidly being accepted as a global measure of functional and structural changes, which accompanies CV disease and is associated with outcomesCitation10.
It is now apparent that lowering LDL-C levels is the main lipid change to result in significant outcome benefits. In this context, statin-related CV risk reduction has been proportional to their LDL-C lowering effects in both the primary and secondary preventionCitation2. However, it has also been proposed that these benefits are related to properties beyond lipid-lowering (the so-called pleiotropic effects). These include anti-oxidant and anti-inflammatory actions as well as atherosclerotic plaque stabilizationCitation11. Statins also improve NO bioavailability, and subsequently endothelial functionCitation11. Nevertheless, it has been debated whether the latter is a lipid-dependent or indeed a pleiotropic effect of statins per se. In this regard, similar LDL-C reductions by either statins or non-statin lipid lowering medications resulted in comparable endothelial function improvement and arterial stiffness reductionCitation12.
Accumulating evidence suggests that boosting LDL-C lowering by adding either ezetimibe or proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitors to statins is associated with greater benefits in CV morbidity and mortality. This was evident even in patients with mildly raised LDL-C levels at baselineCitation13–15. PCSK9 inhibitors (e.g. alirocumab and evolocumab) are very potent lipid lowering medications exhibiting effects at least comparable to statins on LDL-C, when used as monotherapyCitation16. The synergistic effects on LDL-C lowering when combined with statins resulted in very low on-treatment levels, especially in secondary prevention trialsCitation14, Citation15. These levels had not been previously encountered in statin ± ezetimibe trials and were associated with further reductions in CV morbidity and mortalityCitation13–15. To date, the threshold of LDL-C below which no further CV benefit is expected has not been yet identified in secondary prevention trialsCitation17.
In the primary prevention setting, such evidence is even poorer, since hard outcome clinical trials with PCSK9 inhibitors or ezetimibe combined with statins are lacking. It has been widely shown that statin benefits are more apparent in intermediate and high-risk hypercholesterolaemic individuals, while only modest in low-risk onesCitation2. Also, statin benefits on hard outcomes may extend to intermediate- and high-risk normocholesterolaemic populations with subclinical levels of inflammationCitation18, Citation19. This evidence suggests that some extra benefit from LDL-C lowering is expected even within the normal range. However, the LDL-C threshold below which no more benefit is expected in primary prevention remains unknownCitation17.
There is a paucity of data on whether reducing LDL-C can result in CV risk reduction in low-risk normocholesterolaemic individuals. The INTENSITY LOW study (Investigating the lowest Threshold of vascular benefits from LDL-cholesterol lowering with a PCSK9 mAb inhibitor (alirocumab) in healthy volunteers – A mechanistic physiological study) seeks to answer if there are limits to LDL-C reduction in terms of benefits to vascular health from a mechanistic viewpoint. It also evaluates whether there are potential limitations to primary prevention in healthy volunteers by lowering LDL-C using PCSK9 inhibitors. This will determine if there is a plateau beyond which there is no further benefit on early biomarkers of CV disease.
We suggest that identifying this threshold will have major public health implications, by changing the conception of what is ‘normal cholesterol’. The INTENSITY-LOW study will also investigate whether LDL-C lowering via PCSK9 inhibition has comparable benefits in vascular function to the ones achieved by statins. This will provide significant and specific evidence that the so-called pleiotropic effects of statins are mediated by LDL-C lowering per se, and not necessarily by extra-lipid actions.
Study objectives
The primary objective of the study is to assess whether LDL-C reduction with alirocumab improves NO bioavailability as measured by forearm plethysmography in healthy normocholesterolaemic individuals. Assessment of whether: (1) alirocumab is comparable to statin in improving endothelial function when similar LDL-C lowering is achieved; (2) alirocumab + statin is better than statin monotherapy in improving endothelial function, due to more potent LDL-C lowering with the combination therapy; (3) alirocumab reduces markers of systemic inflammation in healthy individuals; (4) to provide inputs to a decision model to explore whether alirocumab has the potential to be cost-effective in healthy individuals.
