Abstract
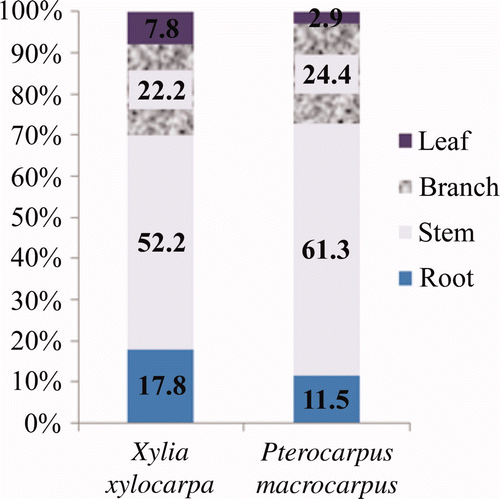
This research was conducted in the Katha District of Myanmar to compare the biomass (carbon) allocation of Xylia xylocarpa (Roxb.) W. Theob. and Pterocarpus macrocarpus Kurz species and to investigate the carbon content of the undergrowth vegetation, litter layer, and soil at these two plantations. A total of 40 trees from both species were selected to estimate the biomass allocation in each component of a tree. The estimated biomass (carbon) allocation for X. xylocarpa was 7.8% in the leaves, 22.2% in the branches, 52.2% in the stems and 17.8% in the roots while the estimated biomass (carbon) allocation for P. macrocarpus was 2.8% in the leaves, 24.4% in the branches, 61.3% in the stems and 11.5% in the roots. The study discovered that the biomass of the trees at the X. xylocarpa plantation (80.4 tons ha−1) was higher than that at the P. macrocarpus plantation (77.2 tons ha−1). The total carbon content at the P. macrocarpus plantation (130.8 tons ha−1) was significantly higher than that at the X. xylocarpa plantation (120.5 tons ha−1). Likewise, the mean annual increment (MAI) of the carbon content in the X. xylocarpa plantation was estimated at about 2.7 tons ha−1 while the P. macrocarpus plantation accounted for 2.5 tons ha−1. This study suggests that it is very important in the management of plantations to focus not only on the planted trees but also on the undergrowth vegetation, litter layer and soil layer, which play a significant role in the stand-level carbon content.
Introduction
The forestry sector plays a key role in the global climate change process (Ciesta Citation1995). Forests not only sequester carbon but also are a source of greenhouse gases (GHGs). Deforestation, forest degradation, burning and soil emission all together contributed approximately 25% of the current increase in atmospheric GHGs (IPCC 2003).
In Myanmar, due to overexploitation and deforestation, the total area of open/degraded forests accounts for 9.9 million ha, representing 13.7% of the total land area (Forest Department of Myanmar Citation2006). In order to supplement the natural forests and to compensate for deforestation, the Forest Department of Myanmar implemented a large-scale reforestation/restoration program in the 1980s. About 30,000 ha of forest plantations have been formed annually since 1984. From 1996 to 2007, the total area of commercial plantations (both pure and mixed plantations) reached 53% of the total planted areas consisting mainly of three species, Teak (Tectona grandis), Pyinkado (Xylia xylocarpa) and Padauk (Pterocarpus macrocarpus), which are 41%, 6% and 2%, respectively, of the total planted area (Forest Department of Myanmar Citation2007).
Carbon sequestration by plantations is 20.3% higher than natural forests based on remote sensing data (Kale et al. Citation2009). Estimating tree and forest biomass is essential for assessing ecosystem yield and carbon stock in compliance with the Kyoto Protocol on GHG reduction (Korner et al. Citation2005). MacDicken (Citation1997) suggested that measuring planted forests is necessary to understand the growth characteristics and to estimate the quantity of carbon sequestered by a forest.
Myanmar has great potential for the future carbon market through its reforestation activities, conservation of existing forests and plantations acting as carbon sinks. As a result, effective investigations and research activities are necessary to explore the status of the carbon content in various tree species and carbon accumulation in different land cover types for future carbon projects. Meanwhile, Kyi (Citation2003) suggested that in order to get better data estimates, further investigations on the carbon content of different woody species at different age classes need to be done.
