?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The research aimed to analyze the distribution of trees species around springs in various environmental factors and the trees-springs interplay possibility in Soloraya region, Indonesia. Investigation was conducted by survey using census method within 10 m radius toward upstream from the point of vegetated springs or previously overgrown by trees. Cluster analysis and discriminant analysis divided 59 springs areas into four (4) groups based on environmental characteristics summarized into two (2) general groups (lowlands and highlands). Only Ficus benjamina was widely distributed shown by high value of relative frequency in entire areas (80%) and high value of indicator species in both lowlands and highlands which was statistically similar. Particular species were obviously dependent on environmental factors (Samanea saman and Innocarpus fagiferus for lowlands, Artocarpus elasticus and Bischofia javanica for highlands). Species dependency on environmental factors was also performed by Canonical Correspondence Analysis showing −0.604, 0.538 and −0.647 of correlation coefficients for elevation, average rainfall and average temperature, respectively. This research also found an association between trees condition and water-flow of springs. It was supported by 0.57 of contingency coefficient which was statistically significant. The information obtained was expected to lead restoration/rehabilitation efforts on the spring protection zone. Adding the number of samples and environmental factors were suggested for future research to get more information. Deeper investigation on the role of the trees around springs was also suggested.
Introduction
Soloraya region in Central Java Indonesia is the upper part of Bengawan Solo watershed (BPDAS Solo Citation2015). This area is also a groundwater basin marked by the existence of many groundwater discharge areas such as springs (BPSDA Bengawan Solo Citation2017). Springs were generally utilized by local people of rural area to fulfill their water needs (Sudarmadji et al. Citation2015). According to MacDonald and Davies (Citation2005), consuming groundwater from spring is a better and cheap way for rural communities. Therefore, spring protection efforts and restoration efforts on the degraded/dried springs in this area are a must. According to Yuliantoro and Siswo (Citation2016), the degraded/dried springs in this region reached 47% within ten years, from 2006 to 2016.
Restoration efforts can be implemented by rehabilitation program (Bradshaw Citation1997) given the influence of vegetation especially trees. Vegetation affects the aquifer recharge (Scanlon et al. Citation2002; Wang et al. Citation2013) although such understanding is often admittedly low (Liu and Clewell Citation2017) and occasionally contradictive especially in hydrological cycle (Wei et al. Citation2008). On the other hand, water availability is an essential factor for the existence of ecosystems (Foster et al. Citation2006) and tree growth (Zhu et al. Citation2009). Therefore, specific discussion on the relationship between trees vegetation and water will include recharge area and discharge area (George et al. Citation1999). According to Le Mailtre et al. (Citation1999), vegetation-groundwater association occurs in two steps i.e. the rainfall reaching aquifer process and groundwater extraction either by deep rooting or by environmental condition causing the groundwater flow to the surface over the springs/seepages, rivers or lakes.
In recharge area, water is controlled by vegetation (Zhang and Schilling Citation2006) and other factors such as topography, geology, climate (Winter Citation2001) and rainfall (Duan et al. Citation2016; Fan et al. Citation2016; Pramono et al. Citation2017). The positive role of vegetation in recharge area was known to control water infiltration (Duan et al. Citation2016) into the root zone (Wu et al. Citation2017) and to flow it laterally in the ground and rocks (Weiler Citation2005). In addition, vegetation also affects water evaporation from the land or forest floor (Wenjie et al. Citation2006; Fan et al. Citation2016). Groundwater will flow to discharge area over the seepages or springs and wetlands (Grielber and Avramov Citation2014) which were usually close and flow into permanent water bodies such as rivers and lakes (Winter et al. Citation1998; University of Calgary Citation2012).
Discharge areas including springs were often indicated by particular species (Goslee et al. Citation1997; Verma et al. Citation2015; Hoyos et al. Citation2016). Although species around water body (riparian plants) were commonly considered as shallow roots, especially due to high water table, the key species explored around springs in Indonesia are usually specific plants i.e. big trees with strong and deep roots (Fiqa et al. Citation2005; Sofiah and Fika Citation2010; Soejono and Arisoesilaningsih Citation2013; Trimanto Citation2013; Ridwan and Pamungkas Citation2015; Kali et al. Citation2015). However, different location of springs may have different vegetation structures or compositions because environmental characteristics determining species distribution (Ludwig and Reynolds Citation1988; Goslee et al. Citation1997). According to Billing (Citation1952) and Kimmins (Citation1987), physical environment variation is one of the determinants of organisms including plants distribution patterns. Therefore, it should be considered in the rehabilitation efforts.
Rehabilitation effort has been widely conducted in Indonesia through many programs of forest and land rehabilitation including springs area (KLHK Citation2017; BPDASHL Solo Citation2017). Official development assistance as applied in the forest greening in Korea (Park et al. Citation2017) has also been provided to support those programs. However, degraded spring areas have not been fully restored yet. A number of mass media (Arum Citation2013; Junaedi Citation2014; Isnanto Citation2017; Iskandar Citation2017) reported the occurrences of water crisis and the decrease of spring numbers where many springs dry/die in the area of Soloraya. Excessive water-drilling which is affecting water table (USGS Citation2018) might lead to drought and water crisis in certain regions. However, in rural people’s perception (traditional knowledge in Java), particular big trees around springs were often recognized for storing and protecting water (Trimanto Citation2013; Ridwan and Pamungkas Citation2015; Siswo et al. Citation2017) and even becoming natural water pumps (Riski Citation2015). One concrete case in Soloraya was the emergence of several springs around the roots of Ficus benjamina after 17 years of planted in Gendol hill (Siswo et al. Citation2017).
Phenomena of the spring occurrence after planting of some particular species around discharge area (dried springs) was very interesting to be investigated. Generally, studies on the effect of vegetation on water cycle or hydrological cycle were limited in term of interception, infiltration, and evapotranspiration which are recognized to have an effect on the recharge area. Several studies focusing on vegetation around springs has been actually carried out in Indonesia (Yulistyarini and Sofiah Citation2011; Soejono and Arisoesilaningsih Citation2013; Trimanto Citation2013; Solikin Citation2013; Ridwan and Pamungkas Citation2015; Kali et al. Citation2015). However, previous studies were commonly limited to species identification. This study observed the distribution of tree species around springs in various environmental conditions/factors and identified the trees-springs interplay possibility. This study highlighted the environmental factors to elevation, rainfall, and air temperature as the factors commonly determining species distribution. Tree-springs interplay possibility was examined from general conditions of trees and springs confirmed by local people statements.
