Abstract
Objective
Femoroacetabular impingement (FAI) is a condition that has been increasingly recognized as a source of hip pain and a possible risk factor to early development of hip osteoarthritis (OA). To our knowledge, the use of HA in the treatment of femoroacetabular FAI has been investigated only by two studies, both using a high molecular weight HA. The aim of this study was to evaluate the efficacy of two weekly injections of an hexadecylamide derivative of HA (HYADD4-G, HYMOVIS, Fidia Farmaceutici) in FAI.
Methods
All patients received two weekly intra-articular injections of Hymovis at baseline and after 7 days. Clinical and functional assessments were performed at baseline and was repeated after 1, 3, 6 and 12 months. Functional measures included visual analogue scale (VAS) for pain, Harris Hip score (HHS), Lequesne Index (LI), Tegner activity level scale (TALS) and monthly consumption of nonsteroidal anti-inflammatory drugs (NSAIDs).
Results
Twenty-one hips (19 patients, 2 bilateral cases) were treated. The variables VAS, HHS as well as Lequesne improved significantly from T0 to T4 (at 12 months) with the best improvement between T0 and T1. At the same time, a reduction in NSAIDs monthly intake was registered. On the other hand, a significant improvement in Tegner scale was not observed. No adverse events were registered.
Conclusion
This study states that one cycle of HYADD4-G could be a safe and effective treatment in patients with FAI, showing significative results in term of pain control as well as hip functionality and quality of life up to 1 year.
Introduction
Femoroacetabular impingement (FAI) was firstly described in the 1990sCitation1,Citation2, and since that time has been increasingly recognized as a cause of groin pain and dysfunction. Nevertheless, the incidence of FAI in the general population is still unknown. A recent cohort study conducted in Netherlands analyzed 31.451 patients showing a prevalence of groin pain of 0.6% among which 17% was later clinically and radiologically diagnosed with FAICitation3. Furthermore, several studies on asymptomatic volunteers presented prevalence rates of radiological characteristics of FAI ranging between 7 and 23%Citation4,Citation5.
FAI is caused by repetitive mechanical stress on a morphologically abnormal proximal femur and/or acetabulum during terminal range-of-motion of the hipCitation6. This pathological process eventually results in characteristic damage to the labrum and acetabular cartilage, and may predispose to hip osteoarthritis (OA)Citation7,Citation8. Growing evidence has recently mounted over the role of FAI in the development of early OACitation9, focusing on labral deterioration and labral calcification as part of this degenerative evolutionCitation10.
Precise guidelines for the treatment of FAI have not been defined yet. Conservative measures including physical therapy, rest, core strengthening, sensory-motor skills improvement as well as pain control with nonsteroidal anti-inflammatories (NSAIDs) are the mainstays of nonsurgical treatmentsCitation11. When nonsurgical treatments show no benefit after 3 months or if the patient becomes refractory to conservative management, surgical intervention may be requiredCitation12.
Viscosupplementation (VS) by intra-articular injection of hyaluronic acid (HA) is proving to be safe and effective for pain control in patients affected from arthritisCitation13, especially in knee osteoarthritis. The latest OARSI guidelines state that intra-articular HA are considered Level 1B/Level 2, thus recommended, treatments for knee OA whereas the same treatment is not recommended for individuals with hip or polyarticular OACitation14. Accordingly, Leite et al., after having conducted a systematic review and meta-analysis, do not recommend VS in hip OA given the scarce evidence of its efficacy against pain and disability up to 3 months and no difference at 6 months despite high evidence of safety compared to placeboCitation15. On the other hand, evidence on the possible use of VS in FAI is still limited: to our knowledge, the use of HA in the treatment of FAI has been investigated only by two studiesCitation16,Citation17, both using a high molecular weight HA, and the efficacy of an hexadecylamide derivate of HA in FAI has never been investigated yet.
