ABSTRACT
A giant dogwood (Cornus controversa) was bleeding transparent sap that turned into gummy orange slime with a pungent odor on the bark in early April. The tree exudation originated from a branch wound that occurred during the most recent spring pruning. Scanning electron microscopy revealed the intact bark surface was irregularly undulating and covered with at least three types of epicuticular waxes: polygonal rodlets; polygonal tubules; and threads, whereas the dried orange plaques were covered with fungi, possibly the co-occurrence of yeast and filamentous forms. The yeast forms were ovoid, lemon- or club-shaped, and approximately 10 μm in length. Obviously constricted septa and clamp connections were rarely detected on the filamentous forms. No bacterial proliferation was detected on the orange slime. X-ray microanalysis revealed a steep increase in calcium concentration in the orange plaques compared with that in the intact bark. These results suggest that bark-dwelling dimorphic red yeasts may constitute one of the dominant microbial consortia of the orange slime of giant dogwood. Given the high amount of sugars and calcium in the sap, and tolerance to diurnal temperature fluctuations in early spring, the red yeasts may be specialist species that thrive under a limited range of environmental conditions.
Woody plants show different responses to biotic or abiotic stresses compared to herbaceous plants (Yamada Citation2001). For example, broad-leaved deciduous trees have two types of exudates: (i) chronic slime-flux; and (ii) spring sap-flow (Weber Citation2006). Chronic slime-fluxes are caused by microbial infections or minor wounding and can persist for months to years. In contrast, spring sap-flows occur because of pruning or puncturing and are active for a short time (Weber Citation2006). Reactivation and mobilization of stored carbohydrates and lipid in parenchyma tissues during budbreak and leaf emergence are associated with spring sap-flows (Westhoff et al. Citation2008).
Spring sap-flows exuding from fresh wounds of broad-leaved deciduous trees become quickly colonized by microbial consortia comprising a planktonic phase of yeasts and biofilm of bacteria and yeasts in a matrix of fungal hyphae (Herz et al. Citation2007). Particularly, spring sap-flows of birch (Betula sp.) display strong orange to red colors if basidiomycetous yeasts dominate the microbial consortium (Herz et al. Citation2007). Basidiomycetous yeasts typically inhabit the plant surface and utilize pentoses, sugar alcohols, tannin, and lignin (Johnson Citation2013). Because of their perennial persistence in forest stands, tree barks frequently covered by epiphytes such as lichens and mosses represent complex habitats with a mosaic of ecological niches, providing stable substrates for microbial colonization across broad taxonomic groups (Aschenbrenner et al. Citation2017). However, knowledge of the bark microbiome dynamics remains relatively scarce compared to that of either leaf or root microbiomes.
Belonging to the family Cornaceae, Cornus controversa Hemsl. is a broad-leaved deciduous tree that is widely distributed across temperate regions. It is commonly referred to as the giant dogwood and has peculiar multi-tiered branches in its canopy. When wounded at the stems in spring, the trees bleed transparent sap that later develops into dry plaques of orange slime. Spring sap-flows and gummy orange slime are not detrimental to tree health in most cases; however, they apparently decrease the aesthetic value of trees and pose a serious concern for growers in terms of arboricultural practice. Nevertheless, few studies have examined the identity and composition of the orange slime of the tree species. We hypothesized that the orange slime may contain abundant bacterial communities and iron oxide, based on the extensive mass and sudden appearance of homogeneous strong orange color. In this study we evaluated the biological and chemical composition of the dried plaques of orange slime of C. controversa using a field emission scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectrometer.
Materials and methods
Site inspections and tree materials
An approximately 4 m tall giant dogwood was grown in a mountainous region of Sangju (36°42′N, 128°16′E), Republic of Korea. Numerous dry plaques of orange slime were present on the tree bark. A range of site inspections for tree diagnosis, from visual tree assessment to witness interviews, were performed. The upper canopy of the tree was reached using an extension stepladder to detect any wounds from which the spring sap-flows had originated. Bark pieces with orange slime on the surface were collected from the stem in early April. They were air-dried in paper envelopes at room temperature for at least 2 months. Bark pieces without orange slime were also collected as control.