Methods
Study site
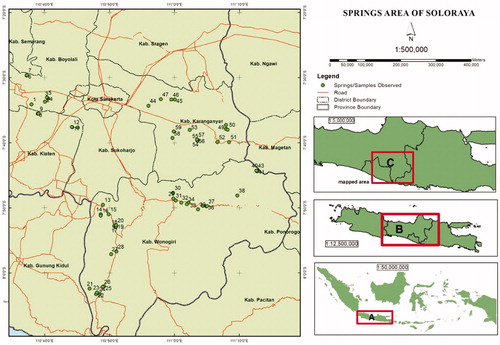
The study was conducted in the groundwater basin area of Soloraya, Central Java, Indonesia (). Plots/springs selected in this exploration were spread in Boyolali district (Teras, Mojosongo, and Banyudono county), Klaten district (Polanharjo and Wonosari county), Wonogiri district (Manyaran, Wuryantoro, Eromoko, Pracimantoro, Ngadirojo, Sidoharjo, Tremes, Jatisrono, Eromoko, Girimarto and Bulukerto county) and Karanganyar district (Jaten, Watujamus, Ngargoyoso, Tawangmangu, Matesih and Jumantono county).
According to BPSDA Bengawan Solo (Citation2017), many springs existed in the study site and utilized by local people. The utilization of spring by local people varied depending on the spring size. As listed in , many springs were used directly on the spot for bathing and taking water for domestic water needs. Some springs were channeled to settlements if the water discharge is possible to flow. Several springs were even used for commercial purposes such as bathing pool, fishing pond, irrigation, and drinking water company. Springs were spread in various environmental characteristics such as topography, lithology, soil type, elevation, rainfall, and air temperature. In lithology, the Soloraya areas are generally sedimentary and volcanic formations with flat, hilly, and mountainous topography (Surono and Sudarni Citation1992). Environmental factors highlighted in this study including elevation, annual rainfall, and temperature varied from 143 m to 1,212 m above sea level, 1,281 mm/year to 3.326 mm/year and 20 °C to 26 °C, respectively ().
Table 1. List of explored springs in the study site.
Data collection
To identify tree vegetation around springs, exploration survey was carried out on several selected areas where the springs exist based on the early information (BPSDA Bengawan Solo Citation2017). We selected springs covered by trees or previously overgrown by trees according to local people information. Vegetation survey was carried out using census method within 10 m radius above the point of spring as the spring protection zone (BPDASHL Solo Citation2017; Hendrayana Citation2013). Therefore, quadratic plots for trees vegetation survey (Barbour et al. Citation1987; Kusmana Citation1997) were created sizing 10 m × 10 m above the point of springs.
Vegetation survey included species characteristics and environmental data in the plots. All trees species with stem diameter (dbh) ≥ 10 cm and bamboo species were noted for the presence and absence, species name, number of species, number of individual and the diameter (dbh). Tree status was also recorded for normal/natural and broken/died condition. This survey included bamboo species because this species was often explored around springs in some previous studies (Soejono and Arisoesilaningsih Citation2013; Ridwan and Pamungkas Citation2015; Yuliantoro and Siswo Citation2016). In addition, bamboo was often found in high dominance because it was a clump/bundle of many species although the individual stem diameter was mostly less than 10 cm. Environmental parameters directly taken from the plots were position, elevation, springs size category, tree vegetation status, and springs condition. Other environmental factors such as rainfall and air temperature were collected from secondary data.
Spring size was classified based on the utilization of springs confirmed by local people information. Seepages and small springs directly utilized on the spot were classified as “small”, higher springs used and/or channeled by local people to their housing were categorized as “medium” and the springs used for commercial utilization such as bathing pool, fishing pond, irrigation, and drinking water company were classified as "big”.
Tree status was categorized as "degrade/damaged" if the key trees found were broken/fallen or died. Inversely, trees in natural condition were categorized “original”. Spring condition was classified based on local people confirmation whether the spring is still in the natural/normal or decreased flow according to their experiences/observations.
Data analysis
Geographical/environmental classification
To analyze the distribution of tree species around springs at various environmental categories, we first classified all sample sites into sample groups. The grouping was run using cluster analysis (Currel Citation2015) based on environmental factors (McCune and Grace Citation2002) including elevation, rainfall and average temperature as listed in . We also employed determinant analysis in the grouping to clarify the contribution of each factor (elevation, rainfall, and air temperature) as a description of group separation and the accuracy of grouping (Rencher Citation2002).
Vegetation analysis
General information of tree species distribution found in the survey was analyzed descriptively by calculating relative frequency (% of frequency) following (Kusmana Citation1997);
(1)
(1)
Where Fj is the number of plots where a species found divided by total plots
For deeper analysis, we calculated important value index (IVI) of all species in each plot reflecting species abundance/dominance (McCune and Grace Citation2002: Ping et al. Citation2015). According to McCune and Grace (Citation2002), important values are an average of two or more parameters on a relative basis of density, frequency, and dominance. Therefore, IVI was computed by summing relative density (% of individual number) and relative dominance (basal area) then divided by two following formulas:
(2)
(2)
(3)
(3)
(4)
(4)
Where RDy is relative density, RDo = relative dominance, nj is the number of individual species j, while BAj is the basal area of species j.
The species abundance supporting IVI data then was also the basis for computing diversity index (Shannon index) by following formula (Maguran Citation1988):
(5)
(5)
Where H′ is Shanon diversity index, n.i is the number of ith species, and N is the total number of species.
Indicator Species Analysis was employed to examine the trees specificity characterizing each geographical group (Dufrêne and Legendre Citation1997) using PC-ORD software (McCune and Grace Citation2002; Peck Citation2010). Indicator values were calculated for each species following Equationequation 6(6)
(6) , Equation7
(7)
(7) , Equation8
(8)
(8) , and Equation9
(9)
(9) then were analyzed for statistical significance of maximal indicator value (IVmax) using Montecarlo Test of significant.
