Abstract
The larvae of Japanese gypsy moth (JGM, Lymantria dispar japonica) are highly polyphagous and considered a serious pest that cause significant ecological and economic losses in forests. Monitoring of egg masses is important to prevent large outbreaks of JGM from occurring in their native range. To investigate oviposition site selection by JGM, we analyzed the occurrence and spatial distribution of egg masses across various evergreen tree species within a secondary forest dominated by evergreen broad-leaved trees in western Japan, following a large outbreak. Egg masses were concentrated on the abaxial surfaces of the leaves of a few evergreen tree species. There was a strong preference for Camellia japonica L., on which more than 75% of the egg masses were found. Egg masses were only found on evergreen tree species with large leaves (leaf area >10 cm2 and leaf dry mass >0.1 g). The spatial distribution of egg masses were clustered at scales around 1‒2 m. For effective monitoring of JGM egg masses in warm-temperate evergreen broad-leaved forests of Japan, the abaxial surfaces of the most abundant evergreen broadleaved trees with large leaves should be checked. If egg masses are found, it is likely that nearby trees of the same species will also have egg masses. Large trees should be checked thoroughly for occurrence of multiple egg masses.
Introduction
The gypsy moth, Lymantria dispar L. (Lepidoptera: Lymantriidae), is a serious insect pest native to Europe, North Africa, and Asia. The European gypsy moth (EGM) was accidentally introduced to North America in 1869 and continues to expand its range (Elkinton and Liebhold Citation1990; Sharov et al. Citation2002; Johnson et al. Citation2006). The larvae of the gypsy moth are polyphagous, feeding on the leaves of a large number of tree species, resulting in severe defoliation during outbreaks, and causing significant ecological and economic losses in not only natural forests, but also suburban and urban environments (Doane and McManus Citation1980; Webb et al. Citation1991; Bigsby et al. Citation2014).
Recently, the Asian gypsy moth (AGM, L. dispar asiatica) and Japanese gypsy moth (JGM, L. dispar japonica) are known as new threats. In contrast to the flightless females of the EGM, females of the AGM and JGM are flight capable and their larvae are more polyphagous than the European strain (Keena et al. Citation2008; Iwaizumi et al. Citation2010). There is heightened interest in the ecology and population dynamics of AGM and JGM to prevent their introduction and spread in North America, as well as in Australasia (Barlow et al. Citation2000; Matsuki et al. Citation2001; Peterson et al. Citation2007). However, the ecology of AGM and JGM remain poorly characterized compared with EGM (Peterson et al. Citation2007).
In general, a female gypsy moth oviposits only one egg mass in summer. Japanese gypsy moth overwinters as egg and larvae emerge in spring. Information on the density and distribution of the egg masses is important for understanding population dynamics of gypsy moths (Liebhold et al. Citation2007). In particular, a detailed observation about the oviposition site selection would greatly aid forest monitoring to prevent large outbreaks, because the oviposition sites may adaptively change depending on the region (Higashiura Citation1989). Some studies concerning JGM egg masses have been conducted in deciduous broad-leaved forests or mixed coniferous–deciduous forests. In those forests, JGM typically deposited egg masses on the trunk and branches of trees (Schaefer Citation1978; Hajek and Tobin Citation2009). In snowy regions of Japan, eggs laid beneath the snow surface escaped avian predation more effectively (Higashiura Citation1989). These results imply that oviposition on sturdy surfaces, and safety from avian predation, are important determinants of larvae survival for diapause eggs of JGM.
In contrast, few studies have been conducted in warm-temperate evergreen broad-leaved forests (but see Jikumaru Citation2008; Jikumaru Citation2013), which are distributed throughout eastern Asia, including western and southern Japan (Kira Citation1991; Tanouchi and Yamamoto Citation1995). About one-third of the forested area of Japan is secondary forest, which had been intensively used by local communities for gathering firewood and fertilizer. Many such forests were abandoned so that in suburban areas of warm-temperate Japan, secondary forests are succeeding toward late-successional evergreen broad-leaved forest (Morimoto and Yoshida Citation2005; Ishii and Asano Citation2010).