Biomass (carbon) estimation was conducted for several forest types and species (Yamakura et al. Citation1986; Lugo et al. Citation1988; Wang et al. Citation1991; Brown Citation1997; Hashimoto et al. Citation2000; Ketterings et al. Citation2001; Kiyono and Hastanlah Citation2005). To reduce uncertainty, accurate carbon accounting methods are required. The development of new species-specific allometric equations is necessary to achieve higher levels of accuracy (Basuki et al. Citation2009). The most accurate method for the estimation of biomass is through the cuttings of trees and weighing their different parts (Basuki et al. Citation2009). However, Poorter and Nagel (Citation2000) mentioned that it is unsatisfactory to describe the allocation of biomass only in terms of roots and shoots. They also stated that the separation of leaves, branches, stems and roots gives better justice to the very different functions of leaves and stems because changes in the allocation pattern are relatively strong when light or nutrient supply varies.
Most of the investigations in Myanmar focused on silvicultural management, growth and yield predictions. There are few studies on the carbon sequestration of plantations (Oo et al. Citation2006; Myo Citation2008; Oo Citation2009) without much information on the carbon content of X. xylocarpa and P. macrocarpus plantations using destructive methods.
Therefore, the objectives of this study were: (1) to compare the biomass (carbon) allocation of the X. xylocarpa and P. macrocarpus species; and (2) to investigate the carbon content of the undergrowth vegetation, litter layer and soil at the two plantations of X. xylocarpa and P. macrocarpus in the Katha District of Myanmar.
Materials and methods
The study area
The study was conducted in the X. xylocarpa and P. macrocarpus plantations situated in Wun Tho in the Katha District of the Sagaing Division, which lies between 23°31' to 24°57' north latitudes and 95°2' to 94°40' east longitudes as shown in . These plantations are located within the Hinthar and Kyautpya reserved forests with an elevation ranging from about 610 to 1220 m above sea level (m asl). The major commercial tree species planted in the study area were X. xylocarpa and P. macrocarpus, and both species were selected for biomass study. In this study, 15-year-old plantations were selected to match with the thinning schedules. The first thinning (mechanical thinning) was done in 2000 (age 5–7 years) while the second thinning will be done after 15–17 years. Both plantations were established in 1995 with an initial spacing of 2.6 m to restore the degraded forests in Myanmar.
Biomass estimation
Inventory of the forest was done in 20 m × 20 m plots (a total of six plots) to examine the species composition, stand structure and biomass accumulation of the study sites. Based on the inventory data, 20 samples of different diameter classes for each plantation species were selected. Measuring of the biomass followed the destructive method (IPCC 2003). Trees were felled as close as possible to the ground and the height of the remaining stumps was exposed to about 0.3 m. Above-ground biomass was divided into different components, such as stems, branches, bough, leaves and bark, and the total fresh weight was determined. Sample slices of wood (about 4 cm in thickness) were taken from every 2 m section and then weighed. About 10-cm long pieces of branches and bough (1 kg) were collected as well and about 200 kg of leaves were randomly chosen. Saplings (DBH >5 cm and height >1.3 m), seedlings (DBH <5 cm and height <1.3 m), shrubs, grasses and litter layers were collected, and samples were taken from each component for biomass estimation. Samples from each component were pooled, sealed in a plastic bag, and transported to the laboratory of the Forest Research Institute (FRI) in Yezin, Myanmar. Samples were dried in an oven at 80°C for a week until constant weights were obtained (KFRI, 2007; Kenzo et al., Citation2009). The biomass of a tree was calculated by the ratio of dry and fresh weight of the samples. A carbon content default value of 0.5 was used to estimate the carbon content of the tree's biomass as proposed by the IPCC (1996).
An allometric regression equation was constructed for each species. A linear equation for each component (leaves, branches, stems and roots) was first developed to estimate the biomass using DBH, DBH2, DBH.H, DBH2.H, and H. This study selected the equation that showed the highest correlation among all the equations and it was used to estimate the biomass for all the studied species.
