Abstract
Mangrove estuaries are claimed to be productive and important breeding grounds for fishery resources. The role of particulate organic matter (POM), especially derived from decomposed litter detritus, is well documented in mangrove estuaries. However, being a primary producer, phytoplankton may play a significant role, which has not been well discussed, in governing the productivity of mangrove estuaries. Based on relevant published literature, this paper focuses on the role of phytoplankton in mangrove estuaries in the tropical coastal region and their interdependency. Analysis reveals that there are two-way interactions between phytoplankton and mangrove estuaries. The POM enriched water in mangrove estuaries acts as an ideal medium for phytoplankton succession. Simultaneously, diversified phytoplankton assemblages play a significant role in the food web of the estuarine mangrove ecosystem. Biomass and diversity of phytoplankton are influenced by nutrient and environmental parameters in mangrove estuaries and, concurrently, phytoplankton play a significant role in fish diversity and primary production in the same system. This review reveals that the inconsistent relationships between mangroves and coastal production could probably be due to the influence of seasonal changes. This paper unveils the latent potential and role of phytoplankton in tropical mangrove estuaries, which could be a source of thought for future research in this arena.
Introduction
Mangrove habitat is one of the most productive ecosystems on Earth, lying between the land and the sea on tropical and subtropical coastlines (Kathiresan and Bingham Citation2001; Kathiresan Citation2002). Mangrove ecosystem's productive characteristics are governed by a complex interaction between its biotic and abiotic components. Mangroves have the capacity to efficiently trap suspended material from the water column. Litter from mangroves (leaves, propagules, and twigs) and subsurface root growth provide significant inputs of organic carbon to mangrove sediments (Alongi Citation1989). According to Bouillon and Dehairs (Citation2000), a range of other sources may also provide important organic carbon inputs including allochthonous riverine or marine material, autochthonous production by benthic or epiphytic micro or macro algae, and local water column production by phytoplankton. Organic materials derived from decaying mangrove leaves are also used as a primary food source, sustaining larval and juvenile stocks in the system. Faunal communities in mangrove ecosystems have traditionally been considered to be driven by the large production of mangrove litter (Odum and Heald Citation1975), but recently several studies have shown that the importance of phytoplankton and benthic micro algae may have been underestimated in this “detritus-based food web” concept (Newell et al. Citation1995; Loneragan et al. Citation1997). However, compared to the well-acknowledged importance of mangrove detritus in these systems, the importance of phytoplankton as carbon and energy sources has only recently begun to be recognized (Chew and Chong Citation2010; Mwashote et al. Citation2005).
Mangroves in the estuarine ecosystem play important roles in biodiversity and energy flow, and in maintaining functioning food chains, with phytoplankton playing a vital role as a primary producer. Phytoplankton initiates the marine food chain by serving as food to primary consumers such as zooplankton, shellfish, and finfish (Sridhar et al. Citation2006). It has been shown that, in comparison to adjacent marine areas, larval retention and high productivity in mangrove-lined estuaries have generally been attributed to the abundant planktonic food supply (Rajkumar et al. Citation2009). The distribution and abundance of commercially important fish and shellfish, and their larvae, are dependent on some species of phytoplankton as their main food source (Mitra et al. Citation2004).
In general, the fertility and health of mangrove environments are reflected through the productivity of phytoplankton and zooplankton as primary and secondary producers, respectively. The availability of phytoplankton influences the distribution of consumer organisms, such as immediate herbivore zooplankton. In addition, phytoplankton have a high nutrient content, which makes them a valuable food item in the aquatic food chain (Khatoon et al. Citation2010). Thus, in the food web of estuarine ecosystems, phytoplankton play an important role.
Research on community structure and diversity of phytoplankton, and their importance in mangrove estuaries, is somewhat limited. In tropical regions, in particular, where the agglomeration of mangrove habitats is high, information on the role of mangrove detritus in the estuary ecosystem is common but is very limited in the context of phytoplankton. Thus, this study aims to investigate the interdependence of phytoplankton and mangroves. The outcome of the study could help to evaluate the contribution of phytoplankton in mangrove estuarine ecosystems.
Material and methods
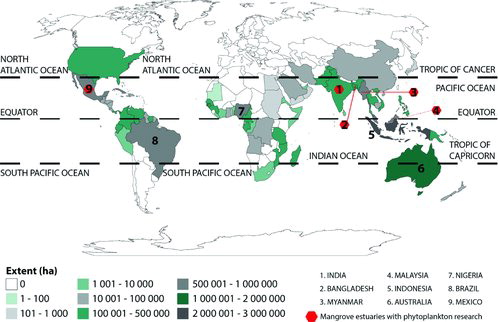
The review gathered information from a range of different online scientific publications. Articles were collected without any time restrictions. Terms such as “phytoplankton”, “mangrove estuary”, and “tropical region” were used for searching research articles in freely available online journals subscribed to by the University of Putra Malaysia. Collected research articles were sorted out and finally synthesis of literature focused on some specific countries in the tropical region (). In addition, conference proceedings and books on relevant issues were also analyzed for common understanding of the addressed issue.
