Abstract
Objective
To explore treatment preferences of patients with Hereditary Angioedema (HAE), a debilitating disorder characterized by potentially life-threatening, recurrent episodes of swelling, resulting in significant physical, emotional, and economic burden. With newer oral prophylactic treatments on the horizon, it is important to understand patients’ preferences.
Methods
An online survey was conducted in 2018 among United States (US) adult patients diagnosed with Type I or II HAE. Respondents were recruited anonymously from online panels and social media.
Results
Online surveys were completed by 75 patients diagnosed with HAE by a healthcare provider, with a mean of 16.7 years since diagnosis. Most patients (64%) report taking at least one medication for prophylaxis of HAE attacks. While almost all patients surveyed agree it is important to take preventative medication as prescribed, over half (52%) of patients report HAE prophylactic treatment to be burdensome. Despite stating that they like their current medications, 98% of the prophylactic HAE medication users would prefer an oral treatment if available; almost all (96%) prophylaxis users agree that oral preventative medication would fit their life better than an injectable medication, with 67% of users citing convenience as the primary reason to try an oral preventative HAE medication. If a more convenient option were available, nearly all (96%) patients currently not treating their HAE prophylactically would feel encouraged to do so.
Conclusions
Most patients with HAE would prefer a newer generation oral prophylactic medication that would decrease treatment burden and allow them to live fuller lives.
Introduction
Hereditary Angioedema (HAE) is a rare genetic condition affecting an estimated 10,000 patients in the USCitation1,Citation2. HAE is a debilitating disease, characterized by potentially life-threatening, recurrent episodes of swelling that can affect many parts of the body, including the face or lips, tongue, larynx, abdomen, back or shoulder, or extremities. These unpredictable attacks often result in emergency room visits or hospitalization and can cause significant physical, emotional, and economic burdenCitation3–9.
There are several FDA-approved medications available for both acute and prophylactic treatment of HAE. Guidelines for the management of HAE recommend that all patients be evaluated for long-term prophylactic therapy to help prevent and reduce the frequency and severity of attacks and improve quality-of-lifeCitation10. The only oral options for HAE prophylaxis are attenuated androgens which were approved in the 1970sCitation11. Due to the risk of significant adverse events and drug interactions, guidelines recommend against the long-term use of androgens in certain patientsCitation10. Although antifibrinolytic agents are not recommended for prophylactic treatment due to poor efficacy, they are still occasionally prescribed for prophylaxisCitation12. In 2008, the first injectable prophylactic treatment for HAE became available when Cinryze (C1 esterase inhibitor [human]) (Takeda Pharmaceutical Company Limited, Lexington, MA) entered the market. Cinryze is administered by intravenous (IV) infusion every 3–4 days by the patient, caregiver, or healthcare provider; patients must be trained in appropriate reconstitution methods as well on how to slowly infuse an IV medication. In mid-2017, another C1 esterase inhibitor (Haegarda, CSL Behring, King of Prussia, PA) became available, offering patients a new option administered by subcutaneous injection that requires reconstitution and has the same dosing frequency as Cinryze. In late 2018, lanadelumab-flyo, a monoclonal antibody (Takhzyro, Takeda Pharmaceutical Company Limited) offered a subcutaneous injectable treatment option administered every 2–4 weeks, that requires refrigeration but no reconstitution.
While more prophylactic treatments options are now available for this debilitating chronic condition, patients participating in research continue to express a need for more convenient options, specifically an oral prophylactic HAE medication, despite generally being satisfied with their current treatmentCitation13. A similar trend has been observed with other diseases where more treatment options are available for patients. In these chronic conditions where multiple treatment options exist, and where efficacy and side-effects are important in treatment decision-making, the route of administration also becomes a key consideration. For example, studies in multiple sclerosis showed route and frequency of administration as two of the most important attributes in treatment decision-makingCitation14,Citation15. For severe asthma, when patients were asked to rank the importance of attributes when considering biologic treatment options, mode of administration ranked second, only behind out-of-pocket costsCitation16. For patients with psoriasis, treatment attributes focusing on convenience and lifestyle compatibility, i.e. treatment location and route of administration, were considered to be more important than adverse effectsCitation17.
Patients’ preference for an oral route of administration has been demonstrated consistently across a variety of conditions and therapy areas. A study of patients with multiple sclerosis revealed a preference for oral administration vs IV infusion or injectionCitation18. Several studies for cancer treatments have found oral treatments to be preferred over intravenous administration across a range of cancer patient populations citing a variety of reasons, including greater flexibility, convenience, less stress, and better quality-of-lifeCitation19–24. Oral treatment was also preferred over subcutaneous or intravenous injections in a survey of ulcerative colitis patients, even when the oral medication had a more frequent dosing scheduleCitation25.