Study outcome measures
The primary outcome measure for the study is the change in forearm blood flow ratio (and additionally, absolute and % change), as measured by venous occlusion plethysmography, in response to intra-arterial Acetylcholine (ACh) infusion, comparing alirocumab with placebo. Secondary outcome measures include change in forearm blood flow ratio comparing various treatment groups, as well as change in arterial stiffness and other haemodynamic measurements. These are detailed in Textbox 1.
Textbox 1. Study outcome measures.
Study design
This is a single-center, randomized, placebo-controlled study, which will include healthy low-risk normocholesterolaemic volunteers. It will take place at the National Institute for Health Research Cambridge Clinical Research Center, Cambridge University Hospitals NHS Foundation Trust and the overall duration of the study for each participant will be approximately 10 weeks. The study is sponsored by Cambridge University Hospitals NHS Foundation Trust. The study protocol was accorded a favorable ethical opinion by the East Midlands – Leicester South Research Ethics Committee (17/EM/0278) and was registered on clinicaltrials.gov (N03273972).
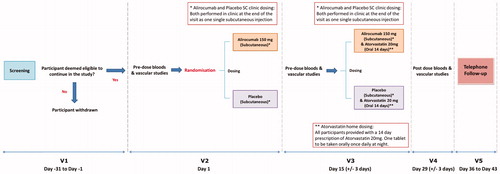
The study design is summarized in .
Figure 1. Synopsis of the study design. After a screening visit all eligible volunteers will attend V2 in which they will have pre-dose blood tests and vascular studies, and then will be randomized to either single sc dose of alirocumab 150 mg or placebo. They will then attend V3 14 ± 3 days later to have pre-dose blood tests and vascular studies and be administered another sc dose of alirocumab 150 mg or placebo. After this all participants in both groups (alirocumab and placebo group) will be prescribed atorvastatin 20 mg once nightly for 14 days. They will then attend V4 for post-dose blood tests and vascular studies. A follow up visit (V5) will take place 7–14 days later. Abbreviations. SC, subcutaneously; V1, Visit 1; V2, Visit 2; V3, Visit 3; V4, Visit 4; V5, Visit 5.
All eligible healthy volunteers (for Inclusion/Exclusion criteria see below) will be randomized in a 1:1 ratio to ONE of the following treatment arms:
Alirocumab treatment arm: Alirocumab 150 mg (single subcutaneous injection in clinic) at Visit 2, followed by alirocumab 150 mg as single subcutaneous injection in clinic + prescription of atorvastatin 20 mg (oral tablet) for 14 days once nightly at Visit 3.
OR
Comparator treatment arm: Placebo (single subcutaneous injection in clinic) in Visit 2, followed by placebo as single subcutaneous injection in clinic + prescription of atorvastatin 20 mg (oral tablet) for 14 days once nightly at Visit 3.
The research team will be blinded as for the administration of alirocumab or placebo at Visits 2 and 3, while atorvastatin will be openly prescribed to all study participants at Visit 3.
Study population
The INTENSITY-LOW study will recruit enough healthy normocholesterolaemic volunteers to ensure that 30 subjects will complete the study (n = 15 in each treatment group). The inclusion/exclusion criteria of the study population are detailed in Textbox 2. In brief, the study population will consist of healthy normocholesterolaemic subjects aged 18–45 years who lack any established CV risk factors. Study participants would not be eligible for cholesterol-lowering treatment according to the existing dyslipidaemia and CV risk management guidelines. Subjects with established CV disease or relevant risk factors as well as subjects who would have any contraindication to any of the study treatments/procedures will be excluded from the study.
Textbox 2. INTENSITY-LOW study population – Inclusion and Exclusion criteria.