Figure 1. Map showing some major tropical countries with mangrove and reported research on phytoplankton in mangrove estuaries (modified from FAO Citation2005).
Discussion
Mangrove and estuarine ecosystem
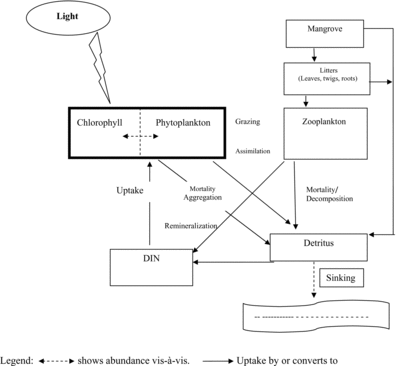
It is well known that mangrove forests represent an important carbon and nutrient resource to lagoonal and coastal systems (Odum and Heald Citation1972, Citation1975; Robertson and Blaber Citation1992). Particulate organic matter (POM) derived from mangrove litter initiates the detrital food chain in the adjacent mangrove estuaries (). The concept of detrital food chains has often been used to explain the relationships between plant detritus (Odum Citation1980), bacterial or meiofaunal communities, and fish production. Only a small percentage of mangrove leaves are consumed directly by terrestrial grazing animals (Lee et al. Citation1990), whereas mangrove detritus constitutes a large carbon reservoir potentially available to the estuarine food web (Tam et al. Citation1990). Mangrove sediments have the ability to retain nutrients, and the efficient cycling of nutrients facilitates high coastal production along mangrove zones. Mangrove ecosystems also help to recycle carbon, nitrogen, and sulfur. It is perhaps the only biotic system that recycles sulfur efficiently in nature, and makes it available in assimilable forms to other organisms. Mangrove habitats contribute various components of biodiversity, which are important to the function and environmental condition of the tropical estuarine ecosystem. A number of studies have shown that the dominant ecological function of mangroves is the maintenance of nearshore marine habitats and the ecological provision of food and refugia to a variety of organisms at different trophic levels (Odum and Heald Citation1972; Rojas et al. Citation1992; Sasekumar et al. Citation1992).
Figure 2. Conceptual flow diagram of the contribution of mangrove leaf to the food chain in an estuary (after Odum Citation1971).
Phytoplankton in mangrove estuaries
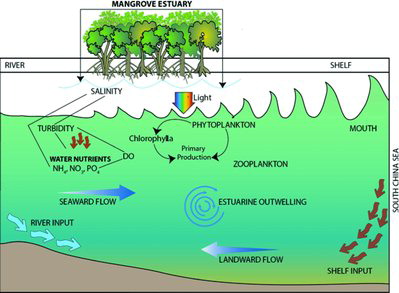
Mangrove estuaries are usually rich in nutrient content. These nutrients, along with other physicochemical parameters such as salinity, turbidity, pH, and total dissolved solids, play a vital role in the assemblage of phytoplankton in the estuarine ecosystem. Studies have revealed that nitrates, phosphates, salinity, and turbidity have a positive correlation with the abundance and distribution of phytoplankton in tropical ecosystems (Saifullah et al. Citation2014a).
It is believed that mangrove estuaries enriched with nutrients can harbor more phytoplankton diversity than other estuaries. Consequently, nutrients influenced by seasonal cycling and the hydrodynamics of water act as a driving factor for phytoplankton abundance (Mohamad-Noor et al. Citation2012). Thus, changes in hydrodynamics and environmental conditions can affect the productivity of phytoplankton (Pradhan and Shaikh Citation2011).