(6)
(6)
(7)
(7)
(8)
(8)
(9)
(9)
Where kj is species j in group k, nk is the number of sample unit in group k, aijk is the abundance species j in sample unit i of group k, b is a matrix of presence (bij = a0ij).
Furthermore, ordination method was carried out to confirm the general relationship between the presence of tree species around springs and the environmental factors of species distribution highlighted (elevation, rainfall, and air temperature) by canonical correspondence analysis (TerBraak and Vendonschot Citation1995; McCune and Grace Citation2002) using PC-ORD software. Elevation was the factor determining species composition or distribution (Yamada Citation1990; Siebert Citation2005; Rozak and Gunawan Citation2015; Afrianto et al. Citation2017). Rainfall and air temperature were also the important factors for species distribution. According to Toledo et al. (Citation2012), climate was a stronger driver of species distribution than soils.
Analysis of trees-springs interplay possibility
Interplay possibility between trees and springs (water-flow) was analyzed by comparison and association analysis approached by direct observation confirmed by local people opinion. We applied a comparison test for density (number of individual trees), dominance (basal area) and diversity index (H′) among springs size category (small, medium and large).
Association analysis between tree status and water conditions (water-flow of springs) was analyzed using coefficient contingency analysis (Rencher 2002). This analysis was based on contingency table 2 × 2 (Trees: degraded and original, water-flow: decreased and normal).
Result and discussion
General characteristic of the study site
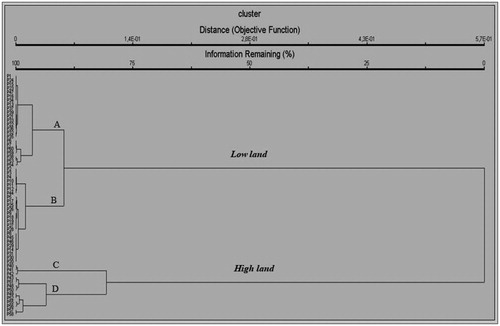
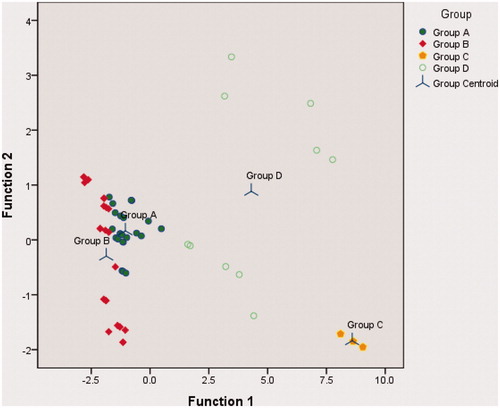
Sample plots surveyed were 59 springs distributed at various elevation, rainfall, and temperature. Hierarchical cluster analysis using Ward's method was run to classify the sample plots (springs sites) into several groups. At the medium cluster distance, the samples were classified into four (4) groups i.e. A, B, C and D where each group showed some differences in environmental conditions. In general, springs (samples) we observed could be summarized into two (2) groups reflecting lowlands and highlands i.e. (A + B) and (C + D), respectively ( and ).
Table 2. Summary of sample groups characteristics.
As shown in , discriminant analysis values satisfactorily strengthened the classification. The high value of “variance explained” and canonical correlation in function 1 reached 96.1% and 0.95, respectively. Discriminant analysis also presented the grouping accuracy (). This was very important to support decision making in prioritizing environmental factors when the restoration efforts will be implemented. Grouping provides direction on environmental suitability for plants growth. According to Billing (Citation1952) and Kimmins (Citation1987), physical environment variation is one of the factors determining organism including plants distribution pattern.
Trees species distribution
In general, trees found around springs (discharge area) in the Soloraya region were 21 species. As shown in , those species were distributed around springs both in lowlands (group 1) and highlands (group 2) with the number of species 19 and 10 respectively. F. benjamina from family Moraceae was widely distributed in the overall area shown by high value of relative frequency (80%). Relative frequency of F. benjamina also prevailed the springs areas in both lowlands and highlands shown by relative frequency value 80% and 77% respectively. This pattern was in line with some publications where banyan (F. Benjamina) was recognized growing in various conditions and elevations (Sosef et al. Citation1998; Yuliantoro et al. Citation2016) and became invasive species in several countries (Gilman and Waston Citation1993; Starr et al. Citation2003). As shown in , other species rather frequently found were S. saman, I. fagiferus, A. pinnata, Bambusa sp., A. elasticus and F. annulata shown by the value of relative frequency above 10%. While another species were rarely found in the exploration (less than 10% relative frequency) and some species were only found in one or two springs.
Table 3. Distribution of tree species from 59 springs explored in the springs areas of Soloraya reflected by Relative frequency and indicator value.
Mann Whitney U analysis () showed a comparison of species characteristics between lowlands and highlands. Density and diversity values in both group areas were in similar conditions while dominance value was different. However, the quality of the environment was not necessarily explained by these values due to the unknown age of trees and the existence of human factor. Several factors such as traditional culture and awareness of local community also play an important rule on water-flow sustainability (Siswadi et al. Citation2011) and forest protection (Tamalene et al. Citation2014).
Table 4. Summary of tree vegetation characteristics of each springs in lowlands and highlands for density, dominancy and diversity analyzed using Mann Whitney U Test.
The density value exhibited a high level of individual number reaching 643/ha and 531/ha for lowlands and highlands, respectively. The presence of bamboo species in several springs contributed to the similarity of diversity value. Bamboo was counted as a clump/bundle and considered as one individual in the calculation of diversity value and important value. Therefore, despite the similarity in the number of individuals, the average value of dominance (basal area) in both groups was different significantly (). In this case, diameter size determined the dominance (basal area) besides the number of individuals (VanLaar and Akca Citation2007). In the diversity aspect (H′), the average value of species diversity index at tree level in both areas shown in was categorized low. According to Shannon and Weaver (Citation1949), H′ value < 2.30 is categorized low.