Study visits and assessments
Subject recruitment
Potential participants will be identified through a variety of advertising sources, including recruitment posters, study websites, newspapers, public areas and social media if applicable. All subjects will be prescreened for eligibility via a telephone call (after expressing interest) and will be provided with a participant information sheet to review for at least 24 h. The research team will then follow up to assess whether each subject is interested in taking part. If so, a screening visit (V1) will be scheduled for the informed consent process to be completed.
Screening visit (visit 1)
Healthy volunteers will attend a screening visit where they will provide full written informed consent. Following informed consent, a full eligibility check will be carried out where the following assessments will be completed: (1) clinical chemistry (as described in the study methods section); (2) hematology (as described in the study methods section); (3) pregnancy test (if applicable); (4) 12-lead electrocardiogram (ECG); (5) blood pressure and heart rate assessment; (5) concomitant medication check, (6) physical examination, and (7) previous medical history. All eligible volunteers will continue with the next phase of the study.
Visit 2 - Day 1
Visit 2 can be conducted on any day following screening provided it does not occur >31 days post screening visit. At Visit 2 the following assessments and procedures will be completed: (1) clinical chemistry sampling (as described in the study methods section), (2) haematology (as described in the study methods section), (3) serum sample for systemic markers [interleukin (IL)-6, IL-2, matrix metalloproteinase 9 (MMP9) and oxidized low density lipoproteins (oxLDL), (4) pregnancy test (if applicable), (5) 12-lead ECG; (6) blood pressure and heart rate assessment, (7) arterial stiffness, (8) central haemodynamics assessment, (9) carotid intima media thickness (IMT) measurement, (10) forearm blood flow studies, (11) concomitant medication check, (12) safety review and reporting, and, (13) randomization.
Dosing: Following randomization eligible subjects will be dosed with either alirocumab or placebo in clinic following completion of the above assessments. Participants will be assessed for any potential post-dose instant reactions (e.g. hypersensitivity).
Visit 3 - Pre-dose, bloods and vascular studies - Day 15 ± 3 days
Approximately 2 weeks following Visit 2, participants will be asked to attend a study visit where the same assessments as in Visit 2 will be completed. In Visit 3 all subjects will also be dosed with either alirocumab or placebo in clinic following completion of the assessments. In addition, participants randomized to both treatment arms will be prescribed atorvastatin 20 mg to be taken every evening for 14 days. A prescription will be provided to ensure enough atorvastatin supply until the next scheduled visit (i.e. 14 days ±3 days). Following dosing, participants will be assessed for any potential instant reactions to the subcutaneous alirocumab or placebo administration (e.g. hypersensitivity).
Visit 4 - Post dose, bloods and vascular studies - Day 29 ± 3 days
Approximately 2 weeks following Visit 3, participants will be asked to attend a post-dose visit where assessments as in Visit 2 and Visit 3 will be completed. No dosing will be provided in this visit and compliance check will be performed for atorvastatin administration via pill count.
Visit 5 - Follow up visit - Day 36 to day 43
A telephone follow-up will be scheduled approximately 7–14 days following Visit 4, where a review of any adverse events (AE)/serious adverse events (SAE) from the last visit will take place.
Study methods
Clinical assessments
Clinic blood pressure and heart rate will be measured in the seated position after 5 min rest. Repeated blood pressure (BP) measurements using an automated BP device will be taken. An average will be used to determine eligibility. A validated machine will be used.
Non-sterilised, pre-menopausal women will undergo urinary beta-human chorionic gonadotropin (HCG) testing at visits 1–4. They will also be given advice on contraceptive use in the Participant Information Sheet.
