Abstract
The effects of gaseous chlorine dioxide fumigation treatment on the quality of Actinidia arguta “Daebo,” a hardy kiwifruit, were investigated. Hardy kiwifruit was fumigated with 30 mg L−1 chlorine dioxide gas for 30 min and stored at 2 °C for 5 weeks. Fruit firmness rapidly decreased the first 2 weeks of storage after fumigation, whereas the content of soluble solids increased. However, the firmness of chlorine dioxide-treated fruits was significantly higher than that of control fruits after 2 weeks of storage, indicating that the treatment helped to maintain the firmness of hardy kiwifruit. The changes in soluble solids content and titratable acidity during storage suggested that the ripening process of the hardy kiwifruit was delayed in gas-treated fruit. Moreover, chlorine dioxide gas treatment reduced the decay incidence and population of microorganisms on fruit surface. These results suggest that treatment with gaseous chlorine dioxide alleviates the deterioration of fruit quality in “Daebo” hardy kiwifruit during cold storage.
Introduction
Hardy kiwifruit (Actinidia arguta), a member of the Actinidiaceae family and a native to Korea, Japan, and north China, is commercially cultivated in New Zealand, USA, Poland, and some other countries (Fisk et al. Citation2008; Latocha et al. Citation2014; Leontowicz et al. Citation2016). In Korea, breeding programs have produced new cultivars of hardy kiwifruit since 2006, such as “Cheongsan,” “Saehan,” “Daesung,” “Chilbo,” “Daebo” and “Autumn Sense,” and their cultivation areas have extended, mainly in Kangwon-do Province (Lim et al. Citation2016). Hardy kiwifruits are smaller than fuzzy kiwifruit (A. deliciosa) and have thin, smooth, and edible skin (Fisk et al. Citation2006). The fruit is known for its high nutritional value as it is rich in vitamin C, lutein, phenolics, and some minerals, especially P, Ca, Fe, and Zn (Nishiyama et al. Citation2004; Latocha and Krupa Citation2008; Latocha et al. Citation2010).
The storage life of hardy kiwifruits is only 1–2 months at 0 °C, within which period they can be consumed fresh or later on used for jam and wine production (Strik and Hummer Citation2006; Latocha et al. Citation2014). Softening, skin pitting, and wrinkling of fruits, which are the most significant disorders during storage, are the main contributors to the short shelf life and loss of quality (Guroo et al. Citation2017; Kim et al. Citation2017). Several studies have investigated the potential of different treatments to prolong storage time of hardy kiwifruits, such as treatment with 1-methylcyclopropene or edible coating, and have identified the effects of controlled atmosphere on hardy kiwifruits (Latocha et al. Citation2014; Kaya et al. Citation2016; Lim et al. Citation2016). Kiwifruit is exposed to fungal pathogen during postharvest shipping and marketing that causes considerable economic losses (Chen et al. Citation2015). Chitosan treatment significantly inhibits gray and blue mold development and maintains their quality during cold and room-temperature storage in kiwifruits (Zheng et al. Citation2017).
Chlorine dioxide (ClO2) is one of the disinfectants used increasingly to control microbiological growth in a number of different industries (Wu and Rioux Citation2010). ClO2 oxidizes both the cell membrane and DNA/RNA and interrupts protein synthesis, disrupting the metabolism of bacterial cells and thereby reducing bacterial and fungal populations (Buschini et al. Citation2004; Sun et al. Citation2014; Praeger et al. Citation2016). Its gaseous and aqueous formulations have been used widely in harvested fruits and vegetables to remove microorganisms, as they do not leave any residue on foodstuff (Kaur et al. Citation2015; Smith et al. Citation2015). Gaseous ClO2 is effective in reducing the production of peroxidases and polyphenol oxidases, which are involved in fruit softening, and inhibits ethylene production by reducing the expression of genes related to ethylene biosynthesis (Wang et al. Citation2011; Guo et al. Citation2014). Additionally, its gaseous form is more effective in inactivating pathogenic cells attached to inaccessible parts as it offers better diffusivity and penetrability than the aqueous form (Wu and Kim Citation2007; Lee et al. Citation2015).
