 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
This study aimed to compare the therapeutic efficacy and safety of dorzolamide/timolol fixed-combination(Cosopt) in newly diagnosed primary open-angle glaucoma (POAG) patients.
Methods
In this prospective, interventional case series, newly POAG patients were included. Patients were started on Cosopt twice a day (BID) for one month and then switched to three times a day (TDS) for an additional month. Patients underwent comprehensive ophthalmic examination, diurnal intraocular pressure (IOP), blood pressure (BP), and 24-h heart rate (HR) measurements at baseline, month 1(BID), and month 2(TDS). Throughout the study, all adverse events were monitored by the investigators.
Results
In 31 POAG patients that completed the study, the mean baseline IOP was 23.1 ± 3.15 mmHg. IOP was decreased significantly 16.5 ± 2.21 at one month (p < .0001) and 13.9 ± 2.23 mmHg at 1 and 2 months follow up (p < .0001). IOP was significantly lower in month 2 compared to month 1 (p = .0004). While Cosopt BID significantly reduced the mean 24-h systolic BP and mean 24-h HR from baseline (p < .0001), the mean 24-h systolic BP and HR remained unchanged with Cosopt TDS compared to BID (p = .62).
Conclusions
Cosopt TDS has a superior IOP-lowering effect than Cosopt BID in POAG patients with comparable safety profiles.
Introduction
Glaucoma is a major public health issue as it is a leading cause of blindness and affects more than 60 million people worldwideCitation1. Lowering intraocular pressure is the only established treatment for glaucoma. While the pendulum is swinging from medical treatment to minimally invasive glaucoma surgeries for mild to moderate glaucoma, medications are still first-line therapy in most practice settingsCitation2.
There are five main classes of topical drugs; these include beta-blockers, carbonic anhydrase inhibitors, prostaglandin derivatives, sympathomimetics, and miotics. Beta-blockers and carbonic anhydrase inhibitors function by reducing the formation of aqueous humor, they may be regarded as “inflow” drugs. The other three classes aim to minimize resistance to the draining away of aqueous humor and are generally considered as “outflow” drugs. These medications are all licensed for the treatment of glaucoma by reducing IOPCitation3.
The ideal medication regimen should address both peak and fluctuation characteristics of the intraocular pressure profile. Typically, first-line treatment involves monotherapy with prostaglandin analogues as they are more efficacious than any other single agent and yield flatter IOP-controlling patternsCitation4. The carbonic anhydrase inhibitor dorzolamide lowers IOP by reducing the synthesis of HCO3 in the ciliary body, thereby decreasing aqueous humor production. Furthermore, dorzolamide can induce relaxation of retinal arterioles with a consequent increase in blood flow and oxygenation of the retina, this effect could be related to the modulation of two important gaseous neurotransmitters nitric oxide and carbon monoxideCitation5,Citation6. Meanwhile, fixed combination (FC) glaucoma medications are introduced to improve the outcome of medical therapy by boosting adherence as well as efficacy. As such several ophthalmologists opted to use them as first-line treatment to avoid ocular adverse events of prostaglandin analoguesCitation7.
Fixed combination of timolol 0.5%-dorzolamide 2.0% is the most frequently prescribed FC medication and perhaps the most studied oneCitation8. It is well established that it reduces IOP more than monotherapy of each agent alone and is as safe and effective as concomitant use of timolol and dorzolamideCitation9,Citation10.
In the light of the results of Early Manifest Glaucoma TrialCitation11 that showed more IOP reduction in early glaucoma results and less disease progression as well as concerns regarding the diurnal IOP-controlling effects of fixed combination medications, some ophthalmologists started to schedule three-times a day Cosopt administration instead of advocated twice-daily dosageCitation12.
Although increasing the dosage of timolol from twice to three times a day resulted in more IOP reduction in one study, concern remains regarding the systemic adverse events, especially cardiovascular, of more frequent exposure to nonselective beta-blocker activity of timololCitation13.