Mangrove estuaries are considered as productive zones where phytoplankton may also play a key role in such productivity (Rahman et al. Citation2013). The estuaries of the Sundarbans mangrove forest in Bangladesh were reported as enriched with diverse phytoplankton. A study recorded 134 phytoplankton species comprising: 99 species from 41 genera of Bacillariophyta; 18 species from six genera of Pyrophyta; 12 species from nine genera of Chlorophyta; four species from four genera of Cyanobacteria; and one species of Ochrophyta in the mangrove estuaries of Sundarbans in Bangladesh (Rahman et al. Citation2013). The Pichavaram mangrove waters on the east coast of India also demonstrate high abundance of phytoplankton with a density of 750–321,000 cells L−1 with maxima in the summer and minima in the monsoon (Rajkumar et al. Citation2009). Similarly, the Kaduviyar estuary is rich in phytoplankton, where population density was found to be 14,135–74,697 cells L−1 (Perumal et al. Citation2009). A total of 85 species of phytoplankton comprising 58 species of diatom, 16 species of dinoflagellates, and seven species of blue-green algae (Chlorophyceae) were recorded in the Kaduviyar estuary. In most cases, diatoms dominate the phytoplankton composition, which is reflected in the Matang mangrove estuary of Malaysia where over 80% of the phytoplankton community is dominated by diatoms (Tanaka and Choo Citation2000). Likewise, on the Kuantan coast, 71 taxa of phytoplankton comprising 63 genera of diatom and seven genera of dinoflagellates were recorded with the highest abundance during the northeast monsoon (7.0 × 104 cells L−1) and the lowest in the inter-monsoon(8.3 × 103 cells L−1) (Mohammad-Noor et al. Citation2013).
Generally, the biomass of phytoplankton can be measured by chlorophyll. The amount of chlorophyll-a present in seawater ranges from a minimum of c. 0.05 µg L−1 in highly oligotrophic areas to a maximum of c. 20 µg L−1 in highly eutrophic areas, although exceptional “red tide” values of several hundred µg L−1 may be found (Strickland Citation1964). Significant amounts of chlorophyll have also been found in mangrove-dominated estuarine water. Estuarine waters of mangrove ecosystems contain more chlorophyll-a than that of other neritic waters (Rahman et al. Citation2013). Tanaka and Choo (Citation2000) found that phytoplankton biomass in a mangrove estuary remained high during the spring tide, which was probably due to nutrients outwelling from the mangrove area by the inundation and tidal mixing of the spring tide, while the decrease in phytoplankton biomass at the neap tide could have resulted from consumption of nutrients. Generally, mangrove estuaries in tropical regions demonstrate higher abundance of phytoplankton and higher concentration of chlorophyll (). Consequently, nutrient outwelling from the sediments of the mangrove swamp and creek (in the form of POM) support high phytoplankton biomass in estuarine waters ().
Table 1. Phytoplankton biomass in some mangrove estuaries in tropical regions.
Primary production and phytoplankton
Phytoplankton alone contribute more than 95% of primary production in oceanic waters (Lewis Citation1974). However, the shallow neritic zones of coastal areas are comparably more productive due to the combined production of unicellular algae, macro algae, symbiotic algae of coral reefs, and seagrasses (Lewis Citation1974). Overall, the drifting micro algal (phytoplankton) population plays a major role in determining the productivity of the coastal and marine environment. Most studies reveal higher productivity in the summer season. High productivity in summer in the estuarine environment is a common phenomenon, which could be attributed to neritic element domination, high light intensity, clear water condition, availability of nutrients, and high phytoplankton biomass (Thillai Rajasekar et al. Citation2005). Primary production in the estuarine area demonstrates variation. For example, primary production values in Pichavaram mangroves in India were found to be high (6.3 gCm−3 d−1) compared to other areas (Krishnamurty and Sundararaj Citation1973). Primary production rate also differed geographically; for example, 5 gCm−3 d−1 in Ivory Coast, 2.4 gCm−3 on the Mexican coast (Robertson and Blaber Citation1992), and 0.0693 gCm−3 d−1 in the Fly River delta in Papua New Guinea (Robertson and Blaber Citation1992) ().
Table 2. Primary production in different estuaries of the world.
Generally, estuaries are very productive zones because they concentrate nutrients from rivers, and have a nutrient trap effect due to mixing with sea water (flocculation) and local retention due to tidal changes (Knox Citation1986). The productivity is on average higher in mangrove estuaries than in other estuaries because of the very high primary productivity of the mangal itself (leaf litter), supplemented by that of cyanobacteria, diatoms, and micro algae (Alongi Citation1989), and that of algae fixed on mangrove prop roots (Rodriguez and Stoner Citation1990).
Seasonal variation of productivity in the mangrove estuarine regime was also found to be distinct in different studies. Higher primary productivity was observed in the summer season in Kaduviyar mangrove estuary in India. It has also been argued that this greater productivity could be due to the influence of population density of phytoplankton, neritic element domination, higher salinity, surface water temperature, and clear water conditions as well as availability of nutrients (Thillai Rajasekar et al. Citation2005). The low primary productivity recorded during winter could be the result of reduced phytoplankton population (Rajasegar et al. Citation2000). In contrast, a study of the Pichavaram mangrove forest found higher productivity in the monsoon and lower in the summer with no relationship to phytoplankton abundance. The abundance of Pichavaram phytoplankton was low in the monsoon when the water column remained, to a large extent, remarkably stratified due to heavy rainfall, high turbidity, reduced salinity, decreased temperature and pH, overcast cloud, and cool conditions. It seems that primary production in an estuary is not only influenced by phytoplankton abundance but also by other environmental factors such as light, turbidity, and rainfall.
