Abstract
Genetic variation patterns of white jabon (Anthocephalus cadamba [Roxb.] Miq.), were evaluated at the population level. Eleven natural populations were examined for variations in fruit, seed, and seedling morphophysiological characteristics. Analysis of variance revealed significant differences among populations for all the characteristics studied, except the radicle length. Genotypic variance and genotypic coefficient of variance for all fruit, seed, and seedling characteristics were found to be higher than corresponding environment variance and environment coefficient of variance, indicating that the genotype explained most of the variance for these characteristics. In particular, high heritability values coupled with high genetic gain were found for fruit weight, seedling height, root collar diameter, sturdiness index, leaf number, leaf length, and leaf width. Principal component analysis and hierarchical clustering of various characteristics of fruit, seed, and seedling revealed that most of the geographically distant populations are genetically close. Since these characteristics appear to be under strong genetic control, considerable scope exists for exploitation of heritable additive genetic components for future breeding and improvement in white jabon.
Introduction
White jabon (Anthocephalus cadamba [Roxb.] Miq., Rubiaceae) is a fast-growing tree species that is native to Indonesia. It can be used for many purposes, and it plays an important role in both commercial and traditional farming systems in several areas of the country (Kallio et al. Citation2011). This species produces timber for pulp, plywood, and light construction (Soerianegara and Lemmens Citation1993), and various parts of the plant have bioactive compounds, including antioxidant, hypoglycemic, hypolipidemic, antibacterial, and antimicrobial properties (Acharyya et al. Citation2011; Xu et al. Citation2011; Mishra and Siddique Citation2011).
White jabon grows widely in the tropical evergreen lowland rain forests of Asia, which extend through India, Nepal, Burma, Sri Lanka and Malaysia, and across Indonesia, Philippines, and New Guinea (Lamprecht Citation1989). In Indonesia, the species is distributed on almost all islands, and it is often found in secondary forests along riverbanks and in the transitional zone between swampy, permanently flooded areas and periodically flooded areas (Soerianegara and Lemmens Citation1993). White jabon can grow on a variety of soils in areas lower than 1000 m above sea level (masl) which normally receive more than 1500 mm of rain per year, but it can survive in drier areas with annual rainfall as low as 200 mm (Martawijaya et al. Citation1989). Evolutionary theory predicts that species with large populations and broad geographic ranges will have high levels of genetic variation. White jabon is therefore expected to harbor a large amount of genetic variation, but little is known about the morphological variation among natural populations. Information on this variation is required for effective collection of genetic materials to establish breeding populations.
Population or provenance variation with respect to seed and seedling morphophysiological characteristics has been studied in many species and locations, such as Pinus wallichiana in India (Rawat and Bakshi Citation2011), Trigonobalanus doichangensis in southwestern China (Zheng et al. Citation2009), and Cedrus deodara in Jammu and Kashmir (Mughal and Thapliyal Citation2012). In several tree species, significant correlations have been shown to exist between specific seed and seedling attributes and specific environmental and geo-climate factors (Cardillo and Bernal Citation2006; Tauchen et al. Citation2011). Such correlations help to identify the environmental factors influencing plant physiology and morphology.
The principal objective of this study was to assess the magnitude of variation in seed and seedling characteristics among 11 populations of white jabon in Indonesia, and the extent to which these characteristics are under genetic control. This information will help evaluate selection criteria for prominent traits of the species for both laboratory and nursery assessments, and could also be used as an index for evaluating a population.
Materials and methods
Sample collection
Samples were collected from 11 natural populations distributed in Sumatra, Java, Borneo, Celebes, and Sumbawa. The geographical coordinates, altitude, mean annual rainfall, and identifying abbreviation for each site are presented in . In each population, 10 to 20 disease- and pest-free seed trees with no visible signs of damage were selected based on their total height, diameter at breast height, and crown spread. To prevent sampling trees from the same parent or pedigree, a minimum distance of 100 m on average was maintained between each tree. Twenty-five fruits were collected from each tree.
Table 1. Geographic origin of the investigated populations.