The purpose of this study is to compare the efficacy and cardiovascular safety of two times and three times a day administration of Cosopt eye drop in treatment of newly diagnosed primary open-angle glaucoma eyes.
Method
This study was conducted at the glaucoma clinic of the Labbafinejad Medical Center from September 2016 to March 2017. The study was approved by the ethics committee and the institutional review board at the Ophthalmic Research Center and followed the tenets of the Declaration of Helsinki. Informed written consent was obtained from each participant.
Patients with newly diagnosed primary open-angle glaucoma who had not received any glaucoma medication were included. POAG has defined an eye with evidence of glaucomatous optic neuropathy (vertical cup-to-disc ratio >0.6, asymmetry of 0.2 or more in the C/D ratios of the eyes) and the presence of neuroretinal rim thinning/notching, or a splinter hemorrhage, or localized or diffuse nerve fiber loss in the absence of any other abnormal finding indicative of ocular disease and open anterior chamber angles associated repeatable visual field (VF) damage. Exclusion criteria were: age ≤ 30, history of using glaucoma medication, history of glaucoma surgery, any ocular or systemic condition that prohibit using timolol or dorzolamide, severe glaucoma that needed more aggressive initial IOP control, and using systemic beta-blockers. The required sample size by is calculated using the following formulaCitation12:
α = 0.05
1-β = 95%
d = 0.35S
n = 33
Thirty-three subjects with bilateral primary open-angle glaucoma were included.
At baseline, all patients underwent a comprehensive ophthalmic examination including best-corrected visual acuity (BCVA), biomicroscopic slit-lamp examination, Goldmann applanation tonometry, gonioscopy, fundus examination, and perimetry (Humphrey visual field analyzer; model 750; Carl Zeiss Meditec, Dublin, California, USA). Diurnal IOP was measured every 4 h from 8 am to 12 pm.
Standard exercise test and a 24-h ECG recording were carried out at the baseline using an Oxford Holter system (Oxford Instruments, Abingdon, UK). Blood pressure (BP) was measured by a cardiologist using a Baumanometer mercury sphygmomanometer (W.A. Baum Co. Inc., Copiague, New York, USA), the patient having been comfortably seated for at least 5 min. Blood pressure was also checked at the time of diurnal IOP measurements.
Patients were started on Cosopt ((Merck & Co, Inc, Whitehouse Station, NJ) eye drop twice a day for one month, and upon their return, all the baseline measurements were repeated by the same examiners. After filling the questionnaires and recording 24 h heart rate and BP, patients were instructed to increase from twice a day to three times a day schedule. After one month of higher dosage therapy, all the previous examinations were performed in the same manner, and data were recorded. All measurements were taken between 9 and 11 am, before the instillation of the morning drop of Cosopt.
The primary outcome measure was IOP, and secondary measures were changes in heart rate and blood pressure.
All analyses were performed using SPSS software (SPSS Statistics for Windows, Version 25, Armonk, NY, IBM Corporation). Frequency, percent, mean ± SD, median, and range were used to describe the data. The normal distribution of the data was checked with the Kolmogorov–Smirnov test. For normally-distributed data, continuous variables were compared using an independent sample Student’s t-test. Concerning the non-normally distributed data, the Wilcoxon signed-rank test was applied. Statistical significance was set at p < .05.
Results
A total of 33 patients were enrolled in this study, but only 31 patients were included in the final analysis. One patient was lost to follow-up, and one patient quitted the study due to bradycardia. Both cases were excluded during the first month of treatment. The demographic data are summarized in . Only the eye with the higher baseline IOP was included for final analysis. Patients were instructed to use one drop of Cosopt in their study eye every 12 h during the first month, and every 8 h during the second month. The patients were scheduled to instill the drops between 10 am and noon and between 10 pm and midnight during the first month, and then between 6 am and 8 am, 2 pm and 4 pm, and 10 pm and midnight during the second month.
Table 1. Baseline demographics and clinical characteristics of the study population.