Biogeochemical cycle and phytoplankton
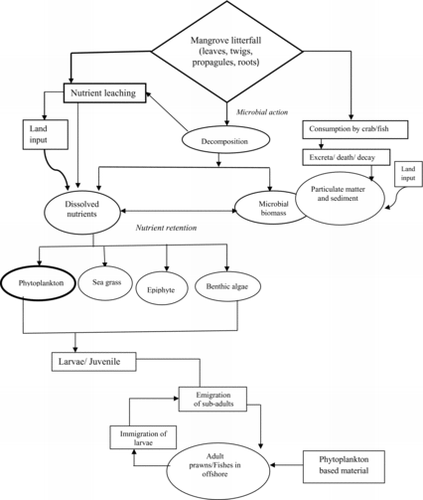
Mangrove ecosystems produce large amounts of litter in the form of falling leaves, branches, and other debris. Decomposition of the litter contributes to the production of dissolved organic matter (DOM) and the recycling of nutrients both in the mangal and in adjacent habitats. This organic detritus and nutrients could potentially enrich the coastal sea, estuary, and, ultimately, support fishery resources. The contribution of mangroves could be particularly important in clear tropical waters where nutrient concentrations are normally low. The nutrient cycling begins when leaves fall from the mangroves () and are subjected to a combination of leaching and microbial degradation (Lee et al. Citation1990). Leaching alone removes a number of substances and can produce high levels of DOM (Benner et al. Citation1990). Dissolved nutrients (POM) derived from nutrient leaching through decomposed mangrove litterfall and land sources are usually utilized by phytoplankton, seagrass, epiphytes, and benthic algae. Subsequently, larvae and juveniles of fishes consume these phytoplankton, epiphytes, and algae, thus forming a food chain (). Potassium is the most thoroughly leached element with up to 95% of the total potassium being removed in a very short time (Steinke et al. Citation1990). Phytoplankton requires both macronutrients and micronutrients for growth, with nitrogen, phosphorus, and potassium playing an important role in fulfilling this nutrient requirement (Davis et al. Citation2006).
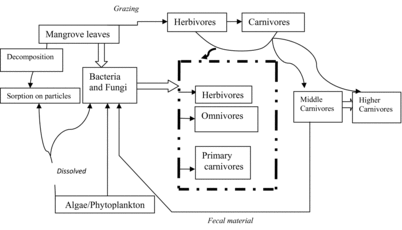
Figure 3. Phytoplankton in the food web of a mangrove ecosystem (after Kathiresan and Bingham Citation2001).
Carbohydrates also leach quickly in mangrove estuaries during early decomposition and subsequent release to the water. Carbohydrates (e.g., water-extractable h-1,3-d-glucan in diatoms) are of key importance for phytoplankton growth in a variable light climate because they facilitate continued growth of the cells in darkness by providing energy and carbon skeletons for protein synthesis (Oijena et al. Citation2005).
Bacteria and fungi contribute to decomposition of the mangrove material and to nutrient cycling. Fungi are the primary litter invaders, reaching their peak in the early phases of decomposition (Rajendran and Kathiresan Citation1997). The phylloplane fungi do not attack living leaves; they begin to break the leaf material down only after it has been submerged. Bacterial colonies appear shortly after the litter has been colonized by fungi. The nitrogen‐fixing azotobacters are an important group in the decomposing litter (Rajendran and Kathiresan Citation1997) and their activities may increase the nitrogen content of the leaves two to three times (Rajendran and Kathiresan Citation1997; Wafar et al. Citation1997). Chale (Citation1993) measured a similar rapid nitrogen increase in leaves after 6 weeks of decomposition and suggested that the litter provides a surface for microbial nitrogen synthesis and acts as a nitrogen reservoir. Studies found phytoplankton a source of c. 70% of global nitrogen assimilation on Earth and therefore a major transformer of incoming solar energy into biomass (Raven et al. Citation1993). A wide variety of nitrogen compounds (nitrate, nitrite, ammonium, molecular nitrogen, and organic nitrogen) of different oxidation states are available and used by phytoplankton. Because of the very dilute nature of the medium in which they live, phytoplankton has developed efficient ways of acquiring nutrients from the surrounding environment. The biogeochemical cycle in a mangrove estuary follows an interlocking pattern () in which mangrove litter and phytoplankton play a vital role. In this cyclic process, both the mangrove and phytoplankton act as initiators of nitrogen-based salt production through their decay and decomposition. The dissolved inorganic nitrogen produced in the process is absorbed by phytoplankton. Phytoplankton is grazed by zooplankton and fishes, thus forming the food chain linking it to other trophic levels.