Collection and analysis of soil samples
Nine points were selected from each plantation, and 100 cm3 of soil samples were collected in 10-cm depth intervals (to a total depth of 50 cm) at each point starting at 0 cm. Representative soil samples from different plantations were collected to measure the soil organic carbon (SOC). There were 90 soil samples in total for the two plantations. The equations used for the estimation of SOC storage are as follows (Lal Citation2000):
Data analysis
All regressions were done using SPSS version 14 for Windows. A t-test was used to compare all components of the two plantations. A Duncan's multiple range test (DMRT) was done to examine the rank of the mean values of the carbon content in the trees' components and in the soil depths.
Results
Biomass and carbon allocation at the tree level
The DBH ranged from 9.9 to 25.6 cm for X. xylocarpa and 7.0 to 28.8 cm for P. macrocarpus species. The DBH classes were arranged in ascending order and 20 trees were selected to estimate the biomass allocation. The mean biomass of the sample-harvested trees from the X. xylocarpa and P. macrocarpus plantations were 191.30 kg and 184.91 kg, respectively ( ). The stem biomass had the highest percentage of biomass among all the biomasses for the components of a tree. In the same manner, the root biomass contributed the highest percentage of biomass to the total biomass of a tree ( ).
Table 1. Mean biomass accumulation in each component of the sampled tree species.
The stock density of the X. xylocarpa plantation was 558 trees ha−1 and 641 trees ha−1 for the P. macrocarpus plantation. X. xylocarpa had 33.15 tons ha−1 and 7.13 tons ha−1 for above-ground and root carbon content, respectively. P. macrocarpus had 32.99 tons ha−1 and 5.62 tons ha−1 for above-ground and root carbon content, respectively. The MAI for the carbon content for the X. xylocarpa plantation was 2.75 tons ha−1 while it was 2.54 tons ha−1 for the P. macrocarpus plantation. The MAI for the growth ring of the X. xylocarpa and P. macrocarpus plantations was 5.9 mm year−1 and 7 mm year−1, respectively. The root and shoot ratio in the excavated trees (6 trees for each species) of the X. xylocarpa plantation had a mean root to shoot ratio of 0.22 while the P. macrocarpus plantation had a mean root to shoot ratio of 0.17. This indicates a variation in the carbon contribution of the different species for above and below ground of a tree. As a result, on average, 20% of the tree carbon was stored in their roots and 80% was in their shoots when the carbon allocation of the different components was considered.
Estimation of the carbon content at the plantation level
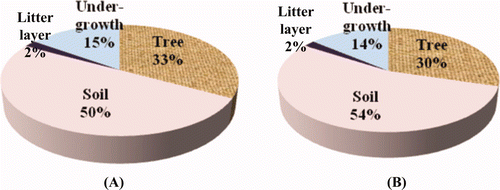
The means of the undergrowth between the plantations were not significantly different with a probability level of 5%. The average carbon content of the undergrowth vegetation (sapling, seedling, shrub and grass) at both plantations was 17.60 tons ha−1 (X. xylocarpa) and 18.33 tons ha−1 (P. macrocarpus). In addition, the average carbon content of the litter layer was 2.83 tons ha−1 in the X. xylocarpa plantation and 2.18 tons ha−1 in the P. macrocarpus plantation. The proportions of the undergrowth and litter layer carbon in the total carbon content were approximately the same at all of the study sites.
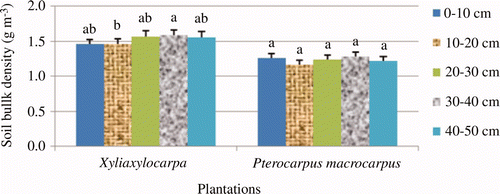
The total SOC (up to 50 cm) accumulated by the X. xylocarpa plantation amounted to 59.77 tons ha−1 while that of P. macrocarpus plantation was 71.69 tons ha−1 ( ). The soil bulk density ranged from 1.35 g cm−3 to 1.67 g cm−3 (average 1.52 g cm−3) in the X. xylocarpa plantation while 1.06 g cm−3 to 1.33 g cm−3 (average 1.23 g cm−3) in the P. macrocarpus plantation ( ). Likewise, both SOC and soil bulk density changed significantly with the depth (P < 0.001 for both). DMRT showed that SOC decreased with increasing soil depth.