However, higher beneficial effects of intra-articular injection of a hexadecylamide HA derivative compared to unmodified 500–730 kDa HA in sheep models have been described in the literature. Particularly, it has been shown an improvement of gait, a stimulation of high molecular weight HA synthesis by the synovia, as well as a reduction in synovial hyperplasia in advanced OACitation18,Citation19. Moreover, another in vitro study has shown better outcomes of derivatized HA compared with unmodified HA on both chondrocyte and synovial fibroblast expression of catabolic enzymes as well as inflammatory cytokines/mediators using human joint tissue cellsCitation19.
Given these considerations, the purpose of this paper is to investigate safety and efficacy of one cycle of the HA derivative Hymovis (HYADD4-G) in patients affected from FAI syndrome.
Methods
Study design
This is a single-center, open label interventional study. 19 patients with clinical and radiological diagnosis of FAI were recruited, treated and evaluated prospectively for up to 1 year of follow-up. All the patients underwent a visitation with an orthopedic specialist. Furthermore, plain radiographs were performed to assess the grade of OA using Tonnis classification systemCitation20,Citation21. Moreover, a magnetic resonance arthrography (MRA) was prescribed to verify the presence of a labral pathology or an acetabular cartilage lossCitation22, using the staging proposed by Czerny et al.Citation23.
This study was conducted following the principles of the Declaration of Helsinki and with the patients’ permission expressed through a written consent.
Inclusion/exclusion criteria
Inclusion criteria were pain for at least 3 months as well as positivity to hip impingement test (FADDIR: flexion and internal rotation). Moreover, we included patients affected from CAM FAI measured with 45° flexion Dunn/Rippstein radiographic view showing an α-angle >55°, obtained to reveal pathomorphology of the anterior femoral head–neck junctionCitation24,Citation25. Radiographic signs of acetabular over-coverage in anteroposterior pelvic view (cross-over sign, ischial spine sign, posterior wall sign), coxa profunda as well as acetabulum protrusion were also inclusion criteria for this studyCitation26. Exclusion criteria were: α-angle ≤55°, age > 55 years, history of hip disease (slipped capital femoral epiphysis, Perthes disease, osteotomy, dysplasia), inflammatory, autoimmune and septic disease (rheumatoid arthritis, connective tissue disease, osteomyelitis), advanced radiographic hip OA (Tonnis ≥2)Citation20,Citation21 as well as previous intra-articular injection with steroids. The flow chart is showed in .
Outcome measurements
At baseline demographic, anthropometric and clinical data (sex, age, weight, height, BMI, symptoms duration, sport and work activities) were collected. All patients underwent two weekly intra-articular injection of Hymovis (HYADD4-G, Fidia Farmaceutici, Abano Terme, Italy) at baseline and after 7 days. The follow-up was set at the following stages: pretreatment (T0), 1 month (T1), 3 months (T2), 6 months (T3) and 12 months (T4), evaluating variations of functional score systems and considering monthly consumption of NSAIDs. Particularly, functional measures included pain during the previous week (visual analogue scale [VAS]), Harris Hip score (HHS), Lequesne Index (LI), Tegner activity level scale (TALS).
Operative technique
Hymovis is a derivative of HA obtained by controlled chemical synthesis (2% partial hexadecylamide). Its chemical structure lends it hygroscopic properties forming a hydrogel with excellent viscoelastic, lubricating as well as rheological properties.
Injections were performed using a musculo-skeletal ultrasound-guided procedure with a low frequency 3.5 − 7.5 MHz linear array probe in sterile conditions. The patient was positioned supine with heels joined and slightly externally rotated legs (10–20° degrees). Using an antero-inferior approach, HA was administered through a 20-G spinal needle inserted at the basis of the femoral neck. The HA intra-articular placement was visualized as a hyperechoic flow by ultrasounds (real time method)Citation27. Patients were asked to rest for a month before resuming sporting activity.