The mean 24-h IOP at baseline was 23.1 ± 3.15 mmHg (95%CI 20.0–29). IOP was decreased significantly to 16.5 ± 2.21 mmHg (95%CI 14–20) after one month of treatment with Cosopt BID and, mean change from baseline was −6.6 mmHg (p < .0001). The average IOP reduction at the end of one month was 28.5%.
After one month of treatment with Cosopt TDS, the mean 24-h IOP level was 13.9 ± 2.23 mmHg (range 10–17 mmHg), mean change from baseline was −8.9 mmHg, and the average IOP reduction was 39.8%. (p < .0001)
The higher dosage of Cosopt resulted in an additional 2.7 ± 1.35 mmHg IOP reduction, corresponding to an 11.7% change. (p = .0004) (95%CI 1.74–3.6)
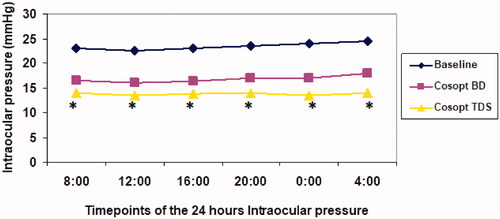
The IOP at each time point of the diurnal IOP with Cosopt TDS was significantly lower than the corresponding time points at baseline, and Cosopt BID ().
Figure 1. Diurnal curve of mean intraocular pressure (IOP) at baseline and during dorzolamide-timolol fixed combination (Cosopt) treatment. *Significant (p < .001) when compared Cosopt three times a day (TDS) versus Cosopt twice a day BID.
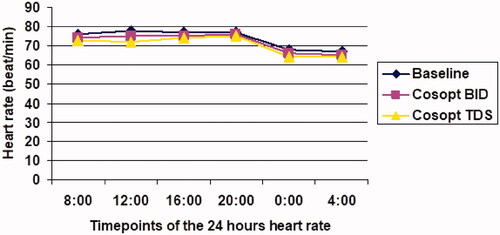
The mean 24-h HR at baseline was 76.6 beats per minute (bpm) (95%CI 65–90) and after one month of treatment with Cosopt BID, it was significantly reduced to 74.4 bpm, (95%CI 65–87; p = .000). After another month of treatment with Cosopt TDS, The mean 24 h HR was 74.06 bpm (range 64–88), which was comparable to Cosopt BID (p = .62) ().
Figure 2. Diurnal curve of mean heart rate at baseline and during dorzolamide-timolol fixed combination (Cosopt) treatment. Twice a day = BID; Three times a day = TDS.
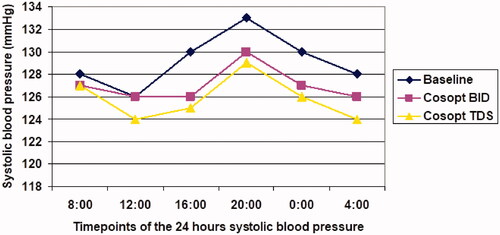
Mean 24 h systolic blood pressure was 131 ± 14.9 mmHg at baseline and was decreased to 128.5 ± 15.7 after one month of treatment with Cosopt BID (p = .03). The 2-month measurement was comparable with 1-month BP reading (177 ± 14[mp1]; 1 mmHg; p = .4) ().
Figure 3. Diurnal curve of mean systolic at baseline and during dorzolamide-timolol fixed combination (Cosopt) treatment. Twice a day = BID; Three times a day = TDS.
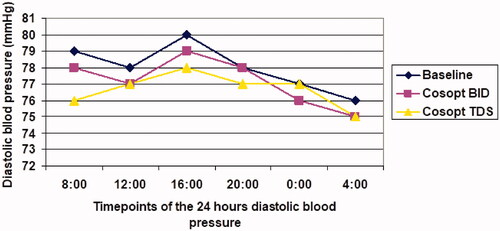
Mean 24 h diastolic blood pressure at baseline, month 1, and month 2 was 82.6 ± 12, 81.3 ± 12.1, and 81.3 ± 14.1 mmHg (ps = .16 and .13, respectively) ().