In a large outbreak of JGM that occurred in 2000 and 2009 in abandoned secondary forests in Hiroshima Prefecture, western Japan, most egg masses were found on the abaxial surfaces of leaves of evergreen broad-leaved trees, and highly concentrated on Quercus glauca Thunb. In the 2009 outbreak, only one among 126 egg masses was on a tree trunk (Jikumaru Citation2013). Avian predation rates for egg masses on the abaxial surfaces of evergreen leaves in Hiroshima were 12.1% in 2008, 0% in 2009, and 0% in 2010 (S. Jikumaru, unpublished data). These observations suggest that, in warm-temperate evergreen broad-leaved forests, oviposition on the abaxial surfaces of evergreen leaves may be effective for survival of JGM eggs. Further accumulation of knowledge regarding gypsy moth oviposition site selection in evergreen broad-leaved forests will serve future region-specific forest management and planning.
In this study, we investigated the occurrence of egg masses of JGM across various evergreen tree species in a warm-temperate, suburban secondary forest in Hyogo Prefecture, western Japan, after a large JGM outbreak. We hypothesized that, as in Hiroshima, the abaxial surfaces of leaves of evergreen broad-leaved trees are the preferred oviposition site by JGM, because they serve as overwintering sites and are well hidden from predation. We investigated whether certain tree species and leaf attributes were preferred and also analyzed whether there is a spatial pattern in the distribution of egg masses.
Study site and methods
Study site
The research was conducted at a secondary forest at Taisanji temple (34°41′ N, 135°04′ E, c. 70–200 m above sea level) in Kobe City, Hyogo Prefecture, Japan. This area is in the warm-temperate zone, where the evergreen broad-leaved forest is regarded as the climax vegetation (Kira Citation1991). Annual precipitation in this region was 1297.5 mm and mean temperature was 17.0 °C, ranging from 5.3 °C (monthly mean) in January to 29.4 °C in August for 2013 (Japan Meteorological Agency Citation2016). This forest had been managed as a coppice forest, was abandoned about 50 years ago, and is now composed of evergreen and deciduous broadleaved trees such as Quercus phillyraeoides A. Gray, Quercus serrata Thunb. ex Murray, Quercus glauca Thunb., and Camellia japonica L. (Iwasaki and Ishii Citation2005). Quercus phillyraeoides is common in coastal forests and low mountain slopes in western and southern Japan. Quercus serrata is a typical component of Japanese secondary forests and, after being abandoned, they grow to large-sized trees. Quercus glauca and Camellia japonica are typical components of Japanese warm-temperate evergreen broad-leaved forests.
In 2005, we established a square research plot (30 × 30 m) about 20 m from the forest edge, which faces east. All trees taller than 1.3 m were numbered and tagged, and species, diameters at breast height (DBH), and tree heights were recorded. The tree locations (X and Y coordinates) were surveyed using a ULD-300 digital compass (Ushikata Co., Tokyo, Japan). Tree mortality and recruitment were surveyed in 2010. There were 34 tree species in the research plot, including 19 evergreen broad-leaved and 15 deciduous broad-leaved species (). Evergreen species dominated the stand, accounting for 77.8% and 68.5% of individual tree density and basal area, respectively.
Table 1. Tree species composition and occurrence of egg masses in the research plot.
Survey for egg masses and leaf attributes
In spring of 2013, an outbreak of JGM occurred in the study site. Defoliation was especially severe for Quercus phillyraeoides, Myrica rubra Sieb. et Zucc., and Vaccinium bracteatum Thunb. From August to October 2013, we surveyed the leaves, trunks, and branches of all the tagged trees with ancillary use of binoculars, and recorded the number of egg masses. After the outbreak, the majority of the larvae were killed by the entomophthoralean fungus, Entomophaga maimaiga. As a result, we found only one egg mass that was newly deposited in 2013. All other egg masses found were those that had been laid in 2012 and hatched in spring of 2013, causing the outbreak.
To identify leaf attributes associated with oviposition site selection, we chose 15 of the dominant evergreen tree species (excluding four species which comprised only individual trees with small DBH), and randomly sampled 20 to 30 leaves from multiple trees of each species. To investigate the leaf attributes associated with the time of oviposition (2012), we sampled previous-year leaves, but for Q. phillyraeoides, M. rubra, and V. bracteatum, the previous-year leaves had been mostly defoliated by JGM larvae in spring. Therefore, we had to select current-year leaves. For the species on which we found egg masses, we additionally sampled some leaves with egg masses.