In 2017, at the Voice of the Patient Summit, as part of the US FDA’s Patient-Focused Drug Development Initiative, patients with HAE and caregivers of patients with the condition were asked for their perspectives on living with and treating HAE, via a live forum. HAE patients cited the route of administration as the most important factor in driving their treatment preference and also expressed a need for the development of less traumatic routes of administration, particularly those that avoid injections and offer ease and convenienceCitation5.
With a newer generation of targeted oral kallikrein inhibitors in clinical development, patient demand for new, oral prophylaxis treatment options for HAE needs to be considered. We sought to identify if unmet needs exist despite the availability of effective HAE prophylactic treatments. It is essential for healthcare providers to explore and investigate patients’ preferences and empathize with patients’ unmet needs as they develop treatment plans with shared treatment goals.
Methods
Study design
An online survey was conducted in 2018 among US patients diagnosed with HAE. Data were collected between November 5 and December 3, 2018. The median length of the survey was 15 minutes. The survey covered a range of topics regarding HAE, including attitudes toward HAE and prophylactic treatment of the condition, perceptions of prophylactic medication, treatment experience and satisfaction, and HAE attack history.
Information about the purpose and nature of the survey was presented to potential participants; only respondents who selected yes (from yes/no options) to indicate consent to participate in the study were allowed to enter the screening portion of the survey. All data were anonymized and analyzed in aggregate. Each participant was offered a nominal honorarium for completing the survey, and reminders were sent to all non-respondents. The screening questions were carefully designed to ensure accurate reporting of diagnoses and treatments; additionally, several strict industry-standard measures were in place to ensure quality.
Participants
Seventy-five patients participated in the survey and met all inclusion criteria: live in the US, aged 18+ years, self-reported diagnosis of Type I or Type II HAE by a healthcare professional, and currently treating HAE or not currently treating their condition and experiencing at least one HAE attack every 3 months. Respondents were recruited via social media or by email through an online panel company to which respondents had provided permission to be contacted for research purposes.
Statistical analysis
We performed descriptive statistical analysis (means, frequencies) using SPSS Statistics for Windows 23 (SPSS, Chicago, IL) and Stata/IC 14.1 (Stata Corp, College Station, TX) of data from the participating HAE patients. Chi-squared tests were used to compare categorical variables; t-tests were used for comparison of continuous variables. Statistics were unweighted and therefore did not account for any demographic variation.
Results
Sample disposition
Characteristics of the survey respondents are summarized in . The mean age of respondents was 39.1 years old and, on average, they had been diagnosed with HAE for almost 17 years. Patients reported a mean of 1.2 attacks in the previous 3 months and 2.6 attacks in the prior 6 months. Most patients (97%) had Type 1 HAE, a proportion slightly higher than that seen in the general HAE patient populationCitation26. Almost all patients (97%) were taking at least one prescription medication to treat their HAE. The demographic characteristics of patients taking medication for HAE prophylaxis and those not taking prophylactic medication were similar except for prescription drug coverage, which was higher among prophylaxis users (96% vs 52%).
Table 1. Characteristics of survey respondents.
HAE prophylaxis treatment experience
Self-reported use of prophylactic medication was 64%. Among those who have taken a HAE medication within 12 months of completing the survey, treatment use included Takhzyro (29%), Haegarda (23%), Cinryze (10%), and oral androgens (8%). Medications indicated for the treatment of acute HAE attacks were also used prophylactically by some patients, with icatibant (Firazyr, Takeda Pharmaceutical Company Limited) being the most commonly used, by 10% of respondents.
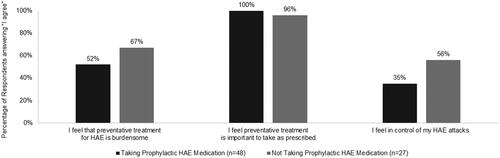
Of the surveyed patients with HAE, all of those taking, and almost all (96%) of those not taking, prophylactic HAE medication agreed it was important to take preventative treatment as prescribed (). However, more than half of all patients surveyed (57%, data not shown) agreed (5–7 on a 7-point scale of “completely disagree” to “completely agree”) that preventative treatment for HAE is burdensome, with those currently treating their HAE prophylactically less likely to agree than those not taking prophylactic medication (52% vs 67%). Furthermore, about two-thirds (65%) of patients taking prophylactic HAE medications reported not feeling in control of their attacks, compared to 44% of those not using HAE prophylaxis. While the reason for the perceived level of control was not assessed in the survey, patients taking prophylaxis reported an average of 3.1 attacks in the 6 months prior to completing the survey, compared to an average of 2.0 attacks in the same time period among those not taking prophylactic medication.