Nutrients and phytoplankton
Mangrove sediments have the ability to retain nutrients, depending on the sediment characteristics and flow patterns of the site. Nutrients are considered one of the most important parameters in the mangrove environment, influencing the distribution of living organisms such as phytoplankton. Generally, nutrient concentration in mangrove estuaries differs according to seasonal changes (Rajkumar et al. Citation2009; Saifullah et al. Citation2014a). Little information is available on the distribution and dynamics of dissolved nutrients in tropical mangrove areas (Tanaka and Choo Citation2000). Some investigations examined nutrients in Malaysian mangrove estuaries using mixing diagrams (Nixon et al. Citation1984). Another study revealed that inorganic nitrogen increases after heavy rainfall or when tides inundate the forest floor in a mangrove creek in Malaysia (Thong et al. Citation1993). It is assumed from nutrient mixing that nutrients have effect on the phytoplankton biomass in the estuary or influence of high abundance of phytoplankton. Tidal variation also very often influences nutrient mixing and abundance of phytoplankton. Studies by Tanaka and Choo (Citation2000) demonstrated high phytoplankton biomass during the spring tide in Matang mangrove estuary, Malaysia, suggesting that this could be due to nutrients outwelling from the mangrove area by inundation and tidal mixing of the spring tide. In contrast, the lower abundance of phytoplankton reported at the neap tide could be due to less nutrients caused by more assimilation by organisms. However, higher nitrite values were observed during the monsoon season, which could be due to variation in phytoplankton fixation, oxidation of ammonia, reduction of nitrate, and recycling of nitrogen, as well as bacterial decomposition of planktonic detritus present in the environment (Rajkumar Citation2009). Another study revealed that the effects of major nutrients on species succession are not obvious (Strickland Citation1964). For no clearly understood reason, whenever nutrient-rich water is present, diatoms nearly always prevail. In a tropical mangrove estuary in Malaysia, higher availability of nutrients was observed, followed by higher abundance of phytoplankton (Saifullah et al. Citation2014b). The same study also revealed an interlinkage among some environmental parameters, phytoplankton, and primary production (). Although no significant relationship between nutrient content, phytoplankton abundance, chlorophyll-a content, and primary production in the estuary was found, total dissolved solids showed a significant relationship to phytoplankton abundance.
Figure 5. Interlinkage among different nutrients, phytoplankton, primary production, and environmental parameters in Sibuti mangrove estuary, Sarawak, Malaysia (Saifullah et al. Citation2014b).
Phytoplankton and fisheries in mangrove estuaries
Phytoplankton, the primary producer in estuaries and seas, enter into the aquatic food chain and thus provide sustenance, directly or indirectly, to fish and other aquatic animals (Castro and Huber Citation2003). Biomass and production of phytoplankton are important in regulating the diversity of organisms at higher trophic levels. Mangrove waters have significantly richer nano-phytoplankton than do estuaries (Subramanian Citation1981). A study in Pichavaram mangrove waters in southeast India revealed that phytoplankton in the 5–10 µm size range contributed 33%–51% of total chlorophyll-a and 20%–22% of total gross production (Kathiresan Citation2000). Likewise, plankton production in mangrove ecosystems is important for fishery resources. Hoque et al. (Citation2015) recorded 65 species of different fisheries resources from Sibuti mangrove estuary in Sarawak, Malaysia. This study also reported the significant influence of environmental variables, phytoplankton abundance, and chlorophyll-a on the seasonal distribution of fisheries resources in the estuary. Another study recorded 54 species of juvenile fish in the two mangrove lined estuaries in Moreton Bay, eastern subtropical Australia (Laegdsgaard and Johnson Citation1995). The same study compared the species richness of fish between mangrove and mudflat habitat where species richness was consistently higher in the former than the latter.
Generally, phytoplankton plays an important role in aquatic food chains where fish are the main consumer. Mangroves consist of a complex and dense trophic network with abundant food and diversified niches where phytoplankton plays a vital role in the first trophic level (). Thus, mangrove estuaries are favored habitat for both adult and juvenile fish species. The assumption is that mangrove litter or mangrove-derived detritus represents the dominant food source for certain groups of fauna. In the Panguil Bay (mangrove estuary of the Philippines) Jimenez et al. (Citation1996) reported 54 tons of fin fishes from 120 species, 2315 tons of molluscs from six species, and 152 tons of crustaceans from four scyllid and eight penaeid species. Canini et al. (Citation2013) speculated that the productivity of fishery resources in mangrove estuaries would be largely linked with the variability of phytoplankton production and ecology. In Florida, it was revealed that the contribution of mangroves to shrimps’ diet depends on the relative productivity of mangroves, when compared to seagrass and phytoplankton (Twilley et al. Citation1996).
