Abstract
Sarawak is endowed with numerous pristine and estuarine mangroves. However, information pertaining to the species composition and diversity of pristine mangroves of Sarawak is scanty. Hence, this study was carried out to assess the plant composition and diversity of Sibuti mangrove forest, Miri, Sarawak to investigate the current status and diversity of vegetation. Nine mangrove plant species were recorded employing the line transect (100–240 m) survey method. Major mangrove species were Rhizophora apiculata, Xylocarpus granatum, and Nypa fruticans. The stand density was recorded as 1938.46 ± 482.24 trees ha−1, 1722.22 ± 254.58 saplings ha−1, and 6222.22 ± 384.90 seedlings ha−1. The mean diameter, height, and basal area for the whole forest stand were 20.83 ± 13.79 cm, 13.53 ± 5.55 m, and 201.83 ± 12.68 m2 ha−1, respectively. The mean diameter of the dominant species R. apiculata was 24.10 ± 13.90 cm, height 15.18 ± 5.09 m, and basal area 176.13 ± 12.73 m2 ha−1. The importance value index (IVI) of R. apiculata was 202.24 followed by 63.85 for X. granatum. Shannon diversity indices (H′), Margalef richness (D), and Peilou evenness (J′) of the forest stand were 1.18, 1.41, and 0.54, respectively. Similarity of species composition showed two major clusters for the whole forest stand. The findings of this study suggest that Sibuti mangrove forest stand is undisturbed and healthy. This forest could be managed and conserved for multi-sectoral uses such as ecotourism, biodiversity, research, and education rather than solely as a wildlife sanctuary.
Introduction
Mangroves are salt tolerant plants comprised of tree, palm, shrub, and fern communities, which grow in intertidal areas, transitional zone of the coasts, estuaries, and along rivers draining into the sea (FAO Citation2007; Naidoo Citation2009; Zhou et al. Citation2010). Mangroves are evergreen plants with a simple structure and mean height of 5–25 m (MacNae Citation1968), with height mainly depending on the age and regional locations of stands (Snedaker Citation1978). Approximately 65 mangrove species have been reported around the world (Kathiresan and Bingham Citation2001).These are mainly divided into three major categories – true mangroves, mangroves, and mangrove associates (Wan Juliana et al. Citation2010). Mangrove forests are distributed in almost 123 countries, covering an area of 15.2 million ha, with 37% in Asia, 27.2% in North and South America, 21% in Africa, and 12.4% in Australia, New Zealand, South Pacific Islands, and Papua New Guinea (FAO Citation2007; Sandilyan and Kathiresan Citation2012).
Mangrove ecosystems sustain the livelihoods of coastal communities and provide essential support for fishery resources and other marine organisms (Kathiresan and Bingham Citation2001; Garcia et al. Citation2014; Hoque et al. Citation2015a). The biomass production and economic valuation of the mangrove forests are quite high (i.e., 200,000 to 9,000,000 US$ ha−1 year−1), including coastal protection and ecological services (McLeod and Salm Citation2006; Wells et al. Citation2006), compared to other plant communities (Rodriguez and Feller Citation2004).
In recent years, mangrove forests have been facing severe threats around the world. Major causes for mangroves degradation are increased aquaculture activities within mangrove forest areas; however, urbanization, conversion of land to agriculture, coastal industrialization, overharvesting of forests for fuel wood and charcoal are also contributing factors to the degradation of mangrove forests (FAO Citation1994; Pillay Citation2004; Boquiren et al. Citation2010; Billah et al. Citation2014; Uddin et al. Citation2014). Continued degradation and depletion of mangrove ecosystems have also caused environmental instability in coastal areas (FAO Citation1994). Rapid declines in mangrove forests are projected to be up to 25% by 2025 in underdeveloped countries (McLeod and Salm Citation2006).