Preference for HAE prophylactic treatments
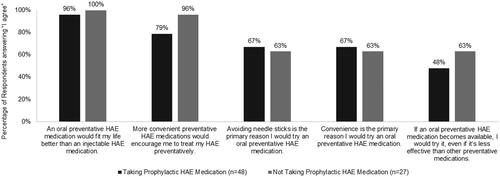
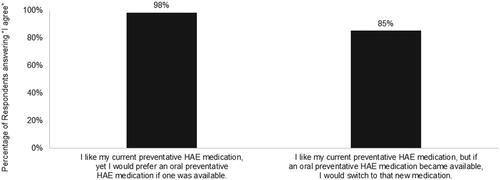
When asked about their preferences for preventative HAE medication, 98% of patients taking prophylactic treatment and 100% of prophylaxis non-users agreed (agreement scale with response options of “I agree” and “I disagree”) they would try an oral preventative medication if one became available. The likelihood to try an oral medication was lower when the treatment was presented as being less effective than other preventative medications; however, almost two-thirds of those not currently treating their condition prophylactically would still be interested in this treatment option. Convenience and fit with lifestyle are important factors driving consideration of a HAE prophylactic treatment medication (). In addition, 67% of patients taking prophylaxis agreed that avoiding needle sticks is the primary reason they would try an oral prophylaxis (). Nearly all of those treating their HAE prophylactically like their current medication, but would prefer an oral medication, and virtually all respondents taking prophylactic medication (98%) would be likely to switch to an oral option if one were available ().
Discussion
Our study sought to explore HAE patients’ treatment perceptions and preferences for the prevention of HAE attacks. The findings from this research identify areas of unmet need regarding prophylactic HAE medication and support treatment strategies that align with patients’ preferences.
We found that patients are very interested in advancements in HAE treatment options and have demonstrated willingness to use more convenient targeted HAE treatments as they have become available, with a strong preference for an oral route of administration. Nearly all patients surveyed who are currently treating their HAE prophylactically would prefer an oral HAE medication if one were available. Among both prophylactic treatment users and non-users, avoidance of needle sticks and convenience are equally cited as the most important reasons why patients would try an oral preventative medication; this finding suggests patients recognize an advantage of a prophylactic medication that decreases treatment burden. This was consistent with previous work citing preference for an alternative route of administration, including subcutaneous injection or oral, in patients dissatisfied with IV injectionsCitation27. When asked about medication developments of interest, half of HAE patients in a recent study indicated a preference for a noninvasive route of administration, including oral therapyCitation28. All patients in our study not taking prophylaxis and almost all (96%) patients taking prophylaxis agree that an oral medication would fit their life better than an injectable therapy. Thus, although our study did not aim to identify specific factors that drive treatment decision-making, it does confirm oral medication preferences as reported in previous research studies conducted among HAE patients.
While HAE is a condition that historically has had few therapeutic options, development of prophylactic therapies over the past 12 years has provided many patients with improved quality-of-life over acute treatment, as demonstrated in a cross-over clinical trial of patientsCitation29 and affirmed by HAE patients at the FDA’s 2017 Voice of the Patient SummitCitation5. Until the past decade, options for both acute and prophylactic HAE treatment were limited to oral androgens and injections or infusions requiring cumbersome reconstitution. In addition, patients have experienced challenges with administration of these medications, including side effects of androgensCitation30 and venous access for IV prophylaxisCitation27. A survey of IV therapy users found that less than half were completely satisfied with the time needed to prepare/administer and with the ease of administering the medication, and the majority reported requiring some assistanceCitation27. Self-administration of HAE therapy can help improve confidence in administering medications in a variety of settings, but learning the proper technique and not feeling confident in the injection process are barriers to self-administration, as reported by both HAE patients and nurses teaching patients to self-administerCitation31,Citation32.
Although the reasons for not using prophylactic treatment vary by patient and circumstance, almost all patients surveyed who are not currently taking prophylactic HAE medications would be encouraged to treat their condition preventatively if there were more convenient options available. Regardless if a patient is currently treating prophylactically, oral administration is preferred even when reduced effectiveness is considered. Two-thirds of the patients who were prophylaxis non-users perceived currently available preventative treatments to be burdensome. While generally accepting their existing therapies, patients with HAE welcome advances in therapies, especially a more convenient preventive oral HAE medication, which would satisfy a persisting unmet need.