Deeper understanding in relation to tree species distribution was expressed by species dependence on environmental categories shown by the value of indicator species analysis. As shown in , most of the species had no strong indicator value. Only three species (F. Benjamina, S. saman and A. elasticus) showed indicator value more than threshold value (25%) suggested by Dufrêne and Legendre (Citation1997). F. benjamina was widely distributed both in lowlands and highlands shown by high indicator value in both areas which were not significantly different. In lowlands, S. Saman was singleton species in group 1 (lowlands) showing high indicator value. A. elasticus was an indicator for highlands even though it was found in both areas. It was shown by high indicator value and statistical significance.
Some singleton species having indicator value close to threshold value were also concluded as dependent species of each area. I. Fagiferus was considered for lowlands and B. Javanica for highlands. Other species were not clearly indicating dependently due to infrequently found resulting low indicator value (<25). clearly showed that some infrequent species were a singleton for each group while another species were infrequent species in both areas. According to McCune and Grace (Citation2002), singleton and infrequent species have no possibility to be species indicator statistically significant.
Indicator value of species will provide guidance in selecting species planted in the restoration efforts to protect springs in different areas (lowlands and highlands). Indicator value of species reflected species preference to the environmental factors (TerBraak and Barendregt Citation1986) and shows the relationship with each other (McCune and Grace Citation2002). Therefore, tree species found having high indicator value in both lowlands and highlands such as F. benjamina can be a great choice. According to Soejono and Arisoesilaningsih (Citation2013), F. benjamina and some species of Moracea family were ecologically recommended for a rehabilitation program in various topographies. Whereas species having significant indicator value in one area such as A. elasticus in highlands, it will be reassuring to be planted in that area. Singleton species including S. saman, I. fagiferus, B. javanica, etc. () was also reasonable to be developed at each area. Commonly, distribution pattern of species depends on environmental factor (Billing Citation1952; Ludwig and Reynolds Citation1988; Goslee et al. Citation1997; Dwire et al. Citation2006).
In relation to environmental factors affecting plant distribution, ordination analysis (canonical correspondence analysis) showed the existence of general relationship between species distribution and environmental factors ( and ). There was a fairly strong correlation between presence/absence of particular species and the environmental factors (elevation, average rainfall, and average temperature) even though the “variance explained” was low. The correlation coefficients were −0.604, 0.538 and −0.647 for elevation, average rainfall, and average temperature respectively. The low value of “variance explained” was common for ecological data related to aspects of presence/absence. “Variance explained” value for ecological data analyzed using CCA is often low even less than 10% (Moller and Jenions Citation2002; Elliot et al. Citation2012). However, as shown in and , particular species were not correlated to elevation, average rainfall, and average temperature even though some of them were infrequent species.
Figure 4. Ordination graph for canonical correspondence analysis (CCA) of the trees around springs at various environmental factors (EL: elevation; AR: average of rainfall; AT: average of air temperature) in Soloraya. Abbreviation of species: FIBE: Ficus benyamina; SASA: Samanea saman; INFA: Inocarpus fagiferus; BIJA: Bischofia javanica; ARPI: Arenga pinata; BAMB: Bambusa sp; AREL: Artocarpus elasticus; FIAN: Ficus anulata; HITI: Hibiscus tiliaceus; BOCE: Bombax ceiba; TAIN: Tamarindus indica; SYDE: Syzigium densiflora; FIMI: Ficus microcarpa; PAED: Pangium edule; PIRE: Ficus retusa; LASI: Laportea sinuata; GADU: Garcinia dulcis; TECA: Terminalia catapa; CAIN: Calopylum inophylum; STEP: Sterculia poetida; LESS: Lesses.
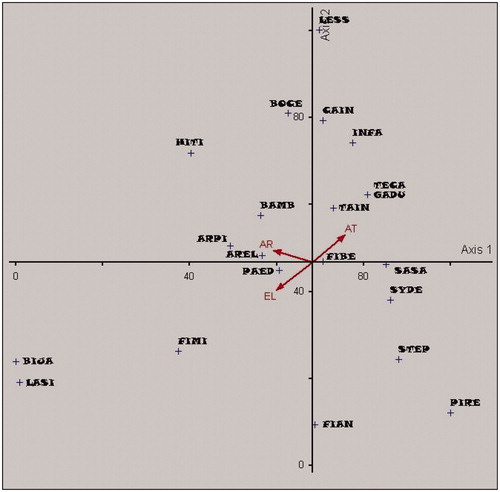
Table 5. Summary statistics of the three canonical correspondence analysis (CCA).
Species existed in both areas also indicated a possibility to be planted as the protective trees for springs. These species have been growing in the two areas for tens or even hundreds of years characterized by the big sizes. Species found also seem to have a big and strong of root system as explained by some previous researches (Fiqa et al. Citation2005; Sofiah and Fika Citation2010; Soejono and Arisoesilaningsih Citation2013; Trimanto Citation2013; Ridwan and Pamungkas Citation2015; Kali et al. Citation2015). According to Sosef et al. (Citation1998) and Yuliantoro and Siswo (Citation2016), species having a deep rooting system were usually able to grow in various conditions and elevations. Deep rooting plant species can extend their roots very deep underground and set it to the part of the ground where the water exists (Meinzer Citation1927; Canadell et al. Citation1996). Those specific characteristics will lead the species to grow easily including in marginal lands such as aquifer rock area or fractured rock where the springs/seepages exist. Therefore, those species can be a choice to be planted in the restoration of the degraded/died spring areas.
Trees-springs interplay possibility
Comparisons among springs categories (small, medium and large) showed differences for density and dominance but similar for diversity (). The similarity of diversity index implied that trees around springs were usually specific with a large in size (Fiqa et al. Citation2005; Yuliantoro and Siswo Citation2016). Human interferences including the role of traditional knowledge in both springs and trees protection might be the main factor of the existence of big trees around springs observed. Based on the local people information, most of the big trees protected in the original state were generally due to the local culture where the trees or springs were considered sacral. This indicated that without human disturbance, tree species were able to grow and survive well. The natural trees around spring commonly from genus Ficus have been known for having high survival ability in various conditions with long lifetime up to hundreds of years (Sastrapraja and Apriastini Citation1984). Thus, most key species around springs were not heavily dependent on the amount of water on the surface around springs (related and unrelated to spring sizes).