In Malaysia there are c. 0.580 million ha of mangrove forest reserves (Chan Citation1987) and the country has a rich plant diversity (Rajpar and Zakaria Citation2014). In terms of mangrove forest cover, Sarawak has the second largest area of mangrove coverage in Malaysia, accounting for 26% of the total (126,400 ha), but only 48% of this is protected as permanent forest reserves (Latiff and Faridah-Hanum Citation2014). However, Sarawak mangrove forests are the least disturbed forests compared to those in other Malaysian states (Murofushi et al. Citation1999; Ashton and Macintosh Citation2002), and very little scientific information has been documented on the ecological aspects of Sarawak mangrove forests. In view of this lack of research, especially on plant community structure, this study aims to assess the composition and diversity of mangroves in Sibuti, Miri, Sarawak.
Materials and methods
Study area
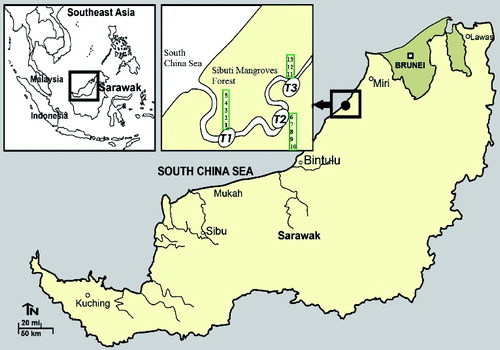
Sibuti mangrove forest is situated at the edge of the South China Sea, 45 km west of Miri town, covering an area of 678 ha. The forest has a mean annual rainfall of 3762.1 mm (ranging on a monthly basis from 141.8 mm to 691.2 mm). The temperature ranges from 23.9 ºC to 32.9 ºC. The forest area is surrounded by Bungai farmlands to the north, the Sungai Sibuti River to the southeast, and the South China Sea to the northwest. The forest was declared a wildlife sanctuary in May 2000 by the Sarawak Forest Department, Malaysia ().
Data collection
Three perpendicular transect lines were established randomly from the river bank inland and upwards into the Sibuti mangrove forest. Transect 1 (3º59′25.76″ N 113º43′51.6″ E) and transect 2 (3º58′53.5″ N 113º44′12.12″ E) were 220–240 m and transect 3 (3º59′22.24″ N 113º44′35.33″ E) was 100 m in length from the shoreline to the end of the forest. In the three transect lines, 13 plots (10 m × 10 m) were established randomly in order to determine species composition and diversity of the stand. In addition, subplots were also established to determine the number of mangrove saplings (2 m × 2 m) and seedlings (1 m × 1 m) in the stand. Enumeration of trees, species name, diameter at breast height (DBH, at 1.3 m), and the tree height were recorded. Plants having height > 4 m were recorded as trees, <1 m as seedlings, and 1–4 m as saplings. Data of tree DBH was recorded using diameter tape, while height of the tree was recorded using clinometers. The data were collected from December 2013 to April 2014. The procedure followed that described by Kathiresan (Citation2000).
Data analysis
Vegetation data were analyzed using importance value index (IVI) of species (Cintron and Novelli Citation1984). IVI was calculated adding the equation for relative density, relative dominance, and relative frequency. The vegetation diversity was measured using Shannon species diversity index (H′), Margelef richness (D), and Peilou evenness (J′) using Species Diversity Richness (SDR) 4.1.2 software (Seaby and Henderson Citation2006). A Bray-Curtis cluster dendrogram was obtained for similarity of species composition using Community Analysis Package (CAP) software v4.0 (Seaby and Henderson, Citation2007).
Results
Species composition
Nine mangrove tree species from eight families were identified at Sibuti mangrove forest, Sarawak (). The dominant mangrove species in the forest stand was Rhizophora apiculata. Like R. apiculata, Xylocarpus granatum was also found in all three transects and Nypa fruticans was observed on the river bank. Five mangrove species (R. apiculata, X. granatum, X.mekongensis, Intsia bijuga, and N. fruticans) were recorded in transect 1, four mangrove species (R. apiculata, X. granatum, Acrostichum speciosum, and N. fruticans) were recorded in transect 2, and six tree species (R. apiculata, X. granatum, Excoecaria agallocha, Phoenix paludosa, A. speciosum, and Thespesia populnea) were recorded in transect 3.