Our study has some limitations. First, patients belonging to an online panel may differ from those who are not panel members, and responders may have different demographic characteristics than non-responders, which may limit the generalizability of the findings to the entire population of patients with HAE. While responder bias could exist, to mitigate it, the topic of the survey was not revealed to respondents until they met all the required screening criteria. Second, it is possible that patients treating their HAE prophylactically are different than patients who are not using preventative HAE therapies. Although these two patient groups were similar with regards to age, gender, US census region, time since HAE diagnosis, HAE type, and number of recent HAE attacks, there may be other differences not assessed in our survey. Third, statistical comparisons between prophylaxis users and non-users were not performed due to the small sample size of the latter group. Lastly, the survey was sponsored by a pharmaceutical company which is developing an oral agent for the prophylactic treatment of HAE; however, the study sponsor was blinded to the study respondents and a third party conducted the data collection and analyzed the results. Despite these limitations, our findings shed light on the prophylactic treatment preferences among patients with HAE, which can be valuable in informing treatment decisions.
Conclusions
Our research demonstrates strong patient preference for an oral prophylactic HAE medication option. Even if patients appear to be satisfied with their HAE treatment, healthcare providers should recognize the context of that perception as these patients are expert at coping with their HAE. When presented with alternatives, actively coping patients are likely to see value in more convenient routes of administration that fit into their lifestyle, improve quality-of-life, and can be successfully maintained. For healthcare providers, recognizing that patients have these preferences is important to help develop optimal, patient-supported treatment plans which, in turn, could ultimately increase adherence and satisfaction and decrease treatment burden.
Transparency
Declaration of funding
This study was funded by BioCryst Pharmaceuticals, Inc.
Declaration of financial/other interests
Daniela Geba, Johan Mohd Sani, Michaela Gascon, and Rebecca Hahn are consultants/employees of KJT Group, Inc., which was commissioned by BioCryst Pharmaceuticals, Inc. to conduct the market research study on which this manuscript is based. KJT Group, Inc. was also commissioned to provide medical writing support. Kavita Aggarwal and Jinky Rosselli are employees of BioCryst Pharmaceuticals, Inc.
A reviewer on this manuscript has disclosed that they have collaborated in research and acted as a paid advisor to BioCryst, the sponsor of this work. They have collaborated in research or educational initiatives with, accepted personal consultancy, speakers fees, or educational grant support from the following companies: Adverum, CSL Behring, GSK, Ionis, Kalvista, Medscape, Pfizer, Pharvaris, Pharming, Takeda. They also act as a medical advisor to the patient support groups HAEUK and on the Scientific steering committee for HAE international. The other peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Mr. Mohd Sani, Ms. Gascon, and Ms. Rosselli designed the study and developed the study materials. All authors provided input into the data analyses, contributed to writing the manuscript, and read and approved the final manuscript.
Ethics and permissions
We conducted an anonymous online survey. The survey was conducted in accordance with the principles and guidelines established by the Office for Human Research Protections and the Insights Association Code of Standards and Ethics. After reviewing information about the purpose and nature of the survey, respondents selected a yes/no option indicating consent to participate in the study prior to entering the screening portion of the survey. If they consented, they continued to the survey questions. Respondents could discontinue the survey at any time.
Previous presentations
Our research has been presented as an abstract, which was published in Journal of Managed Care & Specialty Pharmacy, April 2020, 26(4-a):S24–S25.
Acknowledgements
None stated.
References
- Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001;161(20):2417–2429.
- U.S. National Libray of Medicine. [cited 2020 Feb 5]. Available from: https://ghr.nlm.nih.gov/condition/hereditary-angioedema#statistics.
- Zilberberg MD, Nathanson BH, Jacobsen T, et al. Descriptive epidemiology of hereditary angioedema emergency department visits in the United States, 2006–2007. Allergy Asthma Proc. 2011;32(5):390–394.
- Zilberberg MD, Jacobsen T, Tillotson G. The burden of hospitalizations and emergency department visits with hereditary angioedema and angioedema in the United States, 2007. Allergy Asthma Proc. 2010;31(6):511–519.
- The voice of the patient: hereditary angioedema – report of the U.S. Food and Drug Administration patient-focused drug development initiative public meeting September 2017. Center for Biologics Evaluation and Research (CBER) and U.S. Food and Drug Administration (FDA); 2018.