Field emission scanning electron microscopy
Bark pieces (5 × 10 mm2) with dried plaques of orange slime were excised from the specimens, mounted on a metal stub with double-sided copper tape, and coated with platinum using a sputter-coater (SCD 005; BAL-TEC, Balzers, Liechtenstein). The specimens were observed with a Schottky-type field emission SEM (Supra 55VP; Carl Zeiss, Oberkochen, Germany) operated at an accelerating voltage of 2 kV. Terminology for plant epicuticular waxes was adapted from Barthlott et al. (Citation1998).
X-ray microanalysis
To analyze the element composition in a non-destructive manner, the bark pieces were probed using a high-energy incident electron beam to generate characteristic X-ray signals, as reported previously (Kim et al. Citation2011). Element composition was identified using an energy-dispersive X-ray spectrometer (XFlash 4000; Bruker AXS Microanalysis, Berlin, Germany) equipped with the SEM operated at an accelerating voltage of 15 kV. Both region analysis and mapping of elements were performed on the bark and orange plaques at 1000× magnification. Three regions on the bark and orange plaques were randomly selected for quantitative element analysis. Representative results for each specimen were presented as atomic concentrations of elements measured from X-ray spectra of K-series peaks.
Results
The tree was bleeding abundant transparent sap for several days according to witness interviews (data not shown). The spring sap-flow turned into gummy orange slime on the stem surface and had a pungent odor (A). The orange slime ran down along the bark from the branch of the lower crown to the ground. On the upper part of the stem was a pruning wound from which the sap-flow originated (B). There were pruned twigs and branches around the tree on the forest floor (C). The sap-flow ran along the stem, which could be seen from different views of the tree.
Figure 1. Spring sap-flows of Cornus controversa. Front view of the bleeding tree stem. Note the orange slime solidified on the stem (A), wound on the upper part of the stem. The pruning wound (arrow) was observed after removing the covered orange slime (B), side view of the bleeding tree stem. There were pruned twigs and branches (arrows) around the tree on the forest floor (C).

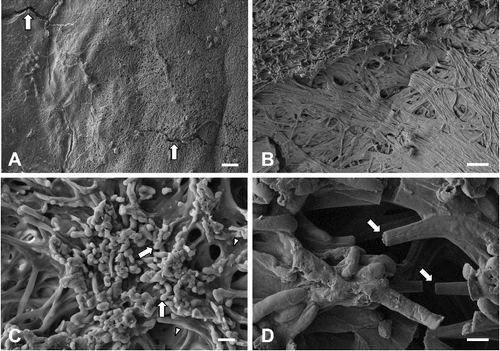
Field emission SEM showed that the bark had an irregularly undulating rough surface (A). No distinct rows of lenticels were present on the bark. Eruption of cork occurred through the bark (B). Tufts of plant epicuticular waxes were frequently observed on the bark (C). Rodlets and threads were the most prevalent types of epicuticular waxes. Higher magnifications revealed polygonal cross-sections and threads of varying thickness and flexibility (D). Most polygonal rodlets had four to six facets and were arranged into clusters. A polygonal tubule with a terminal opening was also observed.
Figure 2. Field emission scanning electron micrographs of the bark of Cornus controversa. Bark surface, which was irregularly undulating. Scale bar = 200 μm (A), magnified view of bark. Note the erupted cork (arrows). Scale bar = 50 μm (B), epicuticular waxes. Note rodlets (arrows) and threads (arrowheads). Scale bar = 20 μm (C), magnified view of epicuticular waxes. Note a tubule with a terminal opening (square inlet), polygonal rodlets (arrows), and threads (arrowheads). Scale bar = 5 μm (D).