We measured all sampled leaves for leaf area, length, width, and thickness. Leaf area was measured using a LI-3100 leaf area meter (Li-Cor Inc., Lincoln, NE). Leaf length and width were measured using a ruler and thickness was measured using a Quickmini thickness meter (Mitsutoyo Inc., Tokyo, Japan). Then, all leaves were oven dried to constant weight at 65 °C for measurement of leaf dry mass to calculate leaf mass per area (LMA), a measure of leaf sturdiness.
Statistical analyses
We first analyzed whether there was a preference for oviposition among species by using the relative basal area of each species as the expected value in a χ2 test, assuming that the total leaf area of each species is proportional to its basal area. To analyze oviposition preferences across species in relation to their leaf attributes, we compared leaf attributes in a two-way ANOVA with species and presence/absence of egg masses as the two main effects. Tukey’s honest significant difference (HSD) test was used for multiple comparison among species.
To clarify quantitatively the spatial distribution pattern of egg masses, we investigated the spatial distribution of trees using the L-function. The L-function is a square root transformation of Ripley's K-function (Ripley Citation1979; Freeman and Ford Citation2002; Loosemore and Ford Citation2006).
where r denotes distance between trees. L (r) can be used to test whether an observed spatial point pattern, such as the horizontal distribution of trees in a forest stand, is spatially random (L (r) = 0), clustered (L (r) > 0), or regular (L (r) < 0). In addition, L (r) can be used to infer the spatial scale (e.g., cluster size) of the observed pattern. Here, we calculated L (r) using the X, Y coordinates of trees, to estimate the distribution pattern of all trees within the plot and that of trees with egg masses, as well as the spatial scale of these patterns.
All statistical analyses were done using R (v3.0.2, R Development Core Team Citation2013). To calculate L (r), we used the spatstat packages in R, and ran Monte-Carlo simulation 99 times to calculate 99% confidence intervals for L (r).
Results
We found JGM egg masses from 2012 on 62 individual trees of 9 species (). All of the egg masses in the plot were found on the abaxial surfaces of 1-year-old leaves of evergreen tree species. The distribution of egg masses among species relative to expected values based on basal area was significantly non-random (χ2 = 335.2, P < 0.001, n = 33). The greatest number of egg masses were found on Camellia japonica with egg masses on 41 (74.5%) of 55 trees. Of the total number of egg masses found, 76.6% were on Camellia japonica. The percentages of trees with egg masses were also high for Q. glauca, Photinia glabra (Thunb.) Maxim., Ilex integra Thunb., Dendropanax trifidus (Thunb.) Makino, and Cleyera japonica Thunb.
There were significant differences in all leaf attributes among species (F > 48.0, P < 0.001, n = 15). Species with egg masses present were clearly distinguished from those without egg masses as having larger leaf area (> 10 cm2) and leaf dry mass (> 0.1 g) (). In this forest, Camellia japonica was the most common species that fulfilled these criteria (A). Castanopsis cuspidata (Thunb.) Schottky was the only exception. However, the single egg mass that was found on C. cuspidata was laid over two partially overlapping leaves (B). For species with egg masses present, the leaves with egg masses had significantly larger area, length, width, and dry mass than leaves without egg masses ().
Figure 1. Boxplots of leaf area (A) and leaf dry mass (B) for 15 evergreen tree species in the research plot.
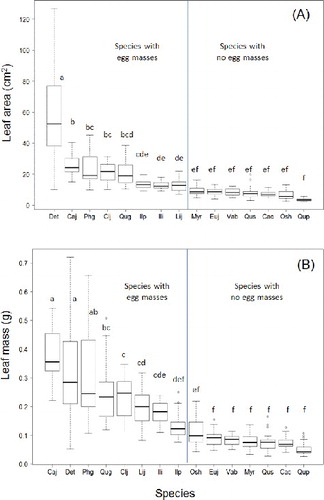
Figure 2. Egg masses of Japanese gypsy moth laid on the abaxial surface of a Camellia japonica leaf (A) and over two overlapping leaves of Castanopsis cuspidata (B). These egg masses were deposited in 2012 and the photos were taken in September–October 2013.