Nutrient availability in mangrove estuaries has always enriched primary producers, especially phytoplankton, which later bonded a strong food chain to make the estuary biologically diversified. Nearly 80% of fish catches are directly or indirectly dependent on mangrove and other coastal ecosystems worldwide (Kjerfve and Macintosh Citation1997). To cite a specific case, the Pichavaram mangroves alone nurture 30 species of prawns, 30 species of crabs, 20 species of molluscs, and 200 species of fish (Kathiresan Citation2002).
Mangrove-generated detritus and phytoplankton may have stronger trophic linkages with epibenthic invertebrates and fish living in the mangal and in nearby habitats. The juveniles feed directly on mangrove detritus and detritivorous invertebrates feed on benthic micro algae growing in the mangal (Newell et al. Citation1995). Shrimp in mangrove estuaries may also feed heavily on epiphytes and phytoplankton (Loneragan et al. Citation1997), while invertebrates may also feed on a variety of cyanobacteria and micro algae that live on submerged portions of the mangroves and on leaf litter. Thus, phytoplankton as a food and nutritional resource augment the composition and diversity of fisheries resources in mangrove estuaries.
Conclusion
As a primary producer, phytolankton has always had a key role to play in aquatic ecosystems, but studies on phytoplankton in mangrove estuaries are lacking. Although the specific role of phytoplankton in mangrove estuaries cannot yet be elucidated, it is nevertheless clear that phytoplankton have an important function in initiating food chains in mangrove estuaries. The POM enriched estuarine environments provide the optimum conditions for phytoplankton growth and sustenance.
Acknowledgements
We thank the Department of Animal Science and Fishery, UPM Bintulu Sarawak campus for providing facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alongi DM. 1989. The role of soft-bottom benthic communities in tropical mangrove and coral reef ecosystem. Crit Rev Aqua Sci. 1(2):243–280.
- Aziz A, Rahman M, Ahmad A. 2012. Diversity, distribution and density of estuarine phytoplankton in the Sundarban mangrove forests in Bangladesh. Bang J Bot. 41(1):87–95.
- Benner R, Hatcher PG, Hedges JI. 1990. Early diagenesis of mangrove leaves in a tropical estuary; bulk chemical characterization using solid state (super 13) CNMR and elemental analyses. Geochimicaet Cosmo Acta. 54(7):2003–2013.
- Bouillon S, Dehairs F. 2000. Estimating spatial and seasonal phytoplankton δ13 C variations in an estuarine mangrove ecosystem. Isot Enviro Health Stud. 36:273–284.
- Canini ND, Metillo EB, Azanza RV. 2013. Monsoon influenced phytoplankton community structure in a Philippine mangrove estuary. Trop Ecol. 54(3):331–343.
- Castro P, Huber Hill M. 2003. Marine biology. 4th ed. St Louis, MO: McGraw-Hill.
- Chale FMM. 1993. Degradation of mangrove leaf litter under aerobic conditions. Hydro. 257(3):177–183.
- Chew LL, Chong VC. 2010. Copepod community structure and abundance in a tropical mangrove estuary, with comparisons to coastal waters. Hydro. 666:127–143.
- Davis, OA, Alfred-Ockiya, JF, Asele, A. 2006. Induced growth of phytoplankton using two fertilizers (NPK and agrolyser) under laboratory conditions. Afr J Biol. 5(4):373–377.
- FAO. 2005. The world's mangroves 1980-2005. A thematic study prepared in the framework of the global forest resources assessment. FAO Forestry Paper. Rome: FAO; p. 153.
- Hoque MM, Abu Hena MK, Idris H, Ahmed OH, Saifullah ASM, Billah MM. 2015. Status of some fishery resources in a tropical mangrove estuary, Sarawak, Malaysia. Mar Biol Res. 11(8):834–846.
- Jimenez CR, Tumanda Jr MI, Laurden AJ. 1996. Post-resource and ecological assessment monitoring and training project in Panguil Bay. Terminal report. Naawan, Naawan, Philippines: Mindanao State University.
- Kathiresan K. 2002. Greening the blue mud! Rev BioTrop. 50:869–874.
- Kathiresan K, Bingham BL. 2001. Biology of mangroves and mangrove ecosystems. Adv Mar Bio. 40:81–251.
- Kjerfve B, Macintosh DJ. 1997. Climate change impacts on mangrove ecosystems. In: Kjerfve B, Lacerda LD, Diop S, editors. Mangrove ecosystem studies in Latin America and Africa. Paris: UNESCO; p. 1–7.