Figure 3. Organic carbon content of the soil with different soil layers from the X. xylocarpa (A) and P. macrocarpus (B) plantations. The different letters indicate significant differences in SOC among the soil depths according to DMRTs at a 5% level of probability. The letters are the rank order from highest to lowest value (alphabetically). Open bar indicates the standard error.
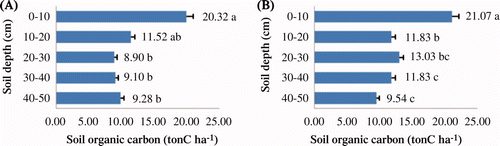
Figure 4. Soil bulk density at the two plantations. Different letters indicate significant differences in soil bulk density among the study sites according to Duncan's multiple range tests at a 5% level of probability. The letters are the rank order from highest to lowest value (alphabetically). Open bar indicates the standard error.
In this study, the total carbon content is the sum of the above-ground, root, litterfall, undergrowth and soil carbon of each plantation site. The total carbon content of the X. xylocarpa plantation was 120.48 tons ha−1 and that of P. macrocarpus plantation was 131.81 tons ha−1. The proportion of carbon storage for the X. xylocarpus and P. macrocarpus plantations is shown in .
Discussion
At the individual tree level, the biomass of X. xylocarpa was higher than that of the P. macrocarpus and T. grandis. It may be due to the different growth patterns of the species and the different wood densities. Redondo-Brenes and Montagnini (Citation2006) suggested that the accumulation of biomass and carbon might be related to the differences in wood specific gravity and growth patterns among fast- and slow-growing species. A wood specific gravity of 0.77 and 0.75 were estimated for X. xylocarpa and P. macrocarpus, respectively (Kyi Citation2003).
Myo (Citation2008) found that the above-ground carbon of a 15-year-old teak plantation was 28.82 tons ha−1. This was lower compared with the present study (P. macrocarpus, 32.99 tons ha−1 and X. xylocarpa, 33.15 tons ha−1). It may be due to the differences in wood densities and growth patterns among fast- or slow-growing species (Thomas Citation1996). Furthermore, the higher wood density species can store much more carbon (Lasco 1988). Oo (Citation2009) found that the above-ground carbon of a 16-year-old teak plantation was 37.6 ± 5.98 tons ha−1, which was higher than in this study. It must be noted that in this study, the second thinning was already done for the plantation. Overall, the specific gravity of the wood could affect the weight of the biomass. In addition, silvicultural operations, such as thinning, improvement felling and weeding at an early age and even the growth patterns among fast- and slow-growing species, can contribute to the weight of the biomass (carbon content) in a plantation.
The results for MAI in this study were slightly higher than Kyi's (Citation2003) investigation based on data from the whole country using a non-destructive method, which was estimated for the X. xylocarpa plantation (2.37 tons ha−1) and P. macrocarpus plantation (2.29 tons ha−1). It may be primarily due to variation in ages and plantation localities. Studies of carbon absorption rates in tropical forest plantations indicate that the maximum growth and carbon uptake occurred during age classes of 0 to 5 and 6 to 10 years (62%). Carbon uptake decreased by about 50% during the next 5 years and decreased even further after 16 years (Lugo and Brown Citation1986).