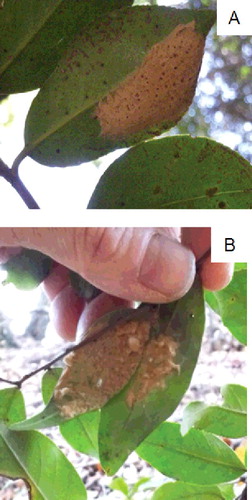
Table 2. Morphological attributes of leaves (mean ± SD) with egg masses present/absent.
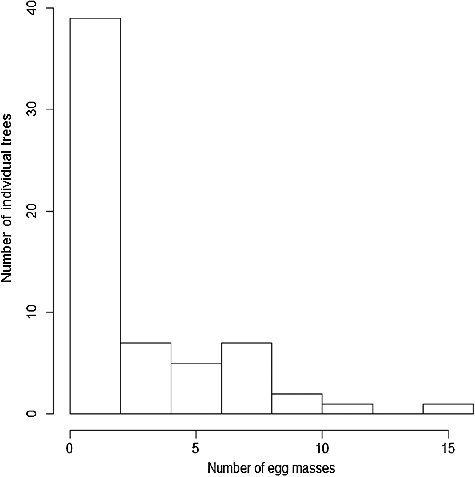
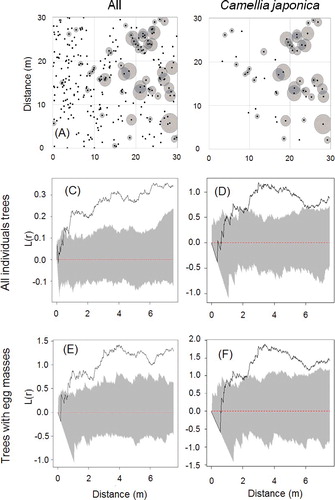
Most trees on which egg masses were found had only one or two egg masses (28 and 11 trees, respectively; ), but the number of egg masses on each tree was positively correlated with DBH (Spearman's rank correlation, rs = 0.322, P = 0.011, n = 62). Many trees with egg masses were found in the northern half of the research plot, where Camellia japonica occurred (A, B). Spatial analyses using the L-distribution indicated that individual trees of all species were clustered at scales greater than about 1 m (C) and trees of Camellia japonica were clustered at scales greater than about 2 m (D). Trees with egg masses present were also clustered at similar spatial scales (E, F).
Discussion
Although we found egg masses on various evergreen tree species, they tended to be concentrated on one species, Camellia japonica, indicating a strong preference in oviposition site selection by JGM. Camellia japonica is a common evergreen tree in natural and secondary forests of warm-temperate regions in Japan (Ueno et al. Citation2000; Osono Citation2008). It has large, thick leaves and leaf longevity is more than 2 years (Ishii and Ohsugi Citation2011). We also found that larger trees tend to have more egg masses, probably due to the greater leaf area available for oviposition, and trees with egg masses present were clustered, but not at spatial scales significantly different from the underlying population.
During the large outbreak in Hiroshima Prefecture in 2009, egg masses were highly concentrated on Q. glauca (Jikumaru Citation2013), which is also a common evergreen tree in natural and secondary forest of warm-temperate Japan. Our observations suggest that females of JGM may commonly oviposit on the abaxial surfaces of leaves of evergreen broad-leaved trees, which are common in forests throughout western Japan. In this study, females of JGM preferred to oviposit on evergreen tree species with large leaves (>10 cm2 and >0.1 g). At the study site in Hiroshima Prefecture, Q. glauca was the most abundant species that fulfilled this criteria (Jikumaru Citation2013), while in this study the corresponding species was Camellia japonica. The average body length of female JGM is about 2.5 cm (Nakane Citation2007). The length and weight of female JGM in the study conducted in Hiroshima Prefecture were c. 2.3 cm and 450 mg, respectively, while the length and width of egg masses were c. 4.0 cm and 2.0 cm, respectively (S. Jikumaru, unpublished data). Therefore, small leaves are unsuitable for oviposition because of insufficient stability and size as ovipositing sites. The egg mass that was laid on two partially overlapping leaves of Castanopsis cuspidata in this study supports the theory that sufficient surface area is needed for oviposition by JGM. As for Q. phillyraeoides, M. rubra, and V. bracteatum, we were only able to sample current-year leaves. In the future, additional survey of the previous-year leaves of these species will be necessary to strengthen this theory.