Figure 4. Diurnal curve of mean diastolic blood pressure at baseline and during dorzolamide-timolol fixed combination (Cosopt) treatment. Twice a day = BID; Three times a day = TDS.
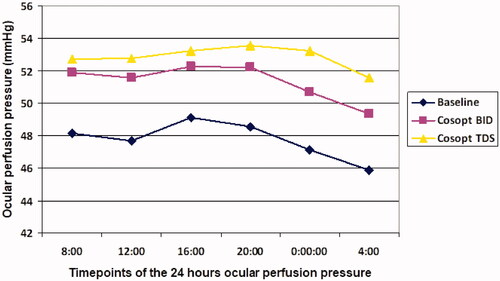
Mean 24 h ocular perfusion pressure at baseline, month 1, and month 2 was 48.12 ± 11, 51.71 ± 10.1, and 53.1 ± 9.6 mmHg (ps = .04 and .03, respectively) ().
Figure 5. Diurnal curve of mean ocular perfusion pressure (mmHg) at baseline and during dorzolamide-timolol fixed combination (Cosopt) treatment. Twice a day = BID; Three times a day = TDS.
One case developed bradycardia three weeks after starting Cosopt BID, and medication was immediately discontinued, which led to normalization of heart rate. No ocular discomfort was reported by the patients during the course of the study.
Discussion
In the current study, Cosopt BID reduced the IOP by almost 28% from the baseline, which is comparable to previous reports on its efficacyCitation8,Citation12,Citation14,Citation15. Increasing the dose of the medication to three times a day provided further IOP reduction by 12% and delivered a 40% reduction from the baseline that agrees with the significant efficacy of Cosopt TDS observed in the previous studyCitation12. This additional IOP lowering effect was not associated with any major systemic adverse effect or any change in heart rate and blood pressure. Our study included newly diagnosed primary open-angle glaucoma patients, but Shemesh et al. Included primary open-angle glaucoma patients after washout period and used Holter monitoring for 24 h in patients.
The introduction of several classes of glaucoma medications since 1979 has rendered medical therapy as the mainstay treatment for early glaucoma casesCitation16.
Recent endeavors revolved around combining already existing medications to increase efficacy and improve adherence.
Fixed combination drugs are an attractive alternative for glaucoma patients as it is shown that more than 50% of glaucoma cases need more than one dropCitation17, and there is a direct correlation between the number of the medication, and the adherenceCitation18.
Fixed combination drugs have at least the same efficacy as concomitant administration, and they increase patient persistence and reduce the cost. The problem though is to sync the different dosage schedule of each of the agents in combination. While timolol is recommended to be administered twice daily, dorzolamide needs three times a day doses to build up to steady-state levels of drug concentration in the ciliary bodyCitation19,Citation20.
Cosopt is recommended to be used twice daily to trade off higher dorzolamide efficacy for less timolol side effectCitation10.
The result of our study showed that increasing the dosage resulted in significant IOP reduction unparalleled to any other single or combined medicationCitation21,Citation22. Furthermore, IOP controlling pattern of more frequent usage tended to have a plateau profile, which by blunting the IOP fluctuations prevents further optic nerve head damage caused by undetected IOP peaksCitation23.
The dynamic behavior of intraocular pressure is thoroughly studiedCitation24–29. It is demonstrated that two-thirds of IOP peaks occur outside office hoursCitation28, and the rise of IOP during the nocturnal period is documented in both glaucoma and normal eyesCitation24,Citation25,Citation28. These undetected, high IOPs are considered a reason for progressing glaucomatous optic neuropathy despite apparently well-controlled IOP. In fact, 24-h IOP reading led to a modification of clinical management in almost 80% of reviewed glaucoma patientsCitation30.
Our findings show that Cosopt significantly decreases heartbeat and blood pressure at all visit points during the day. This finding agrees with previous reports on systemic effects of topical timolol administrationCitation31–33 and corroborates with the high bioavailability of timolol drop through the conjunctival and nasal mucosaCitation34–36.