The MAI for the carbon content of the X. xylocarpa plantation was higher compared to the P. macrocarpus plantation, especially in the root carbon. It may be due to the different organic carbon levels in the soil and the different nutrients. It has been known for a long time that allocation to the roots increases with decreasing nutrient or water availability (Brenchley Citation1916) and that allocation to shoots increases with decreasing irradiance (Shirley Citation1929). Similarly, plants shift allocation towards the roots when there is a low level of below-ground resources, such as nutrients and water. In addition, the soil properties of the P. macrocarpus plantation were higher than at the X. xylocarpa plantation; therefore, root carbon allocation of X. xylocarpa was higher than that of P. macrocarpus. Moreover, this study supports the result of Poorter and Nagel (Citation2000) that at low nutrients and water availability, roots use relatively more of these resources, leaving less for the shoots (leaves).
In this study, the mean root to shoot ratio of both species (0.20) was closer to that of other tropical forest biomass, which was 0.24 (Cairns et al. Citation1997). This study was similar to that of Harris (Citation1992), who found that in most adult trees under normal conditions, the root to shoot ratio was between 0.16 and 0.20. It is also in agreement with a study by Lukac et al. (Citation2010) who stated that the root to shoot ratio tended to stabilize at around a value of 0.20 in all species. Regardless of the species, the root to shoot ratio ranged from 0.22 to 0.30 in Australia (Specht and West Citation2003) and from 0.15 to 0.27 in Myanmar (Oo Citation2009).
The MAI for the growth ring in this study was lower than a 26-year-old plantation with regular thinning of 8.5 mm and 9 mm for the X. xylocarpa and P. macrocarpus plantations (Oo Citation2009). It may be due to the site suitability, the growth characteristics of each species (e.g. leaf area and crown diameter), and the influence of climate variations. The frequency of the thinning operation and tending activities must also be considered when estimating the growth rate of the tropical species. The growth ring increment of the studied species was within the range of 3.2 to 9.4 mm (Worbes Citation1999) based on the data of 37 tropical species.
Undergrowth vegetation of 15% (X. xylocarpa) and 14% (P. macrocarpus) were higher compared with the 4–7% total above-ground biomass in pine stands (John 1973), and 3.4% total above-ground biomass in a 16-year-old teak plantation (Oo Citation2009). The undergrowth is expected to be a small component of the above-ground biomass in managed plantations, but this may vary with the weeding intensity, improvement felling, site characteristics and planting distance. The carbon content of the litter layer in this study was higher than in the study by Oo et al. (Citation2006) who mentioned that the biomass of the litter layer in the central dry zone plantation (Eucalyptus camaldulensis and Acacia catechu) was 1.17 tons ha−1 (0.53 tons ha−1 in terms of carbon). It may be due to the planted species belonging to the Fabaceae family (Leguminosae) which easily accumulates fallen leaves, decomposes and later improves the soil fertility.
Depending on the planted species, the amount of carbon content was different among the different plantations. A 13-year-old pure Diptery panamensis stored 87.10 tC ha−1 while a 13-year-old mixed D. panamensis stored 126.90 tC ha−1 in the Philippines (Redondo-Brenes and Montagnini Citation2006). On the other hand, an 8-year-old Populus deltoids plantation stored 96.23 tC ha−1 in India (Singh and Lodhiyal Citation2009). The differences in carbon storage in this study and other studies may be due to the different study sites and growth characteristic of the species.
Oo et al. (Citation2006) assessed that the biomass of 8-year-old Acacia catechu was 10.62 tons ha−1 (with a carbon content of 5.31 tons ha−1) in Myanmar. Oo (Citation2009) found that the carbon content in a 16-year-old T. grandis plantation was 90.40 tC ha−1 in Myanmar. Different characteristics and wood density affect the carbon content of plantations. Tree species with a high wood density can store higher amounts of carbon than other species with the same diameter size but a lower wood density.
Conclusion
This study investigated the carbon allocation of X. xylocarpa and P. macrocarpus species and estimated the carbon content of the undergrowth, litter layer and soils at both plantations. Based on the 40 harvested trees, it was discovered that the highest percentage of biomass was allocated to the stem at the individual tree level followed by the branches, roots and leaves in both species. The results indicate that the X. xylocarpa stored more biomass (carbon) than in the P. macrocarpus at the tree level. At the plantation level, the carbon content of the P. macrocarpus plantation was higher than in the X. xylocarpa plantation. It was observed that there were more undergrowth species in the P. macrocarpus plantation than in the X. xylocarpa plantation. Overall, this study showed that it is very important in the management of plantations to focus not only on planted trees but also on the undergrowth vegetation, litter layer and soil layer, which play a significant role in the stand-level carbon content.