Statistical analysis
The software used for statistical analysis were R software (R Core Team, 2017) and lmerTest package. All the treated patients were considered for statistical analysis, which was performed using a mixed effects model. Particularly, it is a statistical model containing both fixed effects as well as random effects, mostly useful in settings like longitudinal studies where repeated measurements are made on the same statistical units.
To statistically quantify this improvement, after model selection with the likelihood ratio test, the aforementioned linear mixed effects model was fitted for each outcome variable considering the following independent variables as fixed effects: time (five categories), age (in years), sex (=1 if male), height (in cm), weight (in Kg), side of lesion (=1 if left) as well as duration of symptoms (in months) at first visit. Since the model includes the effect of different intercepts and slope in time for each individual subject, the random slopes and intercepts of time were added.
Results
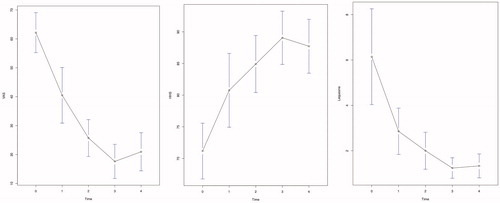
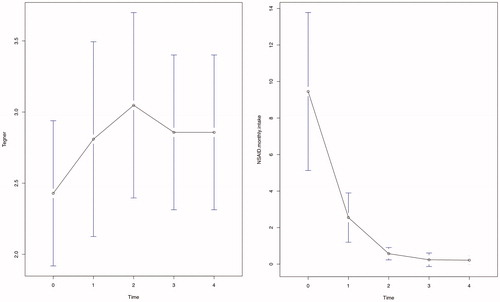
The demographic and clinical data of the patients included in the study are reported in . In 17 patients, hip impingement tests were positive on one side, while in 2 patients, both hips were affected, for a total of 19 patients and 21 hips treated. The FAI characteristics are reported in . The data collected in the follow-up after joint injections show a pattern of marked improvement with time, as can be inferred from , where means and standard deviations are reported for the different variables at different time points. The resulted model fits are summarized in and . p-values were obtained via Satterthwaite’s degrees of freedom method and are highlighted in bold when less than 0.05. Visual inspection of the residuals confirmed the goodness of fit of the aforementioned models.
Table 1. Table showing patients’ demographic and clinical features.
Table 2. Table showing FAI characteristics.
Table 3. Table showing means and standard deviations of Visual Analog Score (VAS), Harris Hip Score (HHS), Lequesne Index (LI), Tegner activity level scale (TALS) as well as NSAIDs monthly intake (number of pills) at each follow-up: pretreatment (T0), 1 month (T1), 3 months (T2), 6 months (T3) and 12 months (T4). p-values between t0 and t4 are summarized in the last line.
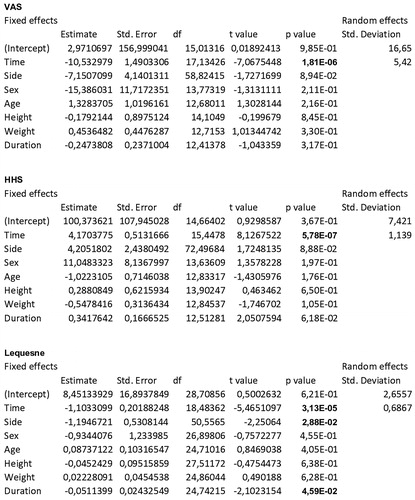
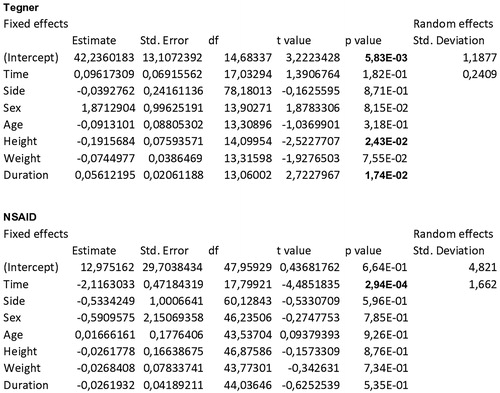
Particularly, the slopes with respect to time for the variables VAS, HHS, Lequesne () and NSAIDs monthly intake () were highly significant (p < .0001; p < .0001; p < .0001; p = .0003). VAS averages decreased constantly from T0 to T3, and the average decrease over the whole follow-up was −10.53 for each time interval. HHS averages increased constantly from T0 to T3, and the average increase over the whole follow-up was 4.17 for each time interval. Lequesne index averages decreased constantly from T0 to T3, and the average decrease over the whole follow-up was −1.10 for each time interval. Monthly NSAIDs intake averages decreased constantly from T0 to T4, and the average decrease over the whole follow-up was −2.12 for each time interval. For all these variables, the steepest increase or decrease was observed between T0 and T1.