- Knox GA. 1986. Estuarine ecosystems: a system approach. Vol. I and II, Boca Raton, FL: CRC Press; p. 520.
- Krishnamurthy K, Choudhury A, Untawale AG. 1987. Status report. Mangroves in India. New Delhi: Ministry of Environment and Forests, Govt. of India; p. 150.
- Krishnamurthy K, Sundararaj V.1973. A survey of environmental features in a section of the Vellar-Coleroon system, South India. Mar Biol. 23:229–237.
- Khatoon H, Banarjee S, Yusoff FM, Shariff M. 2010. Effects of salinity on the growth and proximate composition of selected tropical marine periphytic diatoms and cyanobacteria. Aqua Res. 41:1348–1355.
- Laegdsgaard P, Johnson CR. 1995. Mangrove habitats as nurseries: unique assemblages of juvenile fish in subtropical mangroves in eastern Australia. Mar Ecol Prog Ser. 126:67–81.
- Lee KH, Moran MA, Benner R, Hodson E. 1990. Influence of soluble components of red mangrove (Rhizophora mangle) leaves on microbial decomposition of structural (lignocellulosic) leaf components in seawater. Bull Mar Sci. 46(2):374–386.
- Lewis WM Jr. 1974. Primary production in the plankton community of a tropical lake. Ecol Monog. 44:377–409.
- Loneragan NR, Bunn SE, KeIlaway DM. 1997. Are mangroves and seagrasses sources of organic carbon for penaeid prawns in a tropical Australian estuary? A multiple stable-isotope study. Mar Biol. 130:289–300.
- Mitra A, Banarjee K, Gangopadhyay A. 2004. Introduction to marine plankton. Delhi: Daya Publishing House.
- Mohammad-Noor N, Sing OF, Anwar EW. 2012. Seasonal distribution of harmful algal bloom species in east coast of Sabah, Malaysia. J Fish Aqua Sci. 7:431–438.
- Mohammad-Noor MN, Harun RS, Lazim MZ, Mukai Y, Mohamad T, Saad S. 2013. Diversity of phytoplankton in coastal water of Kuantan, Pahang, Malaysia. Mal J Sci. 32(1):29–37.
- Mwashote BM, Ohowa BO, Wawiye PO. 2005. Spatial and temporal distribution of dissolved inorganic nutrients and phytoplankton in Mida Creek, Kenya. Wet Ecol Manage. 13:599–614.
- NeweIl RIE, MarshaIl N, Sasekumar A, Chong VC. 1995. Relative importance of benthic microalgae, phytoplankton, and mangroves as sources of nutrition for penaeid prawns and other coastal invertebrates from Malaysia. Mar Biol. 123:595–606.
- Nixon SW, Furnas BN, Lee V, Marshall M, E-Ong J, Wong CH, Gong WK, Sasekumar A. 1984. The role of mangrove in the carbon and nutrient dynamics of Malaysian estuaries. Proc As Symp Mangr Env – Res Manage. 534–544.
- Oijena TV, Veldhuisb MJW, Gorbunovc MY, Nishiokad J, Van Leeuwea MA, de Baara HJW. 2005. Enhanced carbohydrate production by Southern Ocean phytoplankton in response to in situ iron fertilization. Mar Chem. 93:33–52.
- Odum WE. 1971. Pathways of energy flow in a south Florida estuary. Univ Miami Sea Grant Bull. 7:162.
- Odum WE, Heald EJ. 1972. Trophic analysis of an estuarine mangrove community. Bull Mar Sci. 22:671–738.
- Odum WE, Heald EJ. 1975. The detritus-based food web of an estuarine mangrove community. In: Cronin LE, editor. Estuarine research. New York: Academic Press, Inc.; p. 265–286.
- Odum EP. 1980. The status of three ecosystem-level hypotheses regarding salt marsh estuaries: tidal subsidy, outwelling, and detritus-based food chains. In: Estuarine perspectives. New York: Academic Press; p. 485–495.
- Perumal VN, Rajkumar M, Perumal PK, Rajasekar KT. 2009. Seasonal variations of plankton diversity in the Kaduviyar estuary, Nagapattinam, south east coast of India. J Env Bio. 30(6):1035–1046.
- Pradhan V, Shaikh JD. 2011. Seasonal fluctuation of plankton population correlated with physico-chemical factors in backwards of Jaikwadi dam (Kaigaon). J Chem Bio Phy Sci. 1:270–274.
- Rahman SMB, Golder J, Rahman MS, Hasanuzzaman AFM, Huq KA, Begum S, Islam SS, Bir J. 2013. Spatial and temporal variations in phytoplankton abundance and species diversity in the Sundarbans mangrove forest of Bangladesh. J Mar Sci Res Dev. 3:126.