This study revealed that a considerable amount of carbon was allocated to the 15-year-old X. xylocarpa and P. macrocarpus plantations, which served as an initial survey of the carbon content in two hardwood species with high economic value (ranged in second and third position). Further clarification on the applicability of the species-pooled equation for X. xylocarpa and P. macrocarpus plantations in other regions and with different ages is still necessary.
Acknowledgement
This study was supported by the ASEAN-Korea Environmental Cooperation Project (AKECOP).
References
- Basuki , T M , Van Laake , P E , Skidmore , A K and Hussin , Y A . 2009 . Allometric equations for estimating the above-ground biomass in tropical lowland Dipterocarp forests . For Ecol Manage. , 257 : 1684 – 1694 .
- Brenchley , W E . 1916 . The effect of the concentration of the nutrient solution on the growth of barley and wheat in water cultures . Ann Bot. , 30 : 77 – 91 .
- Brown , S . 1997 . Estimating biomass and biomass change of tropical forests: a primer. FAO forestry paper 134 Rome , , Italy
- Cairns , M A , Brown , S , Helmer , E H and Baumgardner , G A . 1997 . Root biomass allocation in the world's upland forests . Oecologia , 111 : 1 – 11 .
- Ciesta , W M . 1995 . Climate change, forests and forest management . FAO Publication , : 128 – 138 .
- Forest Department of Myanmar . 2006 . Forestry in Myanmar. Forest Department, Yangon, Myanmar 1 – 20 .
- Forest Department of Myanmar . 2007 . Forestry in Myanmar. Forest Department, Yangon, Myanmar 20 – 40 .
- Harris , R W . 1992 . Root:shoot ratios . J Arboricult. , 18 : 39 – 42 .
- Hashimoto , T , Kojima , K , Tange , T and Sasaki , S . 2000 . Changes in carbon storage in fallow forests in the tropical lowlands of Borneo . For Ecol Manage. , 126 : 331 – 337 .
- IPCC (Intergovernmental Panel on Climate Change) . 1996 . Guidelines for National Greenhouse Gas Inventory: Reference manual Land use and forestry, Section 5.1, September 1996
- IPCC National Greenhouse Gas Inventories Programme . 2003 . Good practice guidance for land use, land use change and forestry , Technical Support Unit IPCC National Greenhouse Gas Inventories Programme, Institute for Global Environmental Strategies, Hayama .
- John , D M . 1973 . Accumulation and decay of litter and net production of forest in tropical West Africa . Oikos , 24 : 430 – 435 .
- Kale , M P , Ravan , S A , Roy , P S and Singh , S . 2009 . Patterns of carbon sequestration in forests of Western Ghats and study of applicability of remote sensing in generation carbon credits through afforestation and reforestation . J Indian Soc Remote Sens. , 37 : 457 – 471 .
- Kenzo , T , Furutani , R , Hattori , D , Kendawang , J J , Tanaka , S , Sakurai , K and Ninomiya , I . 2009 . Allometric equations for accurate estimation of above-ground biomass in logged-over tropical rainforests in Sarawak, Malaysia . J For Res. , 14 : 365 – 372 .
- Ketterings , Q M , Coe , R , Noordwijk , M V , Ambagau , Y and Palm , C A . 2001 . Reducing uncertainty in the use of allometric biomass equations for predicting above-ground tree biomass in mixed secondary forests . For Ecol Manage. , 146 : 199 – 209 .
- Kiyono , Y and Hastanlah . 2005 . Patterns of slash-and-burn land use and their effects on forest succession. Swidden-land forests in Borneo . Bull Forestry For Prod Res Inst. , 4 : 259 – 282 .