Tegner scale () did not show a significant improvement with time.
The quality of life measured considering sport and work activity also showed a marked improvement over time.
Discussion
HA holds mechanical effects and protective properties on articular cartilage commonly known respectively as viscosupplementation and biosupplementationCitation16.
Which lead to a long-lasting effect on articular surface. Specifically, HA improves the overall joint lubrication and viscoelastic properties of the articular cartilage, retaining higher amounts of fluid in the articular space together resulting in anti-inflammatory and anti-nociceptive effectsCitation28,Citation29.
Multiple types of nerve ending have been identified within the labrum, reinforcing the fact that a torn labrum can be a primary cause of hip painCitation30,Citation31. The number and type of nerves and organs in the labrum do not differ based on age. However, more unmyelinated nerve endings, those that sense pain, were found in the superior and anterior quarters of the labrum, the part more involved in the FAI.
Hexadecylamide derivative of HA is a highly viscoelastic hydrogel bioengineered using a proprietary process that increases lubrication and shock absorption properties, resulting in a natural hyaluronan similar to the human synovial fluid hyaluronan. The formulation allows the unique molecule to recover its original structure, even after repetitive mechanical stress. Due to reversible hydrophobic interaction, the non-crosslinked hexadecylamide derivative of HA shows increased elasticity, viscosity as well as a longer half-life in the joint.
Gomis et al.Citation32 observed a reduction of nerve afferents mechanical-related impulse activity in intact guinea pig joints provided by hexadecylamide derivative of HA. Particularly, force transmission on articular mechanotransduction apparatus is likely reduced by its elastoviscous properties, leading to a decrease of the overall force transmission as well as sensory fibers response to mechanical forces. Moreover, hexadecylamide derivative of HA can likely bind to cell membrane receptors and interact with inflammatory mediators of damaged tissues, thus providing a direct analgesic effectCitation33–35. The aforementioned chemical interactions could explain the reduction of impulse activity as well as the consequent marked improvement of the variables analyzed in this study, after use of HYADD4-G.
According to this data, it appears reasonable to speculate that HYADD4-G has a direct effect on joint nociceptors reducing their activity. This effect is both related to its mechanical filter role associated with the aforementioned rheological properties, as well as to its chemical interaction with inflammatory cytokines present in the inflamed joint tissues, thus reducing their sensitizing effect on nociceptor terminals. HYADD-4 has been mainly studied as a treatment for knee and shoulder OA proving to be a safe and effective option for these conditionsCitation36. Moreover, growing interest in HYADD-4 activity was raised when Zorzi et al.Citation37 showed that this hexadecilamide derivate of HA leaded to a significant reduction in length and depth of meniscal knee lesions assessed with MRI compared with a control group.
To our knowledge this is the first evidence of the use of HYADD-4 in hip pathologies and only the third evaluating HA injections as a possible treatment for FAI. Our results are coherent with those of Abate et al.Citation16 and Lee et al.Citation17 in showing that VS with HA is a safe and efficient treatment for this condition. As a future perspective, it would be interesting to compare the efficacy of classical HA with hexadecilamide derivate to investigate the possible advantages of its peculiar composition and structure. In addition to this VS with HA, in the treatment of FAI, should be compared to other promising infiltrative techniques such as PRP that in recent meta-analysis as well as interventional studies showed similar if not superior results compared to HA in hip OACitation38–40.