Table 6. Comparison of tree vegetation characteristics for density, dominancy and diversity at various levels of springs using Kruskall Wallis Test and Mann Whitney U Test as Post-Hoc Test.
Typical roots of deep-rooted trees enable such species to collect a large amount of groundwater very deep (David et al. Citation2013; Lubczynski Citation2009). Deep-rooted plants reaching aquifer can take water over a fissure/crack of rocks (Lewis and Burgy Citation1964). Therefore, particular tree species including riparian trees tend to consume groundwater rather than surface water (Richard et al. Citation2013) although water availability was a vital factor for tree growth (Zhu et al. Citation2009). According to Schwinning (Citation2010), plants water uptake is not only limited to the soil layer but also deeper from the rock layer or fractured rock/bedrock.
Fractured rocks can be a channel of groundwater flow (Selroos et al. Citation2002; Braeter Citation2009). Soft particles of soil and rocks existed between the roots and the rocks give a good contact for a groundwater flow (Zwieniecki and Newton Citation1995). Fractured rocks also might be a media where the groundwater flows from the aquifer to the surface in the form of a springs/seepage. According to Hendrayana (Citation2013), springs can emerge from depressed, perforated, porous and fractured aquifer rocks. On fractured aquifer-rocks overgrown by big trees, seepage/springs were often found around the roots (). For instance, in a case of the emergence of springs in Girimarto and Bulukerto, Wonogiri, Indonesia, the water flowed from the aquifer-rock trough the roots of some big trees planted where the local people explained that the water discharge increased following the growth of F. Benjamina (Siswo et al. Citation2017). A similar case also occurred in Wonosalam East Java where the villagers termed the trees as natural water pumps (Riski Citation2015).
Figure 5. Rooting system of the trees around springs. (a) Roots grown in fractured rock (b) water collected around the roots.

In connection to the above discussion, the second approach for interplay possibility of trees-springs showed an association between trees condition and the flowing of water. The association was known from Coefficient contingency analysis based on observation of trees condition and water-flow of springs confirmed by interview of local people. Coefficient contingency analysis presented a strong positive association shown by contingency coefficient reaching 0.57 followed by the statistically significant value (). There were several springs decreased in water-flow (water discharge) and even some of them became dry as the big trees around died/cut. As shown in , from total of 26 plots with damaged trees, 21 (81%) of them were decreased in the water discharge. Inversely, among the 33 plots of original status in the original status of tree vegetation (33 plots), there were 30 plots (88%) in the normal/original condition of water-flow.
Table 7. Contingency coefficient showed association between trees condition and water-flow.
Association relationship between tree status and spring conditions still needs further study especially on the possibility of deep-rooted trees on supporting water-flow sustainability in particular springs/seepages as believed by the local people. In some cases, big and deep-rooted trees were considered to be a driver causing the water-flow from groundwater stored through the springs/seepages by penetrating and enlarging the fractured aquifer-rock. Another process of the big and deep-rooted trees around springs might relate to the flow of water over the springs/seepage was the hydraulic lift process. Deep-rooted plants lift up water from the depth to the dry topsoil layer (Horton and Hart Citation1998; Burgess et al. Citation2001) at the night and consume it at the day. In this phenomenon, the lifted water might increase the volume of the collected water in the case of small springs (seepages) where the water was coming out and concentrated around the roots ().
Conclusion
Trees species around springs in Soloraya area were generally distributed on various environmental condition as well as the natural distribution pattern shown by relative frequency value, indicator value and ordination analysis using canonical correspondence analysis (CCA). There were three species categories found based on the frequency and value indicator: 1) frequent and infrequent species for all environmental condition both in lowlands and highlands, 2) frequent singleton species for one area, 3) infrequent singleton species for one area. Three species showed high-value indicator (>25) i.e. F. benjamina (both highlands and lowlands), S. saman (lowlands) and A. elasticus (highlands). F. benjamina was showing high frequency (80%) and widely distributed both in lowlands and highlands.
Particular species were obviously dependent on environmental factors. S. saman and I. fagiferus were the main dependent/indicator species for lowlands whereas A. elasticus and B. javanica were the indicators or dependent species for highlands. Some infrequent species were found as singleton of each group while other infrequent species were discovered in both areas. General distribution pattern of tree species around springs in Soloraya was also strengthened by species dependence on environmental factors indicated by the results of CCA analysis showing −0.604, 0.538 and −0.647 of correlation coefficients for elevation, average rainfall, and average temperature, respectively.
Tree species explored commonly big in size and having big-many-deep roots were mostly the remaining old trees protected by local people. Therefore, differences in the dominance of tree species could not be clearly concluded as the effect of the spring categories (spring sizes). In contrast, this study found an association between tree conditions and water-flow of springs where the spring condition decreased after the trees degraded (broken/died). This was shown by contingency coefficient reaching 0.57 which was statistically significant.
For future research, adding the number of samples and environmental gradients including spring characteristics were suggested in order to improve information on the distribution pattern of trees species around springs. Deeper investigation on the role of the trees around springs especially on the water-flow sustainability of the springs was also greatly suggested by considering the root characteristics.
Acknowledgements
Authors would like to thank Korea Forestry Promotion Institute (KoFPI) for providing graduate scholarship to the first author. Authors also thank Wika Ardiyanto, Lathif Brahmantya and Kusrin Sukamto from Watershed Management Technology Center for helping in data collection.
Disclosure statement
Authors declare no potential conflicts of interest with respect to the publication of this article.
Additional information
Funding
References
- Afrianto WF, Hikmat A, Widyatmoko D. 2017. Komunitas floristik dan suksesi vegetasi setelah erupsi 2010 di Gunung Merapi Jawa Tengah [Floristic community and vegetation succession after the 2010 eruption in Mount Merapi, Central Java]. J Biologi Indones. 12(2):265–276.
- Arum TS. 2013. September 24. Berburu air, warga Paranggupito Wonogiri Mengebor 98 meter [Water hunting, residents of Paranggupito Wonogiri Drill it to 98 meters]. Semarangpos. [accessed 2017 August 20]. http://m.semarangpos.com/2013/09/24/bencana-kekeringan-berburu-air-warga-paranggupito-wonogiri-mengebor-98-meter-45081
- Barbour MG, Burk JH, Pitts WD. 1987. Terrestrial plant ecology. Menlo Park: Benjamin/Cummings Publishing Co., Inc.