Table 1. List of plant species recorded in the Sibuti mangrove forest.
Density, diameter, height, and basal area of the stand
The mean tree density of Sibuti mangrove forest was 1938.46 ± 482.24 individuals ha−1 (). The highest density (2340.00 ± 230.21 individuals ha−1) was recorded in transect 2, while the lowest density (1600.00 ± 529.75 individuals ha−1) was found in transect 1. The mean sapling density was 1722.22 ± 254.58 individuals ha−1, with the highest mean sapling density (2000.00 ± 447.21 individuals ha−1) found in transect 1 and the lowest (1500.00 ± 547.72 individuals ha−1) in transect 2. The overall mean density of seedlings was 6222.22 ± 384.90 individuals ha−1, with the highest seedling density (6666.67 ± 577.72 individuals ha−1) recorded in transect 3 and the lowest in transects 1 and 2 (each 6000.00 ± 547.72 individuals ha−1). The tallest tree (R. apiculata) was recorded in transect 3 at 26 m and DBH of 65 cm. There were two scattered trees of R. apiculata found in transect 1 with DBH of 65cm.
Table 2. Tree, sapling, and seedling density, diameter, basal area, and height with respect to transects 1–3 in the Sibuti mangrove forest.
The mean diameter of the Sibuti mangrove stand was 20.83 ± 13.79 cm ().The highest stand diameter (21.39 ± 15.60 cm) was recorded in transect 1 and the lowest stand diameter (20.47 ± 13.16 cm) was found in transect 2. The mean height of the whole forest stand was 13.53 ± 5.55 m. The highest mean tree height (14.39 ± 5.22 m) was recorded in transect 2 and the lowest (12.47 ± 4.83 m) in transect 3. The results showed that the mean basal area of the stand was 201.83 ± 12.68 m2 ha−1, while the highest basal area (237.40 ± 21.94 m2 ha−1) was found in transect 2 ().
The mangrove R. apiculata was the dominant mangrove tree species having a density of 1461.53 individuals ha−1, followed by X. granatum (353.84 individuals ha−1). In contrast, X. mekongensis and T. populnea were the least abundant species of the stand. For R. apiculata, the diameter, height, and basal area were 24.10 ± 13.90 cm, 15.18 ± 5.09 m, and 176.13 ± 12.73 m2 ha−1, respectively ().
Table 3. Diameter, basal area, height, number, and frequency of mangrove species in the Sibuti mangrove forest.
Diversity, richness and evenness for species composition
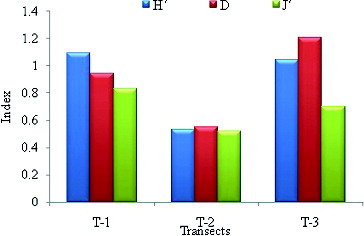
The Shannon diversity index (H′), Margalef richness index (D) and Peilou evenness index (J′) were 1.18, 0.54, and 1.41, respectively. The highest diversity index (H′ = 1.09) and species evenness index (J′ = 0.83) were recorded in transect 1. The highest species richness index (D = 1.20) was recorded in transect 3. The lowest species diversity index (H′ = 0.53), species richness index (D = 0.55), and species evenness index (J′ = 0.52) were recorded in transect 2 ().
Importance value index of species
The IVI results revealed that R. apiculata has the highest IVI value (202.24) followed by X. granatum (63.85) (), while the lowest IVI value was for X. mekongensis (7.19). The data further revealed that R. apiculata had the highest values for relative dominance (88.09%), relative density (75.69%), and relative frequency (38.46%). The lowest values for relative dominance (0.23%), relative density (1.19%), and relative frequency (5.77%) were recorded for X. mekongensis, while T. populnea also had a low value of 1.19% for relative density ().