Limitations
Although this study shows positive results, several limitations must be addressed. A larger sample size would be preferred to avoid selection bias. Moreover, due to the lack of a placebo arm a potential placebo effect cannot be ruled out. However, since the efficacy of an hexadecylamide derivate of HA in FAI has never been investigated yet, the aim of this study is mainly to assess its effects on FAI as well as to give a starting point for further research.
Conclusions
Our results show that intra-articular injection of a HYADD4-G at baseline and after 7 days may provide good results improving hip function, reducing the pain as well as the functional impairment in daily living activities up to 12 months in patients affected from FAI. However, since none of the conservative modalities treat the underlying cause of FAI, surgery is indicated if symptoms persist.
Transparency
Declaration of funding
No funding was received for the production of this article.
Declaration of financial/other relationships
The authors declare that there is no conflict of interest.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
This study was concepted by Marco Ometti. Data collection was conducted by Pietro Conte and Marco Ometti. The statistical analysis was conducted by Davide Schipani. The paper was authored by Pietro Conte. Pierluigi Pironti and Vincenzo Salini provided the final review. All authors read and approved the final manuscript.
Acknowledgements
None reported.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Ganz R, Bamert P, Hausner P, et al. Cervico-acetabular impingement after femoral neck fracture. Unfallchirurg. 1991;94(4):172–175.
- Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:93–99.
- Röling MA, Mathijssen NMC, Bloem RM. Incidence of symptomatic femoroacetabular impingement in the general population: a prospective registration study. J Hip Preserv Surg. 2016;3(3):203–207.
- Fukushima K, Uchiyama K, Takahira N, et al. Prevalence of radiographic findings of femoroacetabular impingement in the Japanese population. J Orthop Surg Res. 2014;9:25.
- Hack K, Di Primio G, Rakhra K, et al. Prevalence of cam-type fem- oroacetabular impingement morphology in asymptomatic volunteers. J Bone Joint Surg Am. 2010;92:2436–2444.
- Ganz R, Parvizi J, Beck M, et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120.
- Cobb J, Logishetty K, Davda K, et al. Cams and pincer impingement are distinct, not mixed: the acetabular pathomorphology of femoroacetabular impingement. Clin Orthop Relat Res. 2010;468(8):2143–2151.
- Tannast M, Goricki D, Beck M, et al. Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res. 2008;466(2):273–280.
- Wyles CC, Heidenreich MJ, Jeng J, et al. The John Charnley Award: redefining the natural history of osteoarthritis in patients with hip dysplasia and impingement. Clin Orthop Relat Res. 2017;475(2):336–350.
- Trisolino G, Favero M, Dallari D, et al. Labral calcification plays a key role in hip pain and symptoms in femoroacetabular impingement. J Orthop Surg Res. 2020;15(1):86
- Samora JB, Ng VY, Ellis TJ. Femoroacetabular impingement: a common cause of hip pain in young adults. Clin J Sport Med. 2011;21(1):51–56.
- Hellman MD, Riff AJ, Frank RM, et al. Operative Treatment of Femoroacetabular Impingement. Phys Sportsmed. 2014;42(3):112–119.
- Abate M, Pulcini D, Di Iorio A, et al. Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly. Curr Pharm Des. 2010;16(6):631–640.
- Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589.
- Leite VF, Amadera JD, Buehler AM. Viscosupplementation for Hip Osteoarthritis: a systematic review and meta-analysis of the efficacy on pain and disability, and the occurrence of adverse events. Arch Phys Med Rehabil. 2018;99(3):574–583.
- Abate M, Scuccimarra T, Vanni D, et al. Femoroacetabular impingement: is hyaluronic acid effective. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):889–892.