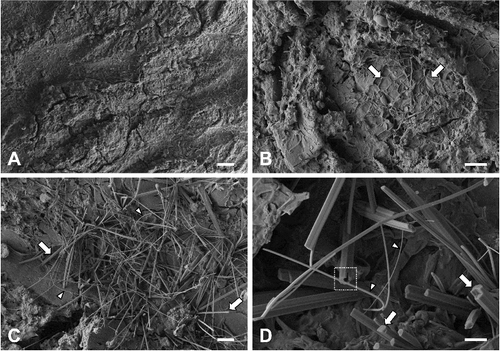
The surface of the dried plaques of orange slime was overall less rough and undulating than that of the bark (A). Microcracks were often observed on the dried orange slime. Biofilm-like microbial mats covered the entire surface. Differences in textures and surface heights were observed on the orange plaques (B). Higher magnifications revealed the fungal growth, seemingly composed of yeast forms and filamentous forms on the surface (C). Neither bacterial proliferation nor biofilm were found on the orange plaques. The yeast forms were ovoid, lemon- or club-shaped, and approximately 10 μm in length. They often formed clusters on the microbial mat where mucilage was present among filamentous forms. Filamentous forms were cylindrical and approximately 5 μm in diameter (D). Obviously constricted septa and clamp connections were rarely observed on the filamentous forms. Some filamentous forms were disconnected, possibly because of innate artifacts in electron microscopy.
Figure 3. Field emission scanning electron micrographs of orange plaques on the bark of Cornus controversa. Orange plaque surface, which showed microcracks (arrows) and was overall less undulating than the bark surface. Scale bar = 200 μm (A), magnified view of the orange plaque. There were differences in textures and surface height. Scale bar = 50 μm (B), yeast forms (arrows). They were lemon- or club-shaped, and often formed clusters on the microbial mat. Note the mucilage (arrowheads) among filamentous forms. Scale bar = 10 μm (C), magnified view of filamentous forms. Filamentous forms were disconnected (arrows). Scale bar = 5 μm (D).
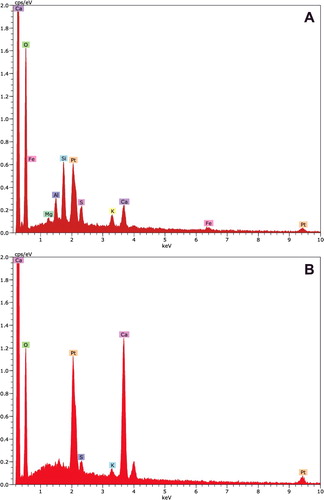
Region analysis of the bark pieces revealed calcium at the peak position of 3.69 keV as K-alpha X-ray signals (A). Potassium was present at the peak position of 3.31 keV as K-alpha X-ray signals. Platinum and other elements were derived from the platinum-coated bark pieces. However, the orange plaques showed different element profiles (B). Relatively high calcium peaks were detected at the peak positions of 3.69 and 4.01 keV as K-alpha and K-beta X-ray signals, respectively. Other elements such as platinum and potassium were also present in the orange plaques.
Figure 4. X-ray spectra of bark and orange plaque of Cornus controversa. X-ray spectrum from bark. Calcium and potassium were detected at the peak positions of 3.69 and 3.31 keV as K-alpha X-ray signals, respectively (A), X-ray spectrum from the orange plaque. Relatively high peaks of calcium were detected at the peak positions of 3.69 and 4.01 keV as K-alpha and K-beta X-ray signals, respectively (B).