- Korea Forest Research Institute . 2007 . Survey manual for biomass and soil carbon http://wwwkfri.go.kr
- Korner , C , Oo , M Z , Shin , T and Yasuo , O . 2005 . Slow in, rapid out-carbon flux studies and Kyoto targets . Science , 300 : 1242 – 1243 .
- Kyi , W . Estimating total carbon storage in forest plantations of Myanmar . Research paper presented in Goettingen University . Germany .
- Lal , R . 2000 . “ Soil aggregation and carbon sequestration ” . In Global climate change and tropical ecosystems , Edited by: Lal , R , Kimble , J M and Steward , B A . 317 – 329 . New York : CRC Press .
- Lasco , R D . 1998 . Management of Philippine tropical forests: implications to global warming . World Res Rev. , 10 : 410 – 418 .
- Lugo , A E and Brown , S . 1986 . Steady state terrestrial ecosystem and the global carbon cycle . Vegetation , 68 : 83 – 90 .
- Lugo , A E , Brown , S and Chapman , C G . 1988 . An analytical review of production rates and stemwood biomass of tropical forest plantations . For Ecol Manage. , 23 : 179 – 200 .
- Lukac , M , Konopka , B , Pajtik , J and Moravcik , M . 2010 . Biomass partitioning and growth efficiency in four naturally regenerated forest tree species . Basic Appl Ecol. , 11 : 234 – 243 .
- MacDicken , K G . 1997 . A Guide to Monitoring Carbon Storage in Forestry and Agroforestry Projects , Vol. 15 , 322 – 327 . Arlington , VA : Winrock International Arling .
- Myo , M T . 2008 . Carbon storage capacity in teak plantation and degraded forest potential for AC-CDM projects (A case study in Yedashe Township, in the eastern part of the Bago Yoma.) MSc (Thesis) , University of Forestry. Yezin, Myanmar .
- Oo , M Z , Shin , T , Oosumi , Y and Kiyono , Y . 2006 . Biomass of planted forest and biotic climax of shrub and grass communities in the central dry zone of Myanmar . Bulletin of FFPRI , 5 : 271 – 288 .
- Oo , T N . 2009 . Carbon sequestration of tropical deciduous forests and forest plantations in Myanmar. , PhD (Thesis), Seoul National University, Republic of Korea .
- Poorter , H and Nagel , O . 2000 . The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review . Aust. J. Plant Physiol. , 27 ( 12 ) : 1191 – 1191 .
- Redondo-Brenes , A and Montagnini , F . 2006 . Growth, productivity, aboveground biomass, and carbon sequestration of pure and mixed native tree plantations in the Caribbean lowland of Costa Rica . For Ecol Manage. , 232 : 168 – 178 .
- Shirley , H L . 1929 . The influence of light intensity and light quality upon the growth of plants . Am J Botany , 16 : 354 – 390 .
- Singh , P and Lodhiyal , L S . 2009 . Biomass and carbon allocation in 8-year-old poplar (Populus deltoids Marsh) plantation in Tarai agroforestry systems of Central Himalaya, India . N Y Sci J. , 2 ( 6 ) : 49 – 53 .
- Specht , A and West , P W . 2003 . Estimation of biomass and sequestered carbon on farmforest plantations in northern New South Wales, Australia . Biomass Bioenergy , 25 : 363 – 379 .
- Thomas , S C . 1996 . Asymptotic height as a predictor of growth and allometric characteristics in Malaysian rain forest trees . Am J Bot. , 83 ( 5 ) : 556 – 566 .
- Wang , D , Bormann , F H , Lugo , A E and Bowden , R D . 1991 . Comparison of nutrient-use efficiency and biomass production in five tropical tree taxa . For Ecol Manage. , 46 : 1 – 21 .
- Worbes , M . 1999 . Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela . J Ecol. , 87 : 391 – 403 .
- Yamakura , T , Hagihara , A , Sukardjo , S and Ogwa , H . 1986 . Tree size in a mature Dipterocarp forest stand in Sebulu, East Kalimantan, Indonesia . Southeast Asian Studies , 23 : 452 – 478 .