Morphophysiological data
The sampling method and design were the same for each population site. From each site, 100 undamaged fruits were randomly collected and measured for diameter (average of the largest and smallest diameter) and fruit weight. The seeds were extracted by the wet extraction method (Bonner et al. Citation1994). Equal weights of seeds composed the samples from individual trees, and seeds were bulked by population for the experiments. From each of the 11 populations, 100 composite seeds were measured for their length and width using a Zeiss Stereo Discovery V.8 Stereo microscope (Carl Zeiss AG, Germany). Seed weight was determined for eight replications of 100 seeds and finally transformed into 1000 seed weight (ISTA Citation2010). Seed germination studies were carried out in greenhouse conditions at 25 ± 3 °C using sand media with six replications of 100 seeds from each population. A seed was considered to be germinated when a pair of leaves developed. Germination was assessed every 24 h for 20 d, and data were recorded for germination capacity, germination rate (Maguire Citation1962), and mean germination time (Edmond and Drapala Citation1958). The hypocotyle and radicle length were recorded at the end of the germination test. The vigor index (total seedling length × germination) was calculated according to Bhattacharya et al. (Citation1991).
Seeds were sown in a greenhouse of SEAMEO BIOTROP (Southeast Asian Regional Center for Tropical Biology), Bogor, Indonesia (http://www.biotrop.org). Seedlings were transplanted into a polybag (diameter 12 cm, height 15 cm) and set up in a randomized block design with eight replications. Twenty-five seedlings formed a square for each replication. When the seedlings were 3 months old, nine seedlings per replication were selected from the center of each block, to minimize the side effect, and marked for measurement of height, root collar diameter, sturdiness index, leaf number, leaf length, and leaf width. For measurements of stomatal density, chlorophyll a, chlorophyll b, aboveground, belowground and total biomass, and root–shoot ratio, one seedling was randomly selected from each replication. Stomatal density was measured using a nail polish impression of the abaxial surface of the first fully developed leaf and counted under a light microscope in three random fields at ×40 magnification. The concentrations of chlorophyll a, chlorophyll b, and total chlorophyll were measured from a single leaf at the second pair from the apices (Lichtenthaler Citation1987) using a UV/visible spectrophotometer (UV-1201, Shimadzu Corporation, Tokyo, Japan).
Statistical analysis
A complete randomized design was used for fruit, seed, and seedling characteristics in the laboratory, while a randomized block design was used for seedling characteristics in the nursery. The fruit, seed, and seedling parameters and population measurements were analyzed by using SPSS (v21) for analysis of variance and Duncan's multiple range test to determine the differences between the leaves, fruits, seeds, seedlings, and populations. Simple correlations (Pearson's) at p < 0.05 were calculated for fruit, seed, and seedling characteristics with geo-climate factors (altitude, longitude, latitude, and mean annual rainfall).
The phenotypic variance, genotypic variance, environment variance, phenotypic coefficient of variance, genotypic coefficient of variance, environment coefficient of variance, broad sense heritability, and genetic gain were determined according to Burton (Citation1952) and Johnson et al. (Citation1955). Principal component analysis (PCA) and hierarchical clustering were used to explain the pattern of variation among the populations.
Results and discussion
Fruit, seed, and seedling traits
Fruit, seed, and seedling characteristics, with the exception of radicle length, varied significantly among the 11 populations of white jabon. The highest coefficient of variation (CV) was recorded for belowground biomass (55.24%) followed by aboveground biomass (48.10%) and total biomass (47.90%). Mean germination time (3.28%) had the lowest CV, followed by the 1000 seed weight (10.56%) (). Variability studies for fruit, seed, and seedling characteristics revealed that the PKC population recorded maximum values for seven characteristics: 1000 seed weight; germination capacity; germination rate; seedling height; aboveground biomass; belowground biomass; and total biomass. The KTB population exhibited the lowest performance for nine characteristics: fruit diameter; seedling height; root collar diameter; leaf number; leaf length; leaf width; aboveground biomass; belowground biomass; and total biomass. The significant differences among populations for most of the seed and seedling morphophysiological characteristics indicated that the variability is under strong genetic influence.
Table 2. Variability estimates for fruit, seed, and seedling characteristics.
Variations in fruit and seed characteristics have been observed in several tree species such as Pinus roxburghii (Mukherjee et al. Citation2004), Pinus wallichiana (Rawat and Bakshi Citation2011), and Cedrus deodara (Mughal and Thapliyal Citation2012). Furthermore, variation in germination according to seed source has been reported in P. roxburghii (Roy et al. Citation2004) and P. wallichiana (Rawat and Bakshi Citation2011). Seed and seedling growth parameters are interdependent, and all are governed by genetic makeup, environmental influences, and seed characteristics (Pathak et al. Citation1984). Variations in fruit, seed, and seedling characteristics among populations of white jabon are probably due to the natural constraints prevailing in their geographic location. The occurrence of white jabon over a wide range of habitats with diverse geo-climatic conditions was expected to be reflected in the genetic constitution of separate populations. This separation in turn influences gene flow.