Laboratory assessments
Blood samples will be obtained after 6–8 h overnight fast for clinical chemistry: Sodium, potassium, glucose, creatinine, bilirubin [total; direct and indirect will be reported if total is >2 × the upper limit of normal (ULN)], alanine transaminase (ALT), γ-glutamyl transferase (γGT), alkaline phosphatase, urea, total creatine kinase (CK), high sensitivity C-reactive protein (hsCRP) and serum lipid profile [total cholesterol, triglycerides, high density lipoprotein cholesterol, LDL-C] and haematology [haemoglobin, haematocrit, platelets, total white blood cells (WBC), neutrophils, lymphocytes, monocytes, eosinophils and basophils] multiple times throughout the study. Thyroid stimulating hormone and HbA1c will also be analyzed at the screening visit. Serum samples will be collected for storage. A total of 195 ml of blood will be taken from each participant over the course of this study, which is intended for analysis and/or storage. Blood and serum samples may be stored for further analysis until study close. This will be used to analyze systemic markers of inflammation [for example (but not limited to IL-6, IL-2, MMP9 etc] and lipid sub-fractions [for example (but not limited to) oxLDL].
Vascular studies
Arterial stiffness and central haemodynamics
Central haemodynamics as well as arterial stiffness [assessed by determination of the Augmentation Index (AIx) and the carotid-to-femoral Pulse Wave Velocity (PWV)] will be determined using a commercially available SphygmoCor system (AtCor Medical, Sydney, Australia), as previously describedCitation20.
Carotid IMT
Carotid IMT will be measured by using a high-resolution ultrasound scanner with a 10 MHz linear-array transducer (MyLab25 Gold Ultrasound Machine, Esaote, Italy), with the volunteer resting in the supine position for at least 5 min. The common carotid artery will be identified and scanned, 2 cm below its bifurcation. Measurements will be repeated 3 times, and the average of the values will be recorded.
Forearm blood flow (FBF) studies
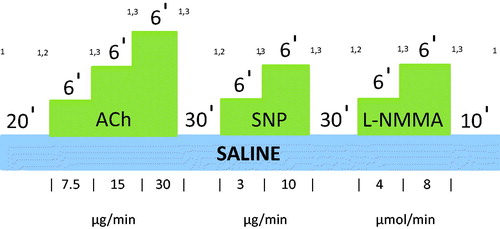
FBF will be measured simultaneously in both arms using venous occlusion plethysmography with indium-gallium strain gauges electrically calibrated, as previously describedCitation8. A 27-gauge unmounted steel needle will be inserted into the non-dominant brachial artery under local anesthesia (1% lignocaine hydrochloride). Saline or drugs will be infused at a constant rate of 1.0 ml/min by means of a constant rate infusion pump. Basal blood flow will be recorded after 20 min of saline infusion. Ach (7.5, 15 and 30 µg/min) will then be infused intra-arterially at 1 ml/min as a measure of endothelial NO activity. Each dose will be infused for 6 min and forearm blood flow will be measured for 3 min at the end of each infusion period. After a further 30 min washout with saline, the NO donor, sodium nitroprusside (SNP) will be infused intra-arterially at 2 doses (3, 10 µg/min) for 6 min each to test for NO-independent mechanisms. Finally, the NO synthase antagonist L-NG-monomethyl-arginine acetate (L-NMMA) will be infused after a 30 min saline washout, again at 2 doses (4 and 8 µmol/min), and FBF measured as before. A final 10 min of saline will then be infused. BP and heart rate will be recorded at baseline, and at the end of each infusion. The ACh, SNP and L-NMMA infusion pattern is described in .