Table 4. Relative dominance (%), relative density (%), relative frequency (%) and importance value index (IVI) of species in the Sibuti mangrove forest.
Tree abundance, diameter class, and stand clustering
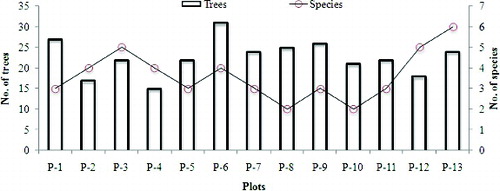
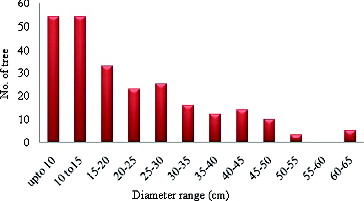
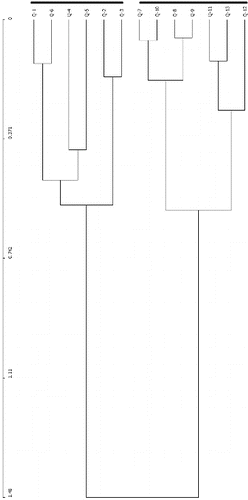
The highest number of trees was found in plot 6 (31 individuals) followed by plot 1 (27 individuals), and plot 9 (26 individuals). Plot 13 had the most species (six) (). Furthermore, relative to diameter class the highest number of trees (54 individuals) were found in the DBH range of <10 cm and 10–15 cm (). Two major groups from the hierarchical cluster analysis were identified from the species number investigated in different plots (). The first group comprised six plots (Q-1, Q-6, Q-2, Q-3, Q-4, and Q-5) and the second group comprised seven plots (Q-7, Q-10, Q-8, Q-9, Q-11, Q-13, and Q-12).
Discussion
There are 41 species of mangrove in Malaysia (FAO Citation2007). However, species diversity in individual forests is low, with the Sibuti forest having just nine species, comparable with the other tropical mangrove forests in Malaysia (Hossain Citation2004; Chandra Citation2013). In this study, the majority of mangrove exclusive plant species were confined to intertidal areas. In terms of plant composition, the results of this study are quite similar to those of Norhayati (Citation1995) for the Kisap Forest Reserve, Langkawi, Kedah and for the Awat Awat mangrove forest, Lawas, Sarawak (Chandra Citation2013). The forest is dominated by R. apiculata, while the bank of the estuary is colonized by Nypa fruticans. The study of Wan Juliana et al. (Citation2014) revealed that R. apiculata is widely distributed in Malaysia. The species R. apiculata is also recorded as the dominant species in the Awat Awat mangrove forest, Lawas, Sarawak (Chandra Citation2013).
The mean tree density (1938.46 ± 482.24 individuals ha−1) of Sibuti mangrove forest is comparable with the recorded value (1821 individuals ha−1) at Ko Yao Tai mangrove forest, Thailand (Chansang Citation1984), and 2175 individuals ha−1 for Kuala Selangor mangrove forest (Hossain Citation2004). Sibuti is therefore similar to other forest stands in the region, with good density coverage. The tallest tree, in transect 1, had a height of 26 m with DBH of 65 cm. This is probably a result of less competition between plants due to the low density coverage (1600 individuals ha−1) and the higher frequency of inundations in the transitional estuary which increases nutrient availability in the soil. The study of Rambok et al. (Citation2010) confirmed high soil nutrients in the Sibuti mangrove forest compared to other mangrove forests in the region.
DBH and height of R. apiculata was recorded as 24.10 ± 13.90 cm and 15.18 ± 5.09 m, respectively, for the whole forest. These values are comparatively higher than those of the Awat Awat mangrove forest of Sarawak (14.45 ± 0.375 cm DBH and 12.16 ± 0.217 m height) (Chandra et al. Citation2011). This indicates that R. apiculata is healthy and in good condition. Besides physicochemical differences between sites (Gandaseca et al. Citation2011), the geographical location and positioning of mangrove forests may influence the height and diameter of R. apiculata.