- Billing W. 1952. The environmental complex in relation to plant growth and distribution. Q Rev Biol. 27(3):251–265.
- BPDAS Solo. 2015. Laporan penyusunan data dan informasi DAS Solo tahun anggaran 2015 [Report on the compilation of the Solo Watershed Data and Information for 2015 s Fiscal Year]. Solo (SOC): Watershed Management Center of Solo (BPDAS Solo).
- BPDASHL Solo. 2017. Rancangan rehabilitasi hutan dan lahan di imbuhan mata air [Design of forest and land rehabilitation in the recharge area of springs]. Solo City, Indonesia: Balai Pengelolaan Daerah Aliran Sungai [Centre of Watershed Management and Protected Forest] Solo (BPDASHL Solo).
- BPS Boyolali. 2017. Kabupaten Boyolali dalam angka tahun 2016 [2016's Boyolali Regency in figures]. Boyolali: Badan Pusat Statistik Boyolali [Statistics center of Boyolali].
- BPS Karanganyar. 2017. Kabupaten Karanganyar dalam angka tahun 2016 [2016's Karanganyar Regency in figures]. Karanganyar: Badan Pusat Statistik Karanganyar [Statistics center of Karanganyar].
- BPS Klaten. 2017. Kabupaten Klaten dalam angka tahun 2016. [2016's Klaten Regency in figures]. Klaten: Badan Pusat Statistik Klaten [Statistics center of Klaten].
- BPS Wonogiri. 2017. Kabupaten Wonogiri dalam angka tahun 2016 [2016's Wonogiri Regency in figures]. Wonogiri: Badan Pusat Statistik Wonogri [Statistics center of Wonogiri].
- BPSDA Bengawan Solo. 2017. Informasi sumberdaya air; mata air: Surakarta. BPSDA Bengawan Solo [Public Works, Water resources, Human Settlements and Spatial Planning Center of Bangawan Solo]. [accesed 2018 July 2]. http://www.bpusdataru-bs.jatengprov.go.id/mata_air.php
- Bradshaw A. 1997. What do we mean by restoration. In: Urbanska KM, Webb NR, & Edwards PJ, editors. Restoration ecology and sustainable development. Cambridge: The Press Syndicate of The University of Cambridge; p. 8–13.
- Braeter C. 2009. Groundwater flow trough fractured rocks. Groundwater. 2:22–42.
- Burgess SS, Adams MA, Turner NC, White DA, Ong CK. 2001. Tree roots: conduits for deep recharge of soil water. Oecologia. 126(2):158–165.
- Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED. 1996. Maximum rooting depth of vegetation types at the global scale. Oecologia. 108(4):583–595.
- CLIMATE-DATA.ORG: Data iklim kota-kota di seluruh dunia [Climate data for cities around the world]. [accesed 2018 August 4]. www.id.climate-data.org
- Currel G. 2015. Scientific data analysis. New York: Oxford University Press.
- David TS, Pinto CA, Nadezhdina N, Kurz-Besson C, Henriques MO, Quilhó T, Cermak J, Chaves MM, Pereira JS, David JS. 2013. Root functioning, tree water use and hydraulic redistribution in Quercus suber trees: a modeling approach based on root sap flow. Forest Ecol Manag. 307:136–146.
- Duan L, Huang M, Zhang L. 2016. Differences in hydrological responses for different vegetation types on a steep slope on The Loess Plateu, China. J Hydrol. 537:356–366.
- Dufrêne M, Legendre P. 1997. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr. 67(3):345–366.
- Dwire K, Kauffman J, Baham J. 2006. Plant species distribution in relation to water-table depth and soil redox potential in montane riparian meadows. Wetlands. 26(1):131–146.
- Elliot D, Pierson J, Roman M. 2012. Relationship between environmental conditions and zooplankton community structure during summer hypoxia in the nothern Gulf of Mexico. J Plankton Res. 34(7):602–613.
- Fan J, Ostergaard KT, Guyot A, Fujiwara S, Lockington DA. 2016. Estimating groundwater evapotranspiration by a subtropical pine plantation using diurnal water table fluctuations: implication from night-time water use. J Hydrol. 542:679–685.
- Fiqa AP, Arisoesilaningsih E. Soejono 2005. Konservasi mata air DAS Brantas memanfaatkan diversitas flora Indonesis [Conservation of the Brantas watershed utilizes the diversity of Indonesian flora]. Seminar Basic Science II. FMIPA UNIBRAW.
- Foster S, Koundouri P, Tuinholf A, Kemper K, Nanni M, Garduno H. 2006. Groundwater dependent ecosystem; the challenge of balanced assesment and adequate conservation. Washington DC: World Bank.
- George RJ, Nulsen RA, Ferdowsian R, Raper GP. 1999. Interaction betseen trees and groundwaters in recharge and discharge areas-A survey of Western Australian sites. Agr Water Manage. 39(2–3):91–113.
- Gilman EF, Waston DG. 1993. Ficus benjamina weeping fig. Gainesville: University of Florida Fact Sheet ST–251.
- Goslee S, Bro.Ks R, Cole C. 1997. Plants as indicator of wetland water source. Plant Ecol. 131(2):199–206.
- Grielber C, Avramov M. 2014. Groundwater ecosystem services: a review. Freshw Sci. 34(1):355–367.
- Hendrayana H. 2013. Hidrogelologi mata air [Hydrogeology of springs]. Yogyakarta. Geological Engineering Dept., Faculty of Engineering, Gadjah Mada University.
- Horton, JL, Hart, SC. 1998. Hydraulic lift: a potentially important ecosystem process. Trends Ecol Evol. 13(6):232–235.
- Hoyos IC, Krakauer NY, Khanbilvardi R. 2016. Estimating the probability of vegetation to be groundwater dependent based on the elevation tree models. Environments. 3(9):1–21.