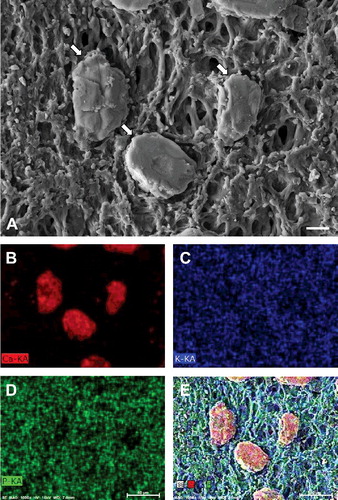
Grain-like particles were frequently found on the orange plaques (A). They were elliptical and approximately 50 μm in length. Element mapping showed prominent localization of calcium in the particles (B). However, potassium and phosphorus were rarely detected on the particles (C–D). Merged images of calcium, potassium, and phosphorus revealed the presence of calcium in the particles (E).
Figure 5. Element maps of orange plaque of Cornus controversa. Field emission scanning electron micrograph of calcium particles (arrows) on orange plaque. Scale bar = 20 μm (A), calcium map of A (B), potassium map of A (C), phosphorus map of A (D), merged image of the secondary electron image, calcium map, potassium map, and phosphorus map (E).
Different element concentrations were found between the bark and orange plaques in quantitative analysis (). Slight differences in element composition were detected from three randomly selected regions. Common elements of bark in forest stands were detected, such as carbon, oxygen, and silicon. Meanwhile, calcium concentration was five to six-fold higher in the orange plaques than in the bark. No marked differences in element composition, except calcium, were found between the bark and orange plaques.
Table 1. Element composition of bark and orange plaque of Cornus controversa determined using an energy-dispersive X-ray spectrometer equipped with a field emission scanning electron microscope.
Discussion
Here we report the in situ characterization of the orange plaques from giant dogwood determined by SEM to unravel their composition in terms of microbial consortia and constituent elements. The site inspections for tree diagnosis were effective for deducing the cause of tree injury. Tree exudation was thought to be caused by the most recent spring pruning in that area according to witness interviews, wound position, and byproducts of pruning easily found around the tree. This indicates that late spring pruning after flowering would alleviate bark exudation of the tree species. Otherwise, the tree is at risk of bleeding or damage by stem borer pests.
High-resolution surface imaging of the orange plaques revealed fungal growth, possibly the co-occurrence of yeast and filamentous forms. Fruiting bodies or spores of filamentous fungi were rarely present. In contrast, the shape, size, and growth characteristics of the cellular structures on the orange plaques were similar to those of yeast species. The filamentous forms (c. 5 μm in diameter) were distinct from those of actinomycetes whose filaments were c. 1 μm in diameter (Wilkinson Citation2003). The mucilage among filamentous forms may function as a shield and prevent filamentous forms from drying from low water activity during dry periods (Bhadra et al. Citation2008).
If the yeast and filamentous forms are derived from the same species, the co-occurrence of the two phases indicates dimorphism. The unicellular yeast phase may be crucial for systemic spread or passive transport through spring sap-flows to distal locations, whereas the multicellular filamentous phase gains entry into its ecological niches via its penetration capacity (Nadal et al. Citation2008). Overall, the filamentous forms appeared to be more prevalent than the yeast form on the orange plaques (). Given the rare occurrence of clamp connection, the filamentous forms may represent an asexual stage of the yeast species. Whether the filamentous forms are pseudohyphae remains unclear, as the absence of septal pores was not confirmed.
The spring sap-flows of C. controversa are acidic and contain high amounts of sugars and calcium (Mori et al. Citation1991; Weilgony and Richter Citation1991; Westhoff et al. Citation2008). This may create a rather harsh environment, acting as a strong habitat filter for the bark microbiome, as suggested for nectar-dwelling yeasts (Wehner et al. Citation2017). Tolerance to high sugar concentrations is a specialized trait possessed by a small fraction (approximately 13%) of yeast species (Herrera et al. Citation2010). Based on the (i) high amounts of sugar and calcium in spring sap-flows, (ii) tolerance to diurnal temperature fluctuations in early spring, (iii) dimorphic cellular structures, and (iv) strong orange pigment, bark-dwelling red yeasts may constitute one of the dominant microbial consortia of orange slime of giant dogwood. Yeasts already present on the bark likely represent the initial colonizers of spring sap-flows (Weber Citation2006). Bark-dwelling red yeasts are thought to degrade and utilize the sugar resource and may affect mutualistic interactions among trees and pollinators. The red yeasts can be regarded as specialist species rather than generalist species in microbial population interactions, as they thrive under a limited range of environmental conditions.