Figure 2. Ach, SNP and L-NMMA infusion pattern in FBF studies. FBF will be measured at baseline and after 20 mins of saline intra-arterial infusion at a rate 1 ml/min. Following this, 3 different concentrations of Ach will be infused intra-arterially at the same rate for 6 mins each. At the last 3 mins of each infusion period FBF will be measured. After 30 mins of washout 2 different concentrations of SNP will be infused intra-arterially for 6 mins each, and FBF will be measured again at the last 3 mins of each infusion period. Following 30 mins washout period 2 different concentrations of L-NMMA will be infused intra-arterially for 6 mins each, and FBF will be measured again at the last 3 mins of each infusion period. A final 10 min of saline will then be infused. BP and heart rate will be recorded at baseline, and at the end of each infusion. 1Blood pressure and heart rate recorded at baseline and at the end of each infusion. 2Basal FBF recorded after 20-min Saline infusion. 3Forearm blood flow recorded after each dose of challenge agent for 3 min. Abbreviations. Ach, acetylcholine; SNP, sodium nitroprusside; L-NMMA, L-NG-monomethyl-arginine acetate; FBF, forearm blood flow.
Safety (AE/SAE reporting)
AE/SAEs will be collected and reported from study enrollment towards the follow up visit for all subjects. AE/SAEs definition and reporting requirement will be consistent with the current International Conference on Harmonisation/Good Clinical Practice ICH GCP E6 (R2)Citation21.
Subjects will be withdrawn from the study if ANY of the withdrawal criteria described in Textbox 3 applies.
Textbox 3. Withdrawal criteria
Statistical analysis
Statistical methods
The primary outcome measure for the intra-arterial infusions studies will be percentage (%) change in FBF, in response to intra-arterial Ach infusion, as measured by venous occlusion plethysmography (infused arm only). Since there is just one primary outcome, no adjustment for multiplicity will be made.
Secondary endpoints will additionally include the % change in FBF (non-infused arm only) and % change in FBF ratio. Three comparisons for each endpoint will be made: alirocumab vs placebo, alirocumab vs statin, and alirocumab + statin vs statin alone. All endpoints will also be analyzed in relation to Ach, SNP and L-NMMA infusion. BP and heart rate will also be reported.
For all outcome measures significance of the global treatment effect across the doses will be determined using mixed effects models. The % change from basal FBF after infusion with challenge agent at each dose will be the repeated outcome measure, adjusting for age, sex, baseline (Visit 2) LDL-C and % change from basal FBF at baseline after infusion with each dose of challenge agent.
Number of participants to be enrolled
The sample sizing for this physiological study assumes that the expected effect size is 25% (between alirocumab and placebo) and the standard deviation of outcome variable is 0.23. To achieve 80% power to detect this difference with a significance level of 5% it is estimated that 13 subjects per group would be requiredCitation22. With a withdrawal/non-evaluable subject rate of 10% a total of 15 per group subjects will be recruited leading to a total required sample size of 30 subjects.
Discussion
The main objective of the INTENSITY-LOW study is to understand whether lowering LDL-C, within the currently accepted normal range, has the potential to reduce CV risk in low-risk healthy individuals. A mechanistic explanation of this benefit might also be provided through an improvement in endothelial function and a reduction in systemic inflammation. In this context, the physiological effects of both alirocumab and atorvastatin on NO bioavailability (as measured by forearm plethysmography) as well as on markers of systemic inflammation will be assessed.
It has widely been shown in clinical trials that ‘pushing’ on-treatment LDL-C to lower levels is linearly associated with improved CV morbidity and mortalityCitation2. This was particularly apparent in secondary prevention studies, in which adding PCSK9 inhibitors to intensive statin treatment ± ezetimibe achieved much lower LDL-C levels than statins ± ezetimibeCitation13–15. Prior to the introduction of PCSK9 inhibitors, the lowest on-treatment LDL-C to further reduce CV morbidity and mortality in secondary prevention had been 1.4 mmol/l. This level was achieved by simvastatin 40 mg + ezetimibe 10 mg dailyCitation13.