Gaseous form of ClO2 is also effective in killing pathogens because of its ability to penetrate the biofilm of hazardous microorganisms (Nam et al. Citation2014). The efficacy of the fumigation treatment with ClO2 gas has been verified in various crops such as apple (Du et al. Citation2003; Sy et al. Citation2005), blueberries (Kingsley et al. Citation2018), carrots (Gomez-Lopez et al. Citation2008), and tomatoes (Park et al. Citation2018). However, the effects of ClO2 fumigation on hardy kiwifruits are yet to be identified. Therefore, the objective of this study was to investigate the effects of fumigation with gaseous ClO2 on quality changes and decay control of hardy kiwifruits.
Materials and methods
Plant material
The hardy kiwifruit (Actinidia arguta) cultivar “Daebo” was harvested in September 2018 from Wonju, Gangwon-do, Korea (). Fruit of uniform size, similar maturity, and no physical damages were selected. They were randomly packed in sixty plastic containers (92 × 92 × 63 mm), nine fruits per one container. Thirty containers were fumigated with chlorine dioxide and the other 30 containers, used as a control, were not exposed to fumigation. Both groups of containers were stored at 2 ± 1 °C in ambient conditions with relative humidity of 75–80%.
ClO2 gas was generated by a chlorine dioxide generator (CA-300, Purgofarm Inc., Hwaseong, Korea; ) using the electrochemical method described by Gates (Citation1998). Briefly, highly pure ClO2 gas produced by the aqueous NaClO2 was passed through a multi-porous membrane electrode assembly. After a series of electrochemical reactions, the gas flowed through a vent into a collection chamber. The gas stream was controlled by a regulator valve to maintain the concentration of ClO2 gas in the chamber (Analytical Technology, Inc, PA, USA) at 30 mg L−1 for 30 min.
Determination of fruit quality parameters
Firmness, soluble solids content (SSC), and titratable acidity of the fruit were determined every week of storage. Flesh firmness was measured by the penetration test with a 5 mmΦ probe to a depth of 10 mm using a rheometer (CR-3000EX-S; Sun Scientific Co., Japan) and defined as the maximum penetration force (N). Fruit juice was extracted by homogenizing three samples in a juicer. The SSC (%) was measured with a digital refractometer (RA-510; Kyoto Electronics MFG Co., Japan). Titratable acidity was determined using a titratable acidity meter (GMK-835N; G-WON Hitech, Seoul, Korea), and the results were expressed as the percentage of anhydrous citric acid. Soluble solid–acid ratio can be used as an efficient instrumental method to determine acceptability by consumers and reveal the taste preference for hardy kiwifruit (Jayasena and Cameron Citation2007). The ratio values were calculated as the ratio between the SSC and acid level.
Measurement of total phenolic content
Total phenolic contents were determined using Folin & Ciocalteu’s phenol reagent (Singleton et al. Citation1999). A sample (5 g) of fresh fruit was extracted in 30 mL of 100% methanol and homogenized for 1 min in a mixer. The mixture was stirred for 1 h in the dark at room temperature. Absorption was measured by a NanoPhotometer (NP80; Implen, Munich, Germany) at 735 nm. All the reactions were performed in triplicate. The total phenolic contents were expressed in gallic acid equivalent (GAE) per kilogram fresh weight. The quantification was performed using a standard curve between 50 and 200 mg L−1 of gallic acid.
Analysis of decay incidence and total microorganism counts
Decay incidence was determined after 1 and 5 weeks and represented by the percentage of infected or moldy fruit. Three grams of hardy kiwifruit pericarp was subjected to mix with 27 mL of distilled water. The obtained solution was then used to prepare a 3-fold serial dilution. Each diluent (1 mL) was inoculated in triplicate on 3 M Petrifilm Aerobic Count Plates and 3 M Petrifilm Yeast and Mold Count Plates (3 M, MI, USA) and incubated at 37 °C for 48 h and 25 °C for 5 days, respectively. After incubation, total microorganisms were counted in red-, blue-, and black-colored colonies for total bacterial, yeast, and mold count, respectively. The results were expressed in log colony forming units (CFU) per gram of hardy kiwifruit pericarp.
Statistical analysis
All data are reported as mean ± standard error of the mean. The figures were produced using Sigma Plot 12.0 software (Systat Software, CA, USA). Independent t-test, using SPSS statistical software version 18 (SPSS, Inc., Chicago, IL, USA), was used for comparison between the control and treatments. A difference was considered to be statistically significant for p < .05.