Variance and coefficient of variability
The genotypic variance ranged from 2858.637 (stomatal density) to 0.009 (chlorophyll b). With respect to variability, the phenotypic and genotypic CVs were the highest for seedling height (76.40 and 76.33, respectively), while the maximum environmental CV was associated with belowground biomass (29.36). The minimum phenotypic and genotypic CVs were recorded for mean germination time (1.87 and 1.56, respectively), and the minimum environmental CV was for 1000 seed weight (0.64). Genotypic variance and genotypic CVs for all fruit, seed, and seedling characteristics were found to be higher than corresponding environmental variance and environmental CVs. The magnitude of error variance was relatively lower than the genotypic variance for all characteristics, while the phenotypic and genotypic CVs were close to each other for all characteristics (). These results indicate that the genotypic component was the major contributor to the total variance for these characteristics.
Table 3. Variances and coefficient of variability for fruit, seed, and seedling characteristics.
In most tree species, the characteristics of fruit, seed, and seedling morphophysiology are primarily controlled very strongly by genotype, while environmental factors, which vary by location and by populations within locations, have only a small effect (Khalil Citation1986). Genetic control of the seed size characteristic has been observed for several tree species such as Juniperus procera (Mamo et al. Citation2006) and Calophyllum inophyllum (Hathurusingha et al. Citation2011). This variability indicates considerable scope for selection. In the present study, the genotypic CV and the genetic gain were found to be high for fruit weight, seedling height, root collar diameter, sturdiness index, leaf number, leaf length, and leaf width. Higher genotypic coefficient of variability indicates that worthwhile improvement could be achieved for this character through simple selection, while higher genetic advance value suggests that population means for seedling height, sturdiness index, and fruit weight may be changed considerably through selection of 5% intensity of superior genotypes.
Estimates of broad sense heritability ranged from 0.52 (for root length) to 0.99 (for fruit weight, seedling height, and sturdiness index). Genetic gain ranged between 2.66% and 157.09% with mean of germination time giving the lowest value and seedling height the highest value. Some parameters such as fruit diameter, weight of 1000 seeds, germination capacity, and germination rate have highest heritability (>0.95) but with lower genotypic CV and genetic gain (). Despite good values of heritability, these characteristics did not show expected genetic gain, which could be explained by the existence of more non-additive genetic effects compared with additive genetic effects. Partitioning the total phenotypic variance (Vp) of each characteristic into a heritable (Vg) and a non-heritable component (Ve) is helpful in determining the proportion of heritable variation that can be exploited to select for prominent characteristics. Estimation of heritability is useful as a gross indicator of the possibility of selection for one or more characteristics (Namkoong et al. Citation1966).
Table 4. Simple correlation between fruit, seed, and seedling characteristics studied and geo-climatic factors.
High heritability values coupled with high genetic gain were found for fruit weight, seedling height, root collar diameter, sturdiness index, leaf number, leaf length, and leaf width, suggesting that these characteristics are highly genetic in origin, with a good amount of heritable additive genetic components that could be used to select the best population for improvement of the species. Johnson et al. (Citation1955) observed that a high heritability value, along with genetic gain in characteristics, is more appropriate for selecting the best individuals from the best populations. In contrast, high heritability values coupled with low genetic gain were seen for fruit diameter, indicating that this characteristic has more non-additive genetic components than additive ones.
Correlation matrix
Simple correlation was calculated between specific parameters studied and the geo-climate factors altitude, longitude, latitude, and annual rainfall (). A positive correlation between seed weight and latitude suggested that seed weight increases with higher latitude. A similar relationship was observed in Picea asperata (Luo et al. Citation2005), with differences in latitude affecting seed size. Southern populations (GSJ, NKJ, APJ, and BHS) generally had better site fertility (pH, N, P, K, Ca, Mg) (Appendix 1) than northern populations, which apparently affected fruit and seed development since the southern populations produced larger seeds. Seed length and vigor index showed positive correlations with longitude, with both measures increasing with higher longitude. Sites in the western populations generally had higher annual rainfalls, leading to nutrient leaching, which was reflected in the soil analysis results. Macronutrients were lower in the western population sites compared with the eastern population sites (Appendix 1), which affected seed development. High rainfall and nutrient leaching were associated with smaller seeds as reported by Rawat and Bakshi (Citation2011) in Pinus roxburghii.