- Iskandar. 2017. Mata Air Soloraya Mati [Springs of Soloraya are dead]. Solopos. [accessed 2017 August 20]. news. [nasional]. http://news.solopos.com/read/20170819/496/844283/198-mata-air-soloraya-mati
- Isnanto BA. 2017. August 16. Kritis, 198 mata air di Surakarta mati dalam 10 tahun terakhir [Precarious, 198 springs in Surakarta have died in the last 10 years]. Detiknews. [accessed 2017 August 20]. berita-jawa-tengah:[detail berita]. http://www.m.detik.com/news/berita-jawa-tengah/d-3602309/kritis-198-mata-air-di-surakarta-mati-dalam-10-tahun-terakhir
- Junaedi E. 2014. 86 Kabupaten Kota di Indonesia Kekeringan [86 Regencies-cities in Indonesia are Drought]. Kompas. [accessed 2017 August 20];news:[regional]. https://regional.kompas.com/read/2014/09/17/22552601/86.Kabupaten.Kota.di.Indonesia.Kekeringan
- Kali FB, Kusuma Z, Leksono AS. 2015. Diversity of vegetation around the springs to support water resource conservation in Belu, East Nusa Tenggara, Indonesia. J Biodivers Environ Sci. 6(4):100–114.
- Kimmins JP. 1987. Forest ecology: the biological basis for management of forest resources. New York: Macmillan Publishing Company. Chapter 2D, The biotic environment; p. 305–338.
- KLHK. 2017. Statistik lingkungan dan kehutanan tahun 2016. [2016's Environmental and forestry statistics]. Jakarta: Kementerian Lingkungan Hidup dan Kehutanan (KLHK) [Ministry of Environment and Forestry].
- Kusmana C. 1997. Metode survey vegetasi [Vegetation Survey Method]. Bogor: Institut Pertanian Bogor (IPB).
- Le Mailtre DC, Scott DF, Colvin C. 1999. A review of information on interaction between vegetation and groundwater. Water SA. 25(2):137–152.
- Lewis DC, Burgy RH. 1964. The relationship between oak tree roots and groundwater in fractured rock as determined by tritium tracing. J Geophys Res. 69(12):2579–2588.
- Liu J, Clewell A. 2017. Management of ecological rehabilitation projects. Beijing: Science Press.
- Lubczynski MW. 2009. The hydrogeological role of trees in water-limited environments. Hydrogeol J. 17(1):247.
- Ludwig J, Reynolds J. 1988. Statistical ecology; a primer on methods and computing. New York: John Wiley and Sons.
- MacDonald A, Davies J. 2005. A brief review of groundwater for rural water supply in sub-Saharan Africa. Nottingham: Britis Geological survey (BGS).
- Maguran AE. 1988. Ecological diversity and its measurement. Pricenton: Pricenton University Press.
- McCune B, Grace JB. 2002. Analysis of ecological communities. Oregon: MJM software.
- Meinzer OE. 1927. Plants as indicator of ground water. Washington: United States Government Printing Office.
- Moller AP, Jenions MD. 2002. How much variance can explained by acologist and evolutionary biologist? Oecologia. 132(4):492–500.
- Park H, Lee JY, Song M. 2017. Scientific activities responsible for successful forest greening in Korea. Forest Sci Technol. 13(1):1–8.
- Peck JE. 2010. Multivariate analysis for community ecologists. Oregon: MjM Software Design.
- Ping A, Xiangjun L, Yuanrun Z, Egrinya EA, Yunus Q, Mingqing Z, Shinobu I. 2015. Distribution of plant species and species-soil relationship in the east central Gurbangtunggut Desert, China. J Geogr Sci. 25(1):101–112.
- Pramono IB, Budiastuti MT, Gunawan T., Wiryanto 2017. Base flow from various area of pine forest at Kedungbulus sub watershed, Kebumen District, Central Java, Indonesia. Int J Dev Sust. 6(3):99–114.
- Rencher A. 2002. Methods of multivariate analysis. New York: John Wilwy & Sons, Inc.
- Richard, A, Galle, S, Descloitres, M, Cohard, JM, Vandervaere, JP, Séguis, L, Peugeot, C. 2013. Interplay of riparian forest and groundwater in the hillslope hydrology of Sudanian West Africa (northern Benin). Hydrol Earth Syst Sci. 17(12):5079–5096.
- Ridwan M, Pamungkas DW. 2015. Diversity of trees around the springs in Panekan Sub-District, Magetan, East Java. Surakarta. Society for Indonesian Biodiversity. Pros Sem Nas Masy BIiodiv Indon. 1(6):1375–1379.
- Riski P. 2015. Belajar konservasi hutan dan mata air di Wonosalam [Learning forest and springs conservation in Wonosalam]. Mongabay; Situs Berita Lingkungan. [accessed 2018 May 25]. http://www.mongabay.co.id/2015/04/30/belajar-konservasi-hutan-dan-mata-air-di-wonosalam/
- Rozak AH, Gunawan H. 2015. Altitudinal gradient affects on trees and stand attributes in Mount Ciremai National Park, West Java, Indonesia. JPKW. 4(2):93–99.
- Sastrapraja S, Afriastini JJ. 1984. Kerabat seri beringin sumber daya alam [Relatives of the banyan series of natural resources]. Bogor. Lembaga Biologi Nasional
- Scanlon BR, Healy RW, Cook PG. 2002. Choosing appropriate techniques for quantifying groundwater recharge. Hydrogeol J. 10(1):18–39.
- Schwinning S. 2010. The ecohydrology of roots in rocks. Ecohydrology. 3(2):238–245.
- Selroos J-O, Walker DD, Ström A, Gylling B, Follin S. 2002. Comparison of alternative modelling approaches for groundwater flow in fractured rock. J Hydrol. 257(1–4):174–188.
- Shannon C, Weaver W. 1949. The mathematical theori of communication. Champaign: Urbana: University of Illonis Press.
- Siebert SF. 2005. The abundance and distribution of rattan over an elevation gradient in Sulawesi, Indonesia. Forest Ecol Manag. 210(1–3):143–158.
- Siswadi S, Taruna T, Purnaweni H. 2011. Kearifan lokal dalam melestarikan mata air (studi kasus di Desa Purwogondo Kecamatan Boja Kabupaten Kendal) [Local wisdom in preserving springs (case study in Purwogondo Village, Boja County, Kendal District)]. J Ilmu Lingk. 9(2):63–68.