Results and discussion
The quality changes (firmness, SSC, titratable acidity, and soluble solid–acid ratio) in hardy kiwifruit treated with ClO2 gas are shown in . The firmness showed sharply declined within 2 weeks of storage after ClO2 fumigation, from 20.4 N to 6.5 N in the control and 7.9 N in treated fruits. This decline in firmness was attributed to ethylene, which is generated through fruit respiration and whose presence generally leads to fruit softening. The firmness of ClO2 gas-treated fruits was higher than that of the control starting from the second to the third week of storage. ClO2 reduces fruit metabolism, thereby inhibiting weight loss and maintaining the firmness in the fruit (Gomez-Lopez et al. Citation2008). ClO2 gas fumigation has been shown to preserve fruit firmness in many plants, including tomato, strawberry, and melon (Aday and Caner Citation2011; Guo et al. Citation2013; Sun et al. Citation2017). Additionally, Guo et al. (Citation2014) reported that the expression of ethylene biosynthesis-related genes, including LeACS2, LeACS4, and LeACO1, was reduced by ClO2 treatment in tomato fruit. However, there is insufficient evidence that the treatment inhibits ethylene production in hardy kiwifruit and further experimental studies are required to demonstrate the effect of ethylene production.
Figure 3. Quality changes in hardy kiwifruit stored at 2 °C and treated with chlorine dioxide (ClO2) and non-treated control. (A) Firmness (N); (B) Soluble solid content (%); (C) Titratable acidity (%); (D) ratio of soluble solid–acid. Vertical bars represent standard error of the means. *, ** represent significant difference according to the independent t-test at p < .05 and .01, respectively.
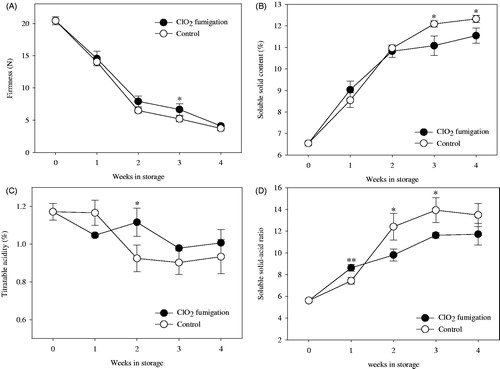
The SSC in the control and ClO2-treated fruit was sharply increased from 6.5% to 11.0% and 10.8%, respectively, with decreasing firmness in the first two weeks of cold storage, which is the time when the ripening process starts after the harvest of hardy kiwifruit. Similar increase in SSC during storage was reported in the hardy kiwifruit cultivars Weiki, 74-49, and D14 (Fisk et al. Citation2006; Krupa et al. Citation2011). In contrast, SSC was significantly lower in ClO2-treated fruits than in the control from third week of storage (p < .05); SSC level did not increase significantly while the fruit maintained its firmness, suggesting that ClO2-treated fruits experienced a delayed ripening process. Similarly, Guo et al. (Citation2013) reported a significantly lower SSC in ClO2-treated fruit than in untreated fruit after 3 days of storage, whereas Kim et al. (Citation2017) noted that ClO2 gas fumigation did not affect SSC in bell pepper.
The titratable acidity of hardy kiwifruit within the range from 1.2% to 0.9% of citric acid equivalent was generally decreased during storage; there was no significant difference between ClO2 gas-treated and control fruit and titratable acidity was not correlated with storage time (). A slightly higher acidity was noted only for ClO2-treated fruits after 2 weeks of storage when compared with the acidity of the control. Krupa et al. (Citation2011) reported a similar continuous decrease in titratable acidity in three cultivars of hardy kiwifruit during storage period. Soluble solid–acid ratio is commonly used as a quality index of fruits (Robertson et al. Citation1992). In plum, the soluble solid–acid ratio ranges between 13.0 and 19.0 and is significantly different among cultivars (Milošević and Miloševi Citation2012). In the present study, the soluble solid–acid ratio in control fruit increased rapidly from 5.6 to 12.4 in the first two weeks, reaching the maximum of 13.9 at 3 weeks of storage. The ratio between SSC and acid in ClO2-treated fruit increased slightly from 5.6 to 11.7 during storage; however, the value continuously increased until the end of the observations. The increasing soluble solid–acid ratio is indicative of the ripening process in hardy kiwifruit after harvest. The different pattern of the changes in the soluble solid–acid ratio between ClO2-treated and control fruit may be caused by ClO2 gas fumigation, which affects the ripening process of hardy kiwifruit.