- Rajasegar M, Srinivasan M, Rajaram R. 2000. Phytoplankton diversity associated with the shrimp farm development in Vellar estuary. Seaweed Res Uti. 22:125–131.
- Rajendran N, Kathiresan K.1997. Biochemical changes in decomposing leaves of mangroves. Chem Ecol. 17:91–102.
- Rajkumar M, Perumal P, Prabu VA, Perumal NV, Rajasekar T. 2009. Phytoplankton diversity in Pichavaram mangrove waters from the south-east coast of India. J Env Bio. 30(4):489–498.
- Raven JA, Wollenwber B, Handley LL.1993. The quantitative role of ammonia/ammonium transport by plants in the global nitrogen cycle. Physiol Plant. 89:512–518.
- Robertson AI, Blaber SJM. 1992. Phytoplankton, epibenthos and fish communities. In: Robertson AI, Alongi DM, editors. Coastal and estuarine studies: tropical mangrove ecosystem. Washington, DC: American Geophysical Union; p. 173–224.
- Rodriguez C, Stoner AW. 1990. The epiphyte community of mangrove roots in a tropical estuary: distribution and biomass. Aqua Bot. 36:117–126.
- Rojas JL, Yanez-Arancibia A, Day JW. Vera-Herrera F. 1992. Estuary primary producers: Laguna de Terminos, a case study. In: Seeliger U, editor. Coastal plant communities of Latin America. New York: Academic Press; p. 141–154.
- Saifullah ASM, Abu Hena MK, Idris MH, Halimah AR, Johan I. 2014a. Composition and diversity of phytoplankton from mangrove estuaries in Sarawak, Malaysia. J Biol Sci. 14(5):361–369.
- Saifullah ASM, Abu Hena MK, Idris MH, Halimah AR. 2014b. Interlinkage among primary production, phytoplankton abundance, chlorophyll and environmental condition of a mangrove estuary in Sarawak, Malaysia. Paper presented at Malaysia International Biological Symposium, 28–29 October, 2014, Putrajaya, Malaysia.
- Sasekumar A, Chong VC, Leh MU, Du Cruz R. 1992. Mangroves as habitat for fish and prawns. In: Jaccarini V, Martens E, editors. The ecology of mangroves and related habitats. Boston, MA: Development in Hydrobiology. 80, Kluwer; p. 195–207.
- Sridhar R, Thangaradjou T, Kumar SS, Kannan L. 2006. Water quality and phytoplankton characteristics in the Palk Bay, southeast coast of India. J Env Biol. 27:561–566.
- Strickland JDH. 1964. Phytoplankton and marine primary production. La Jolla, CA: Institute of Marine Resources, University of California; p. 162.
- Steinke TD, Barnabas AD, Somaru R.1990. Structural changes and associated microbial activity accompanying decomposition of mangrove leaves in Mgeni estuary (South Africa). S African J Bot. 56(1):39–48.
- Subramanian BR. 1981. Studies in net phytoplankton and nano phytoplankton in the mangrove and estuarine water of Porto Novo. PhD thesis, Parangipettai, India: Annamalai University; p. 203.
- Tanaka K, Choo P.2000. Influence of nutrient outwelling from the mangrove swamp on the distribution of phytoplankton in the Matang mangrove estuary, Malaysia. J Oceano. 56:69–78.
- Tam NFY, Vrijmoed LLP, Wong YS. 1990. Nutrient dynamics associated with leaf decomposition in a small subtropical mangrove community in Hong Kong. Bull Mar Sci. 47(1):68–78.
- Thillai Rajasekar K, Perumal P, Santhanam P. 2005. Phytoplankton diversity in the Coleroon estuary, southeast coast of India. J Mar Biol Ass Ind. 47:127–132.
- Thong KL, Sasekumar A, Marshall N. 1993. Nitrogen concentrations in a mangrove creek with a large tidal range, Peninsular Malaysia. Hydro. 254:125–132.
- Tripathy SC, Ray AK, Patra S, Sarma VV. 2005. Water quality assessment of Gautami Godavari mangrove estuarine ecosystem of Andhra Pradesh, India during September 2001. J. Earth Syst Sci. 114(2):185–190.
- Twilley RR, Snedaker SC, Yanez-Arancibia A, Medina E. 1996. Biodiversity and ecosystem processes in tropical estuaries: perspective of mangrove ecosystems. In: Mooney et al., editor. Functional roles of biodiversity: a global perspective. Chichester: John Wiley and Sons; p. 327–370.
- Wafar S, Untawale AG, Wafar M. 1997. Litter fall and energy flux in a mangrove ecosystem. East Coast Shelf Sci. 44:111–124.