The FOURIER (Evolocumab and clinical outcomes in patients with cardiovascular disease) study included 27,564 patients with established atherosclerotic CV disease and baseline LDL-C levels ≥1.8 mmol/l, while on high-intensity statins ± ezetimibe. These patients were randomized to either subcutaneous evolocumab (140 mg every 14 days or 420 mg monthly) or placebo. The median baseline LDL-C of the study participants was 2.4 mmol/l. Evolocumab treatment resulted in an LDL-C reduction to 0.78 mmol/l at 48 weeks. This reduction was associated with a significantly lower by 15% risk for the composite primary endpoint of CV death, myocardial infarction, stroke, unstable angina or revascularisation compared with placeboCitation14.
More recently, the ODYSSEY OUTCOMES (Alirocumab and cardiovascular outcomes after acute coronary syndrome) study included 18,924 patients, who had experienced an acute coronary syndrome up to 12 months earlier. Those subjects had LDL-C ≥ 1.8 mmol/l while on high-intensity or maximum tolerated statins. Study participants were randomized to either alirocumab 75 mg (titrated to LDL-C 0.6–1.3 mmol/l) or placebo every 2 weeks. Alirocumab resulted in a significant LDL-C reduction from a baseline 2.38 mmol/(median) to 0.98, 1.1 and 1.4 mmol/l (median) at months 4, 12 and 48, respectively. The composite of coronary death, non-fatal myocardial infarction, stroke (fatal or non-fatal) or unstable angina requiring hospitalization was the primary endpoint. After 2.8 years (median) these changes were associated with a 15% reduced risk (p < .001) of this endpoint. Likewise, they were related to a 15% further reduction in overall mortality. Interestingly, this benefit was more prominent among patients with baseline LDL-C > 2.6 mmol/l, while also significant for those with LDL-C < 2.07 mmol/lCitation15.
Due to the lack of outcome trials with PCSK9 inhibitors it is unclear whether aggressively reducing LDL-C to very low levels is beneficial on CV outcomes in primary prevention. The ASCOT-LLA (Anglo-Scandinavian Clinical Outcomes Trial - Lipid Lowering Arm) included 10,305 high-risk hypertensive patients with at least 3 other CV risk factors, and a baseline non-fasting total cholesterol ≤6.5 mmol/l. These were randomized to either atorvastatin 10 mg daily or placebo. LDL-C was reduced from a mean baseline 3.4 mmol/l to 2.3 mmol/l at the end of follow up in 3.3 years (median). These changes were associated with a 36% reduced risk of the primary endpoint of fatal coronary heart disease or non-fatal myocardial infarctionCitation23.
The JUPITER (Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein) study assessed the potential benefits of lowering LDL-C in apparently healthy normocholesterolaemic individuals (LDL-C < 3.4 mmol/l) with a raised hsCRP ≥2.0 mg/l. It included 17,802 participants who were randomized to rosuvastatin 20 mg daily or placebo. The lowest on-treatment LDL-C levels achieved by rosuvastatin was 1.4 mmol/l at 48 months. In this study, rosuvastatin was associated with a 44% reduced risk of the composite endpoint of myocardial infarction, stroke, revascularisation, hospitalization for unstable angina or CV deathCitation19.
However, despite the relatively low or normal baseline cholesterol in ASCOT-LLA and JUPITER, all or most participants were at intermediate or high CV riskCitation18,Citation19. Thus, the evidence of potential benefits for effective LDL-C lowering in low-risk hypercholesterolaemic subjects is very poor. There is an even greater paucity of data in normocholesterolaemic subjects.
The INTENSITY-LOW study will include low-risk normocholesterolaemic individuals lacking any of the established CV risk factors or predictors. Therefore, neither treatment intervention is intended for therapeutic benefit, since study participants would not be eligible for lipid lowering treatment according to the current guidelines for the management of dyslipidaemia and CV risk. In this context, results from the INTENSITY-LOW study may provide evidence for or against the concept that there is still a relevant cholesterol-related CV risk in low-risk individuals. This issue has not been addressed by the current management guidelines.