The density and abundance of saplings and seedlings indicate soil nutrient availability and vegetation heredity of the forest (Twilley Citation1995; Pallardy Citation2008). In this study, 1722.22 ± 254.58 individuals ha−1 of saplings and 6222.22 ± 384.90 individuals ha−1 of seedlings were recorded. Generally, a cleared mangrove forest area has the potential to produce 5000–10,000 seedlings ha−1 (Gan Citation1995) and an abundance of saplings and seedlings indicate healthy regeneration (Ashton and Macintosh Citation2002). The Sibuti mangrove forest therefore has good regeneration potential. The mangrove associate (Acrostichum speciosum) was mostly colonized in the trees having large basal area; a similar observation has also been reported from the Samatan mangrove forest (Ashton and Macintosh Citation2002).
The values for Shannon diversity index (H′ = 1.18), Margalef richness index (D = 1.41), and Peilou evenness index (J′ = 0.54) in the Sibuti mangrove forest were similar to those estimated by Ashton and Macintosh (Citation2002) for Sematan mangrove forests (H′ = 1.42, D = 1.50, and J′ = 0.68). This confirms that the Sibuti mangrove forest is similar to other forests in the Borneo region in terms of species diversity, richness, and evenness. The similarity of species composition and diversity could be due to the location of the study area in a tropical region. In tropical ecosystems, climatic as well as other edaphic factors such as soil pH, salinity, nutrients, organic matter, and tidal inundation do not usually fluctuate remarkably (Hoque et al. Citation2015b). The IVI value of the dominant species was calculated as 202.24 for R. apiculata, whereas it was 173.6 for R. apiculata in Semporana mangrove forest, Sabah (Wah et al. Citation2011). Moreover, the relative density of R. apiculata species was 75.69% followed by 18.32% for X. granatum in Sibuti mangrove stands, which are similar to the findings of Norhayati (Citation1995). This confirms that the overall importance of R. apiculata prevailing in the Sibuti mangrove forest is good compared to other mangrove forests in Malaysia. It is also worth mentioning that the stock of R. apiculata in particular, and other species in general, was healthy and undisturbed, and this indicates that the Sibuti mangrove forest is managed effectively for biodiversity and wildlife habitat. Furthermore, research by Saifullah et al. (Citation2014) found that the Sibuti mangrove forest has had less anthropogenic impacts, and is therefore undisturbed and pristine in nature. The vegetative indicators such as density, basal area, and importance value index were evidence that the Sibuti mangrove forest is well conserved.
Conclusion
The Sibuti mangrove forest is a valuable and preserved biosphere in Sarawak and is a declared wildlife sanctuary. The results of this study demonstrate that the forest stand is healthy and well managed. Like other mangrove forests in the region, the plant composition of the stand is dominated by R. apiculata. The flourishing vegetation diversity could be a potential biodiversity site, and a potential location for ecotourism, education, and research. The findings of this study could be used as a primary source of information and to provide baseline data for assessing the environmental parameters of mangrove ecosystems in the region. Furthermore, detailed research on plant ecology as well as the biodiversity aspects of this forest is needed.
Acknowledgements
This study was completed with the cooperation of the Universiti Putra Malaysia Bintulu Sarawak Campus. Sincere thanks to Mr Masum Billah and Mr Khurshid Alam Bhuiyan for their technical support. Special thanks to Sarawak Biodiversity Center (SBC) and Sarawak Forestry Department for their permission to conduct this research work at Sibuti mangrove forest, Miri, Sarawak.
Disclosure statement
No potential conflict of interest was reported by the authors
Additional information
Funding
References
- Ashton EA, Macintosh DJ. 2002. Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For Ecol Manag. 166:111–129.
- Billah MM, Abu Hena MK, Idris MH, Johan I, Bhuiyan MKA. 2014. Cu, Zn, Fe, and Mn in mangrove ecosystems (sediment, water, oyster, and macroalgae) of Sarawak, Malaysia, Zool Ecol. 24:380–388.