- Siswo, Yuliantoro D, Atmoko BD, Yun CW. 2017. Benefit of Moraceae Family Trees in Emergence of Springs; Local Knowledge Perception (Case Study from Gendol Hill, Bulukerto, Wonogiri, Central Java, Indonesia). Koreaan Soc Environ Con. 27(2):8–9. Oct 26–27, Sancheong, South Korea: Koreaan Society of Environment and Ecology.
- Soejono BS, Arisoesilaningsih E. 2013. Proposing local trees diversity for rehabilitation of degraded lowlands areas surrounding springs. Biodiversitas. 14(1):37–42.
- Sofiah S, Fika AP. 2010. Jenis-jenis pohon disekitar mata air dataran tinggi dan rendah (studi kasus Kabupaten Malang) [Tree species around springs at highlands and lowlands (case study of Malang Regency)]. J Berkala Penelitian Hayati Ed Khusus. 4A:1–3.
- Solikin. 2013. Diversity of bamboos around springs in Malang East Java. J Bio Res. 19:38–42.
- Sosef MS, Hong LT, Prawirohatmodjo S. 1998. Plant Resources of South-East Asia. Backhuys.
- Starr F, Starr K, Loope L. 2003. Ficus benjamina. Maui: Haleakala Field Station, US Geological Survey Biological Sciences Division (US); [accessed 2018 June 26]. http://www.hear.org/starr/hiplants/reports/htm/ficus_benjamina.htm
- Sudarmadji S, Darmanto D, Widyastuti M, Lestari S. 2015. Pengelolaan mata air untuk penyediaan air rumah tangga berkelanjutan di lereng gunung merapi [Management of springs for the supply of sustainable household water on the slopes of Mount Merapi]. J Man Ling. 23(1):102–110.
- Surono TB, Sudarni I. 1992. Peta Geologi Regional Lembar Surakarta-Giritontro, Jawa [Geological map of the Surakarta-Giritontro Quadrangles, Jawa]. Pusat Penelitian dan Pengembangan Geologi Bandung.
- Tamalene M, Al Muhdar M, Surasini E, Rochman F. 2014. The practice of local wisdom of Tobelo Dalam (Togutil) tribal community in forest conservation in Halmahera, Indonesia. Int J Plant Res. 4(4A):1–7.
- TerBraak CJ, Barendregt L. 1986. Weighted averaging of specie indicator values: its efficiency in environmental calibration. Math Biosci. 78(1):57–72.
- TerBraak C, Vendonschot P. 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci. 57(3):255–289.
- Toledo M, Peña-Claros M, Bongers F, Alarcón A, Balcázar J, Chuviña J, Leaño C, Licona JC, Poorter L. 2012. Distribution patterns of tropical woody species in response to climatic and edaphic gradients. J Ecol. 100(1):253–263.
- Trimanto. 2013. Diversitas pohon sekitar aliran mata air di kawasan Pulau Moyo Nusa Tenggara Barat. Surakarta: FKIP Universitas Sebelas Maret. Pros Sem Biol. 10(2):434–438.
- University of Calgary. 2012. Groundwater Discharge. Calgary (CAN): University of Calgary. (Groundwater Connection Fact Sheet Series; p. 1–5).
- USGS: USGS Science for a Changing world. 2018. [accessed 2018 October 17]. http://water.usgs.gov/edu/earthgwaquifer.html
- VanLaar A, Akca A. 2007. Forest mensuration (Vol.13). Dordrecht: Springer Science & Bussines Media.
- Verma R, Jayanti T, Vinoda S, Shivappa A. 2015. Tree species as indicators of groundwater recharge and discharge. Int J Eng Tech Res. 3(11):127–135.
- Wang C, Zhao C, Xu Z, Wang Y, Peng H. 2013. Effect of vegetation on soil water retention and storage in semi-arid alpine forest catchment. J Arid Land. 5(2):207–219.
- Wei XH, Sun G, Liu S, Jiang H, Zhou G, Dai L. 2008. The forest-streamflow relationship in China: a 40-years retrospect. J Am Water Resour As. 44(5):1076–1085.
- Weiler M. 2005. An infiltration model based on flow variability in macropores: development, sensitivity analysis and applications. J Hydrol. 310(1–4):294–315.
- Wenjie L, Pengju L, Hongmei L, Weinping D. 2006. Estimation of evaporation rate from soil surface using stable isotopic composition of throughfall and stream water in a tropical seasonal rain forest of Xishuangbanna, Southwest China. Acta Ecol Sinica. 26(5):1303–1310.
- Winter TC. 2001. The concept of Hydrologic Landscapes. J Am Water Resources Assoc. 37(2):335–349.
- Winter TC, Harvey JW, Franke OL, Alley WM. 1998. Ground water and surface water a single resource. Colorado: USGS.
- Wu GL, Liu Y, Yang Z, Cui Z, Deng L, Chang XF, Shi ZH. 2017. Root channals to indicate the increase in soil matrix water infiltration capacity of arid reclamed mine soils. J Forest Hydrol. 546:133–139.
- Yamada I. 1990. The changing pattern of vertical stratification along an altitudinal gradient of the forests of Mt Pangrango, West Java. In: The plant diversity of Malesia. Dordrecht: Springer; p. 177–191.
- Yuliantoro D, Atmoko BD, Siswo. 2016. Pohon Sahabat Air [Trees, water mate]. Surakarta: BPPTPDAS [Watersheed Management Technology Center (WMTC)].
- Yulistyarini T, Sofiah S. 2011. Valuing quality of vegetation in recharge area of seruk spring, Pesanggrahan Valley, Batu City, East Java. Biodiversitas. 12(4):229–234.
- Zhang YK, Schilling KE. 2006. Increasing streamflow and baseflow in Missisippi River since 1940 s: effect of land use change. J Hydrol. 324(1–4):412–422.
- Zhu Y, Ren L, Skaggs T, Lu H, Yu Z, Wu Y, Fang X. 2009. Simulation of Populus euphratica root uptake of groundwater in an arid woodland of the Ejina Basin, China. Hydrol Process. 23:2460–2469.
- Zwieniecki MA, Newton M. 1995. Roots growing in rock fissures: their morphological adaptation. Plant Soil. 172(2):181–187.