Also, our results confirmed the findings of Shemesh et al. and showed that higher dosage has no additional systemic beta-blockade effectCitation12.
Although the undoubtedly higher dosage of medication exposes patients to the higher systemic concentration of timolol, careful patient selection, a thorough review system as well as punctual occlusion and eyelid closure help to avoid undesired systemic Adverse eventsCitation33.
The strength of the current study is its prospective nature and using 24 h heart rate and blood pressure monitoring.
But there are limitations to this study. We did a baseline comprehensive cardiology exam and would have excluded the patient if there was an unusual finding, but in real practice, systemic evaluation is rarely done for healthy individuals, and higher exposure may affect susceptible patients. Also, respiratory function test was not performed in this study, so we cannot comment on the possible respiratory side effect of the higher dosage. Furthermore, the effect of a higher dosage schedule on lipid profile, central nervous system, exercise intolerance, and the endocrine system was not evaluated in our study. One potential disadvantage of a higher dosage schedule is the late-night dosing of timolol. It may reduce ocular perfusion pressure, which is linked to progressive glaucomatous damage by some studiesCitation37. Although we did not observe any change in blood pressure by increasing the dose of timolol, aggravated nocturnal hypotension remains a concern for administering Cosopt three times a day. Another limitation was having no control group and a drop may reach maximum effect after one month.
Moreover, we only followed our patients for two months, and it is possible that a longer follow-up, and more exposure to timolol could result in inadvertent cardiac and respiratory adverse events.
In conclusion, our study showed that Cosopt administered three times a day has a significantly higher IOP reduction than 2-times a day schedule.
Transparency
Declaration of funding
No funding was received to produce this article
Declaration of financial/other relationships
No potential conflict of interest was reported by the author.
Authors do not have any financial interest in any products mentioned in this article
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None stated.
References
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267.
- Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206.
- Bucolo C, Platania CB, Drago F, et al. Novel therapeutics in glaucoma management. Curr Neuropharmacol. 2018;16(7):978–992.
- Russo A, Riva I, Pizzolante T, et al. Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review. Clin Ophthalmol. 2008;2(4):897–905.
- Bucolo C, Drago F. Carbon monoxide and the eye: implications for glaucoma therapy. Pharmacol Ther. 2011;130(2):191–201.
- Handra S, Muir ER, Deo K, et al. Effects of dorzolamide on retinal and choroidal blood flow in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2016;57(3):826–831.
- Sakamoto M, Kitamura K, Kashiwagi K. Changes in glaucoma medication during the past eight years and future directions in Japan based on an insurance medical claim database. J. Ophthalmol. 2017;2017:1–6.
- Crichton ACS, Harasymowycz P, Hutnik CML, et al. Effectiveness of dorzolamide–timolol (COSOPT) in patients who were treatment naive for open-angle glaucoma or ocular hypertension: the COSOPT first-line study. J Ocul Pharmacol Ther. 2010;26(5):503–511.
- Boyle JE, Ghosh K, Gieser DK, et al. A randomized trial comparing the dorzolamide-timolol combination given twice daily to monotherapy with timolol and dorzolamide. Dorzolamide-Timolol study group. Ophthalmology. 1998;105(10):1945–1951.
- Francis BA, Du LT, Berke S, et al. Comparing the fixed combination dorzolamide-timolol (cosopt) to concomitant administration of 2% dorzolamide (trusopt) and 0.5% timolol – a randomized controlled trial and a replacement study. J Clin Pharm Ther. 2004;29(4):375–380.
- Leskea MC, Heijl A, Hyman L, et al. Factors for progression and glaucoma treatment: the early manifest glaucoma trial. Curr Opin Ophthalmol. 2004;15(2):102.
- Shemesh G, Moisseiev E, Lazar M, et al. Intraocular pressure reduction of fixed combination timolol maleate 0.5% and dorzolamide 2% (cosopt) administered three times a day. OPTH. 2012;6:283–287.
- Mäenpää J, Pelkonen O. Cardiac safety of ophthalmic timolol. Expert Opin Drug Saf. 2016;15(11):1549–1561.