Genetic divergence
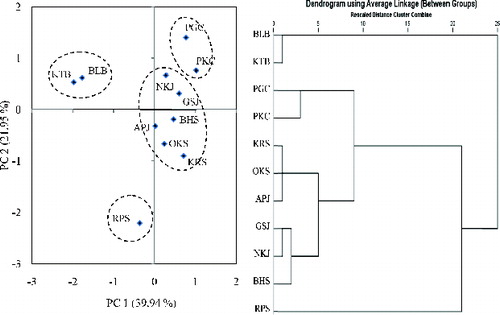
As shown in , PCA and hierarchical clustering had a similar trend and classified the genotypes into four main groups, highlighting considerable genetic diversity for seed and seedling characteristics in white jabon. Group 1 consisted of two populations (KTB, BLB), as did group 2 (PGC, PKC). Group 3 was the largest, with six populations (KRS, OKS, GSJ, NKJ, APJ, BHS), while group 4 had only one population (RPS). Geographically distant populations belonging in the same group revealed that they are genetically close. However, the RPS population was separate from other populations on the same island (Sumatra). The concept of provenance has been adopted in forestry to indicate the origin of planting stock, which is distinctively different due to evolutionary processes. The concept is useful to explain the clustering results in this study. Groups 1, 2, and 4 might be of true provenance from the area, as they clustered according to their geographical origins. Group 34, on the other hand, might be a mixture of provenances, indicating the recent origin of those populations due to high reforestation activities in Sumatra, Java, and Sumbawa islands. Deforestation and forest degradation have been quite intense in the isolated RPS population, and the genetic diversity of remnant populations may already be affected by genetic drift and inbreeding, resulting in high diversity between populations.
Conclusion
Significant differences in the fruit, seed, and seedling characteristics among white jabon populations indicated that evolutionary processes had shaped the populations into different provenances. As the populations differentiated into different provenances it is expected that most of the characteristics are different genetically, as shown by their wide range of variability in terms of mean values, critical difference, variance component, and CV. Strong genetic influence shaped by evolutionary processes was also substantiated by evidence that environmental factors, which vary by location and by populations within a location, have only a small effect on the studied characteristics. PCA and hierarchical clustering provided further evidence for provenance formation of three groups, with the exception of one group only, which could be explained by recently high reforestation activity in the region. The higher amount of heritability in a broad sense, coupled with considerable genetic advance for fruit weight, seedling height, root collar diameter, sturdiness index, leaf number, leaf length, and leaf width, indicated additive genetic effects in the heritage of these characteristics. The investigation has important practical implications for genetic management of resources and for future breeding programs to improve white jabon.
Acknowledgements
This work was supported by SEAMEO BIOTROP, Bogor, Indonesia (grant number 047.20/PSRP/ST-PNLT/III/2012).
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Acharyya S, Rathore DS, Kumar HKS, Panda N. 2011. Screening of Anthocephalus cadamba (Roxb.) Miq. root for antimicrobial and anthelmintic activities. Int J Res Pharmaceut Biomed Sci. 2:297–300.
- Bhattacharya AK, Lakhari AK, Basu RN. 1991. Improvement of germinability of Eucalyptus sp. by pre germination treatments. Indian J For. 117:661–663.
- Bonner FT, Fozzo JA, Elam WW, Land SB Jr. 1994. Tree seed technology training course. Instructors manual. Southern Forest Experiment Station. US Department Agriculture; p. 28–30.
- Burton GW. 1952. Quantitative inheritance of grass. Proc. 6th, Int. Grassland Cong. Held at Pennsylvania State College. Pa. US. 1:74–83.
- Cardillo E, Bernal CJ. 2006. Morphological response and growth of cork oak (Quercus suber L.) seedlings at different shade levels. For Ecol Manag. 222:296–301.
- Edmond JB, Drapala WJ. 1958. The effects of temperature, sand and soil, and acetone on germination of okra seed. Proc Am Soc Hort Sci. 71:428–434.