In addition to embryo survival, proximity to suitable habitat for offspring and maternal survival are important determinants of oviposition site selection in insects (Refsnider and Janzen Citation2010). Newly hatched larvae of gypsy moths move to their feeding sites by ballooning (Diss et al. Citation1994). Our field observations suggest that larvae prefer to feed on small, soft-leaved trees, which is in contrast to their oviposition sites, suggesting that proximity to a suitable feeding site may also be an important factor influencing oviposition site selection by JGM. In association with maternal survival, behaviors of mated females to select oviposition sites and of virgin females to select mating sites also affect female survival, copulation success, and subsequent oviposition site selection (Koshio Citation1996; Iwaizumi et al. Citation2010).
In order to effectively manage and monitor pest species such as JGM in forests, it is important to understand their ecology and population dynamics. Together with previous studies (Jikumaru Citation2008, Citation2013), our findings indicate that, for effective monitoring of JGM egg masses in warm-temperate evergreen broad-leaved forests in Japan, the abaxial surfaces of the most abundant evergreen broad-leaved trees with large leaves should be checked. If egg masses are found, it is likely that nearby trees of the same species will also have egg masses. Large trees should be checked thoroughly for occurrence of multiple egg masses. Further studies, including those conducted in other regions of the warm-temperate zone, and those investigating the influence of larvae feeding sites and female flight behavior on egg mass distribution, are needed to fully understand the adaptive significance of oviposition site preferences of JGM and to effectively monitor and control this pest species.
Acknowledgements
We thank Taisanji Temple for facilitating the study and members of the Forest Resources Lab, Kobe University for field assistance. Drs K. Maeto and S. Sugiura provided constructive comments on earlier versions of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Barlow ND, Caldwell NP, Kean JM, Barron MC. 2000. Modelling the use of NPV for the biological control of Asian gypsy moth Lymantria dispar invading New Zealand. Agr For Entomol. 2:173–184.
- Bigsby KM, Ambrose MJ, Tobin PC, Sills EO. 2014. The cost of gypsy moth sex in the city. Urban For Urban Gree. 13:459–468.
- Diss AL, Kunkel JG, Montgomery ME, Leonard DE. 1994. Effects of maternal nutrition and egg provisioning on parameters of larval hatch, survival and dispersal in the gypsy moth, Lymantria dispar L. Oecologia 106:470–477.
- Doane CC, McManus ML. 1980. The gypsy moth: research toward integrated pest management. Technical Bulletin 1584. Washington, DC: USDA
- Elkinton JS, Liebhold AM. 1990. Population dynamics of gypsy moth in North America. Annu Rev Entomol. 35:571–596.
- Freeman EA, Ford ED. 2002. The effects of data quality on analysis of ecological pattern using the K(d) statistical function. Ecology 83:35–46.
- Hajek AE, Tobin PC. 2009. North American eradications of Asian and European gypsy moth. In: Hajek AE, Glare TR, O'Callaghan M, editors. Use of microbes for control and eradication of invasive arthropods. Netherlands: Springer; p. 71–89.
- Higashiura Y. 1989. Survival of eggs in the gypsy moth Lymantria dispar. II. Oviposition site selection in changing environments. J Anim Ecol. 58:413–126.
- Ishii H, Asano S. 2010. The role of crown architecture, leaf phenology and photosynthetic activity in promoting complementary use of light among coexisting species in temperate forests. Ecol Res. 25:715–722.
- Ishii H, Ohsugi Y. 2011. Light acclimation potential and carry-over effects vary among three evergreen tree species with contrasting patterns of leaf emergence and maturation. Tree Physiol. 31:819–830.
- Iwaizumi R, Arakawa K, Koshio C. 2010. Nocturnal flight activities of the female Asian gypsy moth, Lymantria dispar (Linnaeus) (Lepidoptera: Lymantriidae). Appl Entomol Zool. 45:121–128.
- Iwasaki A, Ishii H. 2005. Vegetation structure of fragmented shrine/temple forests in southeastern Hyogo Prefecture – Estimation of edge-effect distance and minimum conservation area. Humans and Nature 15:29–42.
- Japan Meteorological Agency. 2016. Electronic database for climate [cited 2016 Feb 15]. Available from: http://www.data.jma.go.jp/obd/stats/etrn/index.php (in Japanese).