- Boquiren R, Di Carlo G, Quibilan M, editors. 2010. Climate change vulnerability assessment of the Verde Island Passage, Philippines. Arlington, VA: Technical Report Conservation International; p. 100.
- Chan HT. 1987 Country report on mangroves in Malaysia. In: Umali RM, Zamora PM, Gotera RR, Jara RS, Camacho RS, Vannuchi M, editors. Mangroves of Asia and the Pacific: status and management. Quezon City, Philippines: JMC Press Inc.; p. 131–150.
- Chandra IA, Seca G, Abu Hena MK. 2011. Aboveground biomass production of Rhizophora apiculata Blume in Sarawak mangrove forest; Am J Biol Scie. 6:469–474.
- Chandra I.A. 2013. Above-ground biomass and carbon storage in Awat Awat mangroves forest, Sarawak, Malaysia. [MSc thesis]. Universiti Putra Malaysia.
- Chansang H. 1984. Structure of mangrove forest at Ko Yao Yai, Southern Thailand. In: Soepadmo E, Rao NA, Macintosh JD, editors. Proceedings of the ASIAN symposium on Mangrove Environment: Research and Management. Kuala Lumpur: University of Malaya; p. 86–105.
- Cintron G, Novelli YS. 1984. Methods for studying mangrove structure. In: Snedaker SC, Snedaker JG editors. The mangrove ecosystem: research methods. Paris: UNESCO; p. 91–113.
- [FAO] Food Agriculture Organization of United Nations. 1994. Mangrove forest management guidelines FAO.
- [FAO] Food Agriculture Organization of United Nations. 2007. The world's mangroves 1980–2005: a thematic study in the framework of the Global Forest Resources Assessment 2005.
- Gan BK. 1995. A working plan for the Matang Mangrove Forest Reserve (fourth revision). Malaysia: The State Forest Department of Perak Darul Ridzuan.
- Gandaseca S, Rosli N, Ngayop J, Chandra IA. 2011. Status of water quality based on the physico-chemical assessment on river water at wildlife sanctuary Sibuti mangrove forest, Miri Sarawak. Am J Envirno Sci. 7:269−275.
- Garcia KB, Malabrigo JrPL, Gevaña DT. 2014. Mangrove ecosystems of Asia: status, challenges and management strategies. New York: Springer. Chapter 5, Philippines' mangrove ecosystem: status, threats and conservation; p. 81–94.
- Hoque MM, Abu Hena MK, Idris MH, Ahmed OH, Saifulah ASM, Billah MM. 2015a. Status of some fishery resources in a tropical mangrove estuary of Sarawak, Malaysia. Mar Biol Res. doi: 10.1080/17451000.2015.1016970
- Hoque MM, Abu Hena MK, Idris MH, Ahmed OH, Hoque ATMR, Billah MM. 2015b. Litterfall production in a tropical mangrove of Sarawak, Malaysia. Zool Ecol. 25:157–165.
- Hossain M. 2004. Biomass, litter production and selected nutrients in Bruguiera parviflora (Roxb.): weight and arn. dominated mangrove forest ecosystem at Kuala Selangor, Malaysia [PhD dissertation]. Serdang, Selangor DE, Malaysia: Universiti Putra Malaysia.
- Kathiresan K, Bingham BL. 2001. Biology of mangrove and mangrove ecosystems. Adv Mar Biol. 40:81–251.
- Kathiresan K. 2000. Flora and fauna in mangrove ecosystems: a manual for identification. Parangipettai, India: Ministry of Environment and Forests, CAS in Marine Biology.
- Latiff A, Faridah-Hanum I. 2014. Mangrove ecosystems of Asia: status, challenges and management strategies. New York: Springer. Chapter 1, Mangrove ecosystems of Malaysia: status, challenges and management strategies; p. 1–17.
- MacNae W. 1968. A general account of the fauna and flora of mangrove swamp and forest in the Indo-West Pacific region. Adv Mar Biol. 6:73–270.