- Hathurusingha S, Ashwath N, Midmore D. 2011. Population variations in seed-related characters and oil content of Calophyllum inophyllum L. in Northern Australia and Sri Lanka. New Forest. 41:89–94.
- ISTA. 2010. International Rules for Seed Testing Edition 2010. Bassersdorf, Switzerland: The International Seed Testing Association; p. 101–103.
- Johnson HW, Robinson HF, Comstock RF. 1955. Estimates of genetic and environmental variability in soybean. Agron J. 47:314–318.
- Kallio MH, Krisnawati H, Rohadi D, Kanninen M. 2011. Mahogany and kadam planting farmers in South Kalimantan: the link between silvicultural activity and stand quality. Small-Scale Forestry. 10:115–132.
- Khalil MAK. 1986. Variation in seed quality and some juvenile characters of white spruce (Picea glauca Moeneu Voss). Silvae Genet. 35:78–85.
- Lamprecht H. 1989. Silviculture in the tropics; Tropical forest ecosystems and their tree species – possibilities and methods for their long-term utilization. Eschbor, Germany: Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ).
- Lichtenthaler HK. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148:350–382.
- Luo JX, Zhang XL, Gu WC. 2005. Biogeographic differences in cone, needle and seed morphology among natural Picea asperata populations in Western China. Forestry Study in China. 7:1–6.
- Maguire JD. 1962. Speed of germination – aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 2:176–177.
- Mamo N, Mihretu M, Fekadu M, Tigabu M, Teketay D. 2006. Variation in seed and germination characteristics among Juniperus procera populations in Ethiopia. For Ecol Manag. 225:320–327.
- Martawijaya A, Kartasujana I, Mandang YI, Prawira SA, Kadir K. 1989. Atlas kayu Indonesia (Indonesian wood atlas), Vol. 2. Bogor: Forest Products Research and Development Centre; p. 55–59.
- Mishra RP, Siddique L. 2011. Antibacterial properties of Anthocephalus cadamba fruits. Asian J Plant Sci Res. 1:1–7.
- Mughal AH, Thapliyal RC. 2012. Population variation in cone and seed characteristics of Cedrus deodara (D.DON) G.DON in Jammu and Kashmir. Forestry Study in China. 14:193–199.
- Mukherjee S, Thapliyal P, Phartyal SS. 2004. Seed sources variation in cone, seed and seedling characteristics across the natural distribution of Himalayan low level pine Pinus roxburghii Sarg. Silvae Genet. 5:116–123.
- Namkoong G, Synder EB, Stonecypher RW. 1966. Heritability and gain concepts for evaluating breeding system such as seedling orchards. Silvae Genet. 15:76–84.
- Pathak PS, Debroy R, Rai P. 1984. Autecology of Leucaena leucocephala (Lam) de Wit. seed polymorphism and germination. Trop Ecol. 15:1–10.
- Rawat K, Bakshi M. 2011. Population variation in cone, seed and seedling characteristics in natural populations of Pinus wallichiana A.B. Jacks (Blue Pine) in India. Ann For Res. 54:39–55.
- Roy MS, Thapliyal RC, Phartyal SS. 2004. Seed source variation in cone, seed and seedling characteristics across the natural distribution of Himalayan low level pine Pinus roxburghii sarg. Silvae Genet. 53:116–122.
- Soerianegara I, Lemmens RHMJ. 1993. Plant resources of Southeast Asia 5 (1): Timber trees: Major commercial timbers. Wageningen, Netherlands: Pudoc Scientific Publishers; p. 107.
- Tauchen J, Lojka B, Hlasna-Cepkova P, Svobodova E, Dvorakovaz Z. 2011. Morphological and diversity of Calycophyllum spruceanum (BENTH) K. SCHUM (Rubiaceae) in Peruvian Amazon. Agric Trop Et Subtrop. 44:212–218.
- Xu XY, Yang XH, Li SZ, Song QS. 2011. Two new triterpenoid glycosides from the leaves of Anthocephalus chinensis. J Asian Nat Prod Res. 13:1008–1013.
- Zheng YI, Sun WB, Zhou Y, Coombs D. 2009. Variation in seed and seedling traits among natural populations of Trigonobalanus doichangesis (A. Camus) Forman (Fagaceae), a rare and endangered plant in Southwest China. New Forest. 37:285–294.
Appendix 1. Results of soil analysis of the 11 populations of white jabon