We have utilized a PCSK9 inhibitor (i.e. alirocumab) as our choice of a non-statin lipid-lowering treatment to achieve similar levels of LDL-C lowering as atorvastatin. The doses were based on previous evidence showing that single-dose alirocumab 150 mg at steady state is expected to reduce LDL-C by approximately 40% in healthy normocholesterolaemic individualsCitation24, Citation25, akin to atorvastatin 20 mg daily. We assume three different on treatment LDL-C levels according to the current design: one after alirocumab single dose at Visit 3, one after placebo at Visit 3 and one after alirocumab + atorvastatin at Visit 4.
Placebo + atorvastatin at Visit 4 is expected to achieve similar LDL-C levels to alirocumab single dose at Visit 3. Comparing the differential effects of these regimens on endothelial function, inflammation and arterial stiffness at these timepoints will allow the assessment of differential effects of statin vs non-statin treatment on vascular function for similar LDL-C reduction. A lack of significant difference between treatment arms will suggest that vascular effects of the regimens are LDL-C-dependent only and disprove the theory of statin-related pleiotropic effects.
Transparency
Declaration of funding
This INTENSITY-LOW study is funded by the Jon Moulton Charitable Trust, and will be conducted at the National Institute for Health Research (NIHR) Cambridge Clinical Research Facility. The funder had no role in the conception, design and conduct of the study.
Declaration of financial/other relationships
None of the authors declare any relevant conflict of interest. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
The authors acknowledge the support of the NIHR Cambridge Biomedical Research Center and the NIHR Clinical Research Network, as well as the Core Biochemical Assay Laboratory, Cambridge. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
References
- Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104(10):1108–1113.
- Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590.
- Lind L, Berglund L, Larsson A, et al. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123(14):1545–1551.
- Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102(9):994–999.
- Wierzbicki AS, Chowienczyk PJ, Cockcroft JR, et al. Cardiovascular risk factors and endothelial dysfunction. Clin Sci. 2004;107(6):609–615.
- Kostapanos MS, Florentin M, Elisaf MS, et al. Hemostatic factors and the metabolic syndrome. CVP. 2014;11(6):880–905.
- Sun ZDY, Cheriyan J. Non-invasive measurements of arterial function: What? When? Why should we use them? Heart. 2019;105(15):1203–1211.
- Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52(6):631–646.
- Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678.
- Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605.
- Kostapanos MS, Milionis HJ, Elisaf MS. An overview of the extra-lipid effects of rosuvastatin. J Cardiovasc Pharm T. 2008;13(3):157–174.
- Westerink J, Deanfield JE, Imholz BP, et al. High-dose statin monotherapy versus low-dose statin/ezetimibe combination on fasting and postprandial lipids and endothelial function in obese patients with the metabolic syndrome: The PANACEA study. Atherosclerosis. 2013;227(1):118–124.
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397.
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722.
- Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107.
- Roth EM, Taskinen MR, Ginsberg HN, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176(1):55–61.
- Soran H, Dent R, Durrington P. Evidence-based goals in LDL-C reduction. Clin Res Cardiol. 2017;106(4):237–248.
- Kostapanos MS, Elisaf MS. JUPITER and satellites: Clinical implications of the JUPITER study and its secondary analyses. WJC. 2011;3(7):207–214.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207.
- Lind L. Relationships between three different tests to evaluate endothelium-dependent vasodilation and cardiovascular risk in a middle-aged sample. J Hypertens. 2013;31(8):1570–1574.
- https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice.
- O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95(5):1126–1131.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158.
- Stern RH, Yang BB, Hounslow NJ, et al. Pharmacodynamics and pharmacokinetic-pharmacodynamic relationships of atorvastatin, an HMG-CoA reductase inhibitor. J Clin Pharmacol. 2000;40(6):616–623.
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366(12):1108–1118.
- Stewart J, Breslin WJ, Beyer BK, et al. Birth control in clinical trials: industry survey of current use practices, governance, and monitoring. Drug Inf J. 2016;50(2):155–168.