- McLeod E, Salm RV. 2006. Managing mangroves for resilience to climate change. Gland, Switzerland: IUCN.
- Murofushi T, Chiew FCY, Wat YH, Miyagi T, Mochida Y, Fujimoto K, Ishihara S. 1999. Mangrove forest dynamics in relation to sediment input at the mouth of Sematan River, Sarawak, Malaysia. Tropics. 8:275–289.
- Naidoo G. 2009. Different effects of nitrogen and phosphorus enrichment on growth of dwarf Avicennia marina mangroves. Aquat Bot. 90:184–190.
- Norhayati A. 1995. Biomass and species composition of mangrove forest Pulau Langkawi. [MSc dissertation]. Bangi, Selangor, Malaysia: Universiti Kebangsaan Malaysia.
- Pallardy SG. 2008. Physiology of woody plants. 3rd ed. Columbia: Academic Press.
- Pillay T. 2004. Aquaculture and the environment. UK: Wiley-Blackwell.
- Rajpar MN, Zakaria M. 2014. Assessing the effects of logging activities on avian richness and diversity in different aged post-harvested hill Dipterocarp tropical rainforest of Malaysia. Am J Appl Sci. 11:1519–1529.
- Rambok E, Gandaseca S, Ahmed OH, Majid NMA. 2010. Comparison of selected soil chemical properties of two different mangrove forests in Sarawak. Am J Environ Sci. 6:438–441.
- Rodriguez W, Feller IC. 2004. Mangrove landscape characterization and change in Twin Cays, Belize using aerial photography and IKONOS satellite data. Atol Resear Bull. 509:1–22.
- Saifullah ASM, Abu Hena MK, Idris MH, Rajaee AH, Johan I. 2014. Seasonal variation of water characteristics in Kuala Sibuti river estuary in Miri, Sarawak, Malaysia. Malays J Sci. 33:9–22.
- Sandilyan S, Kathiresan K. 2012. Mangrove conservation: a global perspective. Biodivers Conserv. 21:3523–3542.
- Seaby RM, Henderson PA. 2006. Species Diversity and Richness version 4.0. Lymington, England: Pisces Conservation Ltd.
- Seaby RM, Henderson PA. 2007. Community Analysis Package version 4.0. Lymington, England: Pisces Conservation Ltd.
- Snedaker SC. 1978. Mangroves: their value and perpetuation. Nat Res. 14:6–13.
- Twilley RR. 1995. Properties of mangrove ecosystems related to the energy signature of coastal environments. In: Hall CAS, editor. Maximum power: the ideas and application of Odum HT. Boulder: University of Colorado Press; Colorado; p. 43–62.
- Uddin SMM, Hoque ATMR, Abdullah SA. 2014. Changing landscape of mangrove in Bangladesh and its comparison with other four countries in tropical region. J of For Resear. 25:605–611.
- Wah LM, Mojiol AR, Saleh E. 2011. Diversity of mangroves ecosystem in Semporna mangrove forest. Borneo Sci. 28:8–17.
- Wan Juliana WA, Damanhuri A, Razali MS, Norhayati A, Latiff A. 2010. Mangrove flora of Langkawi. Langkawi Research Centre, Institute for Environment and Development (LESTARI), Kuala Lumpur, Malaysia: Universiti Kebagsaan Malaysia, and Langkawi Development (LADA).
- Wan Juliana WA, Razali MS, Latiff A. 2014. Mangrove ecosystems of Asia: status, challenges and management strategies. New York: Springer. Chapter 2, Distribution and rarity of Rhizophoraceae in peninsular Malaysia; p. 24–34.
- Wells S, Ravilious C, Corcoran E. 2006. In the front line: shoreline protection and other ecosystem services from mangroves and coral reefs. Cambridge (UK): UNEP-WCMC.
- Zhou Y, Zhao B, Peng Y, Chen G. 2010. Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments. Mar Poll Bull. 6:1319–1324.