- Jikumaru S. 2008. Outbreaks of Lymantria dispar in Hiroshima Prefecture for the first time in ca. 40 years and their mortality factors. Forestry Chemicals. 183:8–13. (in Japanese)
- Jikumaru S. 2013. Oviposition preferences of the Japanese Gypsy moth, Lymantria dispar japonica (Motschulsky, 1860) (Insecta: Lepidoptera: Erebidae: Lymantriinae), on evergreen broad-leafed tree leaves in Hiroshima Prefecture, Japan. Life Excit Biol. 1:225–240.
- Johnson DM, Liebhold AM, Tobin PC, Bjørnstad ON. 2006. Allee effects and pulsed invasion by the gypsy moth. Nature 444:361–363.
- Keena MA, Cote MJ, Grinberg PS, Wallne WE. 2008. World distribution of female flight and genetic variation in Lymantria dispar (Lepidoptera: Lymantriidae). Environ Entomol. 37:636–649.
- Kira T. 1991. Forest ecosystems of East and Southeast Asia in a global perspective. Ecol. Res. 6:185–200.
- Koshio C. 1996. Pre-ovipositional behaviour of the female Gypsy Moth, Lymantria dispar L. (Lepidoptera, Lymantriidae). Appl Entomol Zool. 31:1–10.
- Liebhold AM, Sharov AA, Tobin PC. 2007. “Slow the spread”: a national program to manage the gypsy moth. Chapter 2, Population biology of gypsy moth spread; p. 15–109. USDA Forest Service Northern Research Station General Technical Report NRS-6. Newtown Square, PA: USDA Forest Service. Available from: http://www.nrs.fs.fed.us/pubs/gtr/gtr_nrs6.pdf
- Loosemore BN, Ford ED. 2006. Statistical inference using the G or K point pattern spatial statistics. Ecology 87:1925–1931.
- Matsuki M, Kay M, Serin J, Floyd R, Scott JK. 2001. Potential risk of accidental introduction of Asian gypsy moth (Lymantria dispar) to Australasia: effects of climatic conditions and suitability of native plants. Agr Fore Entomol. 3:305–320.
- Morimoto J, Yoshida H. 2005. Dynamic changes of native Rhododendron colonies in the urban fringe of Kyoto city in Japan: detecting the long-term dynamism for conservation of secondary nature. Landscape Urban Plan. 70:195–204.
- Nakane T. 2007. Colored illustrations of the insects of Japan, 47th ed. Tokyo: Hoikusha.
- Osono T. 2008. Endophytic and epiphytic phyllosphere fungi of Camellia japonica: seasonal and leaf age-dependent variations. Mycologia 100:387–391.
- Peterson AT, Williams R, Chen G. 2007. Modeled global invasive potential of Asian gypsy moths, Lymantria dispar. Entomol Exp Appl. 125:39–44.
- R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org/
- Refsnider JM, Janzen FJ. 2010. Putting eggs in one basket: ecological and evolutionary hypotheses for variation in oviposition-site choice. Annu Rev Ecol Evol Syst. 41:39–57.
- Ripley BD. 1979. Tests of ‘randomness’ for spatial point patterns. J Roy Stat Soc B. 41: 368–374.
- Schaefer PW. 1978. Betula platyphylla: the preferred oviposition host of Lymantria dispar japonica in Hokkaido, Japan. Environ Entomol. 7:168–170.
- Sharov AA, Leonard D, Liebhold AM, Roberts EA, Dickerson W. 2002. ‘Slow the spread’: a national program to contain the gypsy moth. J For. 100:30–35.
- Tanouchi H, Yamamoto S. 1995. Structure and regeneration of canopy species in an old-growth evergreen broad-leaved forest in Aya district, southwestern Japan. Vegetatio 117:51–60.
- Ueno S, Tomaru N, Yoshimaru H, Manabe T, Yamamoto S. 2000. Genetic structure of Camellia japonica L. in an old-growth evergreen forest, Tsushima, Japan. Mol Ecol. 9:647–656.
- Webb RE, Ridgway RL, Thorpe KW, Tatman KM, Wieber AM, Venables L. 1991. Development of a specialized gypsy moth (Lepidoptera: Lymantriidae) management program for suburban parks. J Econ Entomol. 84:1320–1328.