- Lee Y-K, Lee GY, Lee JW, et al. Intra-articular injections in patients with femoroacetabular impingement: a prospective, randomized, double-blind, cross-over study. J Korean Med Sci. 2016;31(11):1822–1827.
- Smith MM, Cake MA, Ghosh P, et al. Significant synovial pathology in a meniscectomy model of osteoarthritis: modification by intra-articular hyaluronan therapy. Rheumatol. 2008;47(8):1172–1178.
- Cake MA, Read R, Edwards S, et al. Changes in gait after bilateral meniscectomy in sheep: effect of two hyaluronan preparations. J Orthop Sci. 2008;13(6):514–523.
- Tönnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81(12):1747–1770.
- Pascual-Garrido C, Li DJ, Grammatopoulos G; ANCHOR Group. The pattern of acetabular cartilage wear is hip morphology-dependent and patient demographic-dependent. Clin Orthop Relat Res. 2019;477(5):1021–1033.
- Anderson LA, Peters CL, Park BB, et al. Acetabular cartilage delamination in femoroacetabular impingement risk factors and magnetic resonance imaging diagnosis. J Bone Joint Surg Am. 2009;91(2):305–313.
- Czerny C, Hofmann S, Neuhold A, et al. Lesions of the acetabular labrum: accuracy of MR imaging and MR arthrography in detection and staging. Radiology. 1996;200(1):225–230.
- Meyer DC, Beck M, Ellis T, et al. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res. 2006;445:181–185.
- Ito K, Leunig M, Ganz R. Histopathologic features of the acetabular labrum in femoroacetabular impingement. Clin Orthop Relat Res. 2004;429:262–271.
- Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis-what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540–1552.
- Micu Mihaela C. 2012. Ultrasound Guided Hip Injection Techniques, Osteoarthritis – Diagnosis, Treatment and Surgery, Prof. Qian Chen (Ed.), InTech, Available from.
- Bagga H, Burkhardt D, Sambrook P, et al. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33:946–950.
- Takahashi K, Goomer RS, Harwood F, et al. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta(IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthr Cartil. 1999;7(2):182–190.
- Hosekawa O. Histological study on the type and distribution of the sensory nerve endings in human hip joint capsule and ligament. Nippon Seikeigeka Gakkai Zasshi. 1964;38:887–901.
- Kim YT, Azuma H. The nerve endings of the acetabular labrum. Clin Orthop Relat Res. 1995;320:176–181.
- Gomis A, Miralles A, Schmidt RF, et al. Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthr Cartil. 2009;17(6):798–804.
- Balazs EA, Darzynkiewicz Z. The effect of hyaluronic acid on fibroblast, mononuclear phagocytes and ymphocytes. In: Kulonen E, Pikkarainen J, Eds. Biology of the Fibroblast. London: Academic Press; 1973. 237–e52.
- Gotoh S, Miyazaki K, Onaya J, et al. Experimental knee pain model in rats and analgesic effect of sodium hyaluronate (SPH). Nihon Yakurigaku Zasshi. 1988;92(1):17–e27.
- Punzi L, Schiavon F, Cavasin F, et al. The influence of intra-articular hyaluronic acid on PGE2 and cAMP of synovial fluid. Clin Exp Rheumatol. 1989;7(3):247–e50.
- Priano F. Early Efficacy of Intra-Articular HYADD® 4 (Hymovis®) Injections for Symptomatic Knee Osteoarthritis. Joints. 2017;05(02):079–084.
- Zorzi C, Rigotti S, Screpis D, et al. A new hydrogel for the conservative treatment of meniscal lesions: a randomized controlled study. Joints. 2015;03(03):136–145.
- Ye Y, Xiang Z, Shuiwei M, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials . Int J Surg. 2018;53:279–287.
- Dallari D, Tschon M, Sabbioni G, et al. PRP and HA for hip osteoarthritis: response. Am J Sports Med. 2016;44(9):NP44–NP46.
- Battaglia M, Guaraldi F, Vannini F, et al. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 2013;36(12):e1501–e1508.