Several yeast species have been detected in the sap and bark surface of trees depending on the phase of spring sap-flows. The first planktonic phase is mainly dominated by Basidiomycota such as Cryptococcus magnus and C. skinneri, followed by a white yeast Trichosporon pullulans and red yeast Xanthophyllomyces dendrorhous becoming dominant (Weber Citation2006). The analysis of dried plaques in this study suggests similar yeast species composition to that described above. Originally isolated from tree exudates, X. dendrorhous, the teleomorph of Phaffia rhodozyma, is among the moderately psychrophilic basidiomycetous yeasts (Sharma et al. Citation2015). This non-Saccharomyces red yeast uniquely ferments glucose to alcohol under oxidative conditions and produces the carotenoid astaxanthin (3,3ʹ-dihydroxy-ß,ß-carotene-4,4ʹ-dione), which is responsible for the strong orange color of solidified sap-flows (Weber et al. Citation2006; Libkind et al. Citation2011). Astaxanthin serves as an antioxidant, quenching reactive oxygen species to protect the yeast from cellular damage by oxidative stress (Sharma et al. Citation2015).
X-ray microanalysis identified the chemical composition of the orange plaques of C. controversa. The most prominent element in the orange slime was calcium, whereas little calcium was detected on the bark. Calcium is known to significantly influence the onset of cambial reactivation after winter dormancy as well as wood structure and chemistry (Fromm Citation2010). Particularly, an increase in calcium concentration was detected in the xylem sap of two different species of Cornus at the time of budbreak (Weilgony and Richter Citation1991). These reports are consistent with the observed increase in calcium concentration in this study. Taken together, the grain-like particles were predicted to be calcium deposits in the spring sap-flows of C. controversa.
The waxes on the bark surface of C. controversa were verified in this study. At least three types of waxes were detected in the bark of C. controversa: (i) polygonal rodlets; (ii) polygonal tubules; and (iii) threads. The major chemical composition of each type includes triterpenoids, nonacosan-10-ol, and flavonoids, respectively (Barthlott et al. Citation1998). These results have potential applications in various fields such as polishes, ointments, lubricants, and soaps (Oleson and Schwartz Citation2016).
As an innate response to mechanical wounding, spring sap-flows are distinct from bleeding canker symptoms accompanied by tissue necrosis under bark and tree decline (Nelson and Hudler Citation2007; Yun et al. Citation2013). Whether and how the planktonic or biofilm phases of spring sap-flows affect the development of bleeding canker symptoms remains unclear in allied tree species. Following this initial microscopy study, species identification and spatiotemporal analysis of the microbial consortia will be addressed in further studies. Efforts to employ available technologies to a range of tree injuries will enhance our understanding of tree health diagnosis as well as expand and consolidate the field of forest pathology.
Acknowledgments
We are grateful to Young Il Kim and Ji Sun Lee at Kyungpook National University for notifying the Tree Diagnostic Center of the tree injury.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aschenbrenner IA, Cernava T, Erlacher A, Berg G, Grube M. 2017. Differential sharing and distinct co-occurrence networks among spatially close bacterial microbiota of bark, mosses and lichens. Mol Ecol. 26:2826–2838.
- Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H. 1998. Classification and terminology of plant epicuticular waxes. Bot J Linn Soc. 126:237–260.
- Bhadra B, Rao RS, Singh PK, Sarkar PK, Shivaji S. 2008. Yeasts and yeast-like fungi associated with tree bark: diversity and identification of yeasts producing extracellular endoxylanases. Curr Microbiol. 56:489–494.