- Longhurst H, Bygum A. The humanistic, societal, and pharmaco-economic burden of angioedema. Clin Rev Allergy Immunol. 2016;51(2):230–239.
- Lumry WR, Castaldo AJ, Vernon MK, et al. The humanistic burden of hereditary angioedema: impact on health-related quality of life, productivity, and depression. Allergy Asthma Proc. 2010;31(5):407–414.
- Toscani M, Riedl M. Meeting the challenges and burdens associated with hereditary angioedema. Manag Care. 2011;20(9):44–51.
- Wilson DA, Bork K, Shea EP, et al. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104(4):314–320.
- Maurer M, Magerl M, Ansotegui I, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2017 revision and update. Allergy. 2018;73(8):1575–1596.
- Gelfand JA, Sherins RJ, Alling DW, et al. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med. 1976;295(26):1444–1448.
- Siles R. Hereditary angioedema: Cleveland Clinic Center for continuing education; 2017 [cited 2020 Dec 4]. Available from: https://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/allergy/hereditary-angioedema/#bib12.
- Banerji A, Li Y, Busse P, et al. Hereditary angioedema from the patient's perspective: a follow-up patient survey. Allergy Asthma Proc. 2018;39(3):212–223.
- Visser LA, Louapre C, Uyl-de Groot CA, et al. Patient needs and preferences in relapsing-remitting multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2020;39:101929–101929.
- Hincapie AL, Penm J, Burns CF. Factors associated with patient preferences for disease-modifying therapies in multiple sclerosis. J Manag Care Spec Pharm. 2017;23(8):822–830.
- Gelhorn HL, Balantac Z, Ambrose CS, et al. Patient and physician preferences for attributes of biologic medications for severe asthma. PPA. 2019;13:1253–1268.
- Schaarschmidt M-L, Schmieder A, Umar N, et al. Patient preferences for psoriasis treatments: process characteristics can outweigh outcome attributes. Arch Dermatol. 2011;147(11):1285–1294.
- Mansfield C, Thomas N, Gebben D, et al. Preferences for multiple sclerosis treatments: using a discrete-choice experiment to examine differences across subgroups of US patients. Int J MS Care. 2017;19(4):172–183.
- Ciruelos EM, Díaz MN, Isla MD, et al. Patient preference for oral chemotherapy in the treatment of metastatic breast and lung cancer. Eur J Cancer Care (Engl). 2019;28(6):e13164.
- Chiba T, Hiraoka A, Mikami S, et al. Japanese patient preferences regarding intermediate to advanced hepatocellular carcinoma treatments. Patient Prefer Adherence. 2019;13:637–647.
- Eek D, Krohe M, Mazar I, et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence. 2016;10:1609–1621.
- Twelves C, Gollins S, Grieve R, et al. A randomised cross-over trial comparing patient preference for oral capecitabine and 5-fluorouracil/leucovorin regimens in patients with advanced colorectal cancer. Ann Oncol. 2006;17(2):239–245.
- Borner MM, Schoffski P, de Wit R, et al. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer. 2002;38(3):349–358.
- Liu G, Franssen E, Fitch MI, et al. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–115.
- Boeri M, Myers K, Ervin C, et al. Patient and physician preferences for ulcerative colitis treatments in the United States. Clin Exp Gastroenterol. 2019;12:263–278.
- Ghazi A, Grant JA. Hereditary angioedema: epidemiology, management, and role of icatibant. Biologics. 2013;7:103–113.
- Riedl MA, Banerji A, Busse PJ, et al. Patient satisfaction and experience with intravenously administered C1-inhibitor concentrates in the United States. Ann Allergy Asthma Immunol. 2017;119(1):59–64.
- Jose J, Lehman EB, Craig T. Evaluating satisfaction of patients with hereditary angioedema with their past and present treatments: implications for future therapies. Allergy Asthma Proc. 2018;39(1):74–80.
- Lumry WR, Miller DP, Newcomer S, et al. Quality of life in patients with hereditary angioedema receiving therapy for routine prevention of attacks. Allergy Asthma Proc. 2014;35(5):371–376.
- Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100(2):153–161.
- Wang A, Fouche A, Craig TJ. Patients perception of self-administrated medication in the treatment of hereditary angioedema. Ann Allergy Asthma Immunol. 2015;115(2):120–125.
- Tuong L-AC, Olivieri K, Craig TJ. Barriers to self-administered therapy for hereditary angioedema. Allergy Asthma Proc. 2014;35(3):250–254.