- Gugleta K, Orgül S, Flammer J. Experience with cosopt, the fixed combination of timolol and dorzolamide, after switch from free combination of timolol and dorzolamide, in Swiss ophthalmologists’ offices. Curr. Med. Res. Opin. 2003;19(4):330–335.
- Clineschmidt CM, Williams RD, Snyder E, et al. A randomized trial in patients inadequately controlled with timolol alone comparing the dorzolamide-timolol combination to monotherapy with timolol or dorzolamide. Ophthalmology. 1999;106(12 Suppl):17–24.
- Geringer CC, Imami NR. Medical management of glaucoma. Int Ophthalmol Clin. 2008;48(4):115–141.
- Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953.
- Djafari F, Lesk MR, Harasymowycz PJ, et al. Determinants of adherence to glaucoma medical therapy in a long-term patient population. J Glaucoma. 2009;18(3):238–243.
- Lippa EA, Carlson LE, Ehinger B, et al. Dose response and duration of action of dorzolamide, a topical carbonic anhydrase inhibitor. Arch Ophthalmol. 1992;110(4):495–499.
- Strohmaier K, Snyder E, DuBiner H, et al. The efficacy and safety of the dorzolamide-timolol combination versus the concomitant administration of its components. Dorzolamide-Timolol Study Group. Ophthalmology. 1998;105(10):1936–1944.
- Schuman JS, Katz GJ, Lewis RA, et al. Efficacy and safety of a fixed combination of travoprost 0.004%/timolol 0.5% ophthalmic solution once daily for open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2005;140(2):242–250.
- Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol. 2004;15(2):132–135.
- Susanna R, Jr, Vessani RM, Sakata L, et al. The relation between intraocular pressure peak in the water drinking test and visual field progression in glaucoma. Br J Ophthalmol. 2005;89(10):1298–1301.
- Wilensky JT. Diurnal variations in intraocular pressure. Trans Am Ophthalmol Soc. 1991;89:757–790.
- Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39(13):2707–2712.
- Liu JHK, Zhang X, Kripke DF, et al. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44(4):1586–1590.
- Renard E, Palombi K, Gronfier C, et al. Twenty-four hour (nyctohemeral) rhythm of intraocular pressure and ocular perfusion pressure in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2010;51(2):882–889.
- Pajic B, Pajic-Eggspuchler B, Haefliger I. Continuous IOP fluctuation recording in normal tension glaucoma patients. Curr Eye Res. 2011;36(12):1129–1138.
- Molaei A, Karamzadeh V, Safi S, et al. Upcoming methods and specifications of continuous intraocular pressure monitoring systems for glaucoma. J Ophthalmic Vis Res. 2018;13(1):66–71.
- Hughes E, Spry P, Diamond J. 24-hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma. 2003;12(3):232–236.
- Stewart WC, Castelli WP. Systemic adverse events of topical beta-adrenergic blockers. Clin Cardiol. 1996;19(9):691–697.
- Rolle T, Curto D, Alovisi C, et al. Timogel® vs timolol 0.5% ophthalmic solution: efficacy, safety, and acceptance. Eur J Ophthalmol. 2012;22(1):28–33.
- Farkouh A, Frigo P, Czejka M. Systemic adverse events of eye drops: a pharmacokinetic perspective. OPTH. 2016;10:2433–2441.
- Chang SC, Lee V. Nasal and conjunctival contributions to the systemic absorption of topical timolol in the pigmented rabbit: implications in the design of strategies to maximize the ratio of ocular to systemic absorption. J Ocul Pharmacol. 1987;3(2):159–169.
- Korte J-M, Kaila T, Saari KM. Systemic bioavailability and cardiopulmonary effects of 0.5% timolol eyedrops. Graefes arch. Graefes Arch Clin Exp Ophthalmol. 2002;240(6):430–435.
- Nieminen T, Lehtimäki T, Mäenpää J, et al. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand. J Clin Lab Invest. 2007;67(2):237–245.
- Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol. 1996;80(10):864–867.