- Fromm J. 2010. Wood formation of trees in relation to potassium and calcium nutrition. Tree Physiol. 30:1140–1147.
- Herrera CM, Canto A, Pozo MI, Bazaga P. 2010. Inhospitable sweetness: nectar filtering of pollinator-borne inocula leads to impoverished, phylogenetically clustered yeast communities. Proc R Soc B. 277:747–754.
- Herz S, Weber RWS, Anke H, Mucci A, Davoli P. 2007. Intermediates in the oxidative pathway from torulene to torularhodin in the red yeasts Cystofilobasidium infirmominiatum and C. capitatum (Heterobasidiomycetes, Fungi). Phytochemistry. 68:2503–2511.
- Johnson EA. 2013. Biotechnology of non-Saccharomyces yeasts – the basidiomycetes. Appl Microbiol Biotechnol. 97:753–7577.
- Kim KW, Koo K, Kim P-G. 2011. Seawater spray injury to Quercus acutissima leaves: crystal deposition, stomatal clogging, and chloroplast degeneration. Microsc Res Tech. 74:449–456.
- Libkind D, Moline M, van Broock M. 2011. Production of the UVB-absorbing compound mycosporine-glutaminol-glucoside by Xanthophyllomyces dendrorhous (Phaffa rhodozyma). FEMS Yeasts Res. 11:52–59.
- Mori A, Haibara K, Aiba Y. 1991. Seasonal and diurnal variations of mineral concentrations in the branch xylem sap of Cornus controversa. J Jpn For Soc. 73:466–470.
- Nadal M, García-Pedrajas MD, Gold SE. 2008. Dimorphism in fungal plant pathogens. FEMS Microbiol Lett. 284:127–134.
- Nelson AH, Hudler GW. 2007. A summary of North American hardwood tree diseases with bleeding canker symptoms. Arboric Urban For. 33:122–131.
- Oleson KR, Schwartz DT. 2016. Extractives in Douglas-fir forestry residue and considerations for biofuel production. Phytochem Rev. 15:985–1008.
- Sharma R, Gassel S, Steiger S, Xia X, Bauer R, Sandmann G, Thines M. 2015. The genome of the basal agaricomycete Xanthophyllomyces dendrorhous provides insights into the organization of its acetyl-CoA derived pathways and the evolution of Agaricomycotina. BMC Genomics. 16:233.
- Weber RWS. 2006. On the ecology of fungal consortia of spring sap-flows. Mycologist. 20:140–143.
- Weber RWS, Davoli P, Anke H. 2006. A microbial consortium involving the astaxanthin producer Xanthophyllomyces dendrorhous on freshly cut birch stumps in Germany. Mycologist. 20:57–61.
- Wehner J, Mittelbach M, Rillig MC, Verbruggen E. 2017. Specialist nectar-yeasts decline with urbanization in Berlin. Sci Rep. 7:45315.
- Weilgony P, Richter H. 1991. Concentrations of macronutrient cations in xylem sap of Cornus mas L. and Cornus sanguinea L. Flora. 185:296–304.
- Westhoff M, Schneider H, Zimmermann D, Mimietz S, Stinzing A, Wegner LH, Kaiser W, Krohne G, Shirley St, Jakob P, et al. 2008. The mechanisms of refilling of xylem conduits and bleeding of tall birch during spring. Plant Biol. 10:604–623.
- Wilkinson HP. 2003. Fossil actinomycete filaments and fungal hyphae in dicotyledonous wood from the Eocene London Clay, Isle-of-Sheppey, Kent, England. Bot J Linn Soc. 142:383–394.
- Yamada T. 2001. Defense mechanisms in the sapwood of living trees against microbial infection. J For Res. 6:127–137.
- Yun HY, Lee YW, Kim YH. 2013. Stem canker of giant dogwood (Cornus controversa) caused by Fusarium lateritium. Plant Dis. 97:1378.