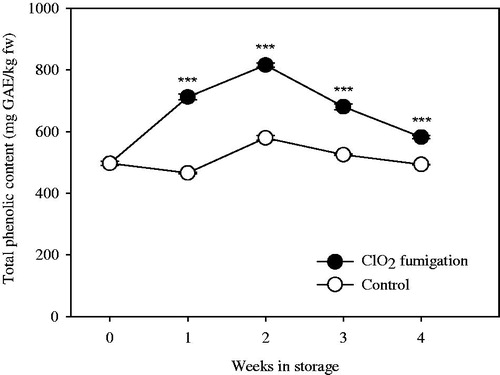
The average total phenolic content in control and ClO2-treated fruits was 512.2 ± 43.0 mg GAE/kg fresh weight and 657.5 ± 122.4 mg GAE/kg fresh weight, respectively. The total phenolic contents were significantly higher in ClO2-treated fruit than in the control during the entire storage period (p < .05; ). In ClO2-treated fruit, the total phenolic content increased sharply in the first two weeks and decreased afterward, whereas the total phenolic content in the control remained constant during storage. Gaseous ClO2 can react with phenolic contents in fruits and vegetables, which may cause nutritional changes (Napolitano et al. Citation2005). The total phenolic contents in Red Delicious and Granny Smith apples that were dipped in a ClO2 solution (Remorini et al. Citation2015) and in ClO2-fumigated “Daw” longan fruit (Chomkitichai et al. Citation2014) were higher compared with their levels in the respective non-treated fruits during storage period, which is consistent with our results.
Figure 4. Effects of fumigation with chlorine dioxide gas on total phenolic content of hardy kiwifruit during storage. Vertical bars represent standard error of the means. ***represents significant difference according to the independent t-test at p < .001.
The decay incidence and mean populations of microorganisms are listed in . Fruit treated with ClO2 gas showed a 1.5-fold lower decay incidence compared with the control hardy kiwifruit stored at 2 °C. Trinetta et al. (Citation2013) reported a significant reduction in tomato decay caused by Alternaria alternata and Stemphylium vesicarium in tomato fruit treated with ClO2 gas. The populations of aerobic bacteria, aerobic yeast, and mold in hardy kiwifruit were 3.51, 3.94, and 3.13 log CFU/g in the control and 2.50, 2.50, and 2.50 log CFU/g in ClO2-treated fruit, respectively, after 5 weeks of storage. The mean population size of the bacteria, yeast, and mold was about 1.5-fold higher in control than in ClO2-fumigated fruit. Many fruits and vegetables, such as apple, blueberry, green pepper, and tomato showed a considerable log reduction in various foodborne pathogens due to ClO2 gas fumigation (Han et al. Citation2001; Du et al. Citation2007; Popa et al. Citation2007; Park et al. Citation2018). Gaseous ClO2 fumigation treatment was effective in the control of decay incidence and reduction of microorganisms in hardy kiwifruit.
Table 1. Percentage decay and microbial populations (log colony forming units, log CFU/g) according to the ClO2 treatment in hardy kiwifruit stored at 2 °C for 5 weeks.
Conclusion
This study demonstrated that postharvest treatment with gaseous chlorine dioxide fumigation significantly affected the quality changes of “Daebo” hardy kiwifruit. Decay incidence and growth of microorganisms on fruit were effectively reduced during cold storage. Moreover, gas treatment retarded the ripening process of hardy kiwifruit by maintaining fruit quality such as firmness, soluble solid content, and titratable acidity. Further studies using increasing gas concentrations above 30 mg L−1 and extending the fumigation over 30 min might help to assess the optimal concentration and optimal duration of gaseous chlorine dioxide fumigation of hardy kiwifruit and thus provide more specific results for decay control of this fruit.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aday MS, Caner C. 2011. The applications of “active packaging and chlorine dioxide” for extended shelf life of fresh strawberries. Packag Technol Sci. 24(3):123–136.
- Buschini A, Carboni P, Furlini M, Poli P, Rossi C. 2004. Sodium hypochlorite-, chlorine dioxide- and peracetic acid-induced genotoxicity detected by the Comet assay and Saccharomyces cerevisiae D7 test. Mutagenesis. 19(2):157–162.
- Chen H, Cheng Z, Wisniewski M, Liu Y, Liu J. 2015. Ecofriendly hot water treatment reduces postharvest decay and elicits defense response in kiwifruit. Environ Sci Pollut Res Int. 22(19):15037–15045.
- Chomkitichai W, Faiyue B, Rachtanapun P, Uthaibutra J, Saengnil K. 2014. Enhancement of the antioxidant defense system of post-harvested ‘Daw’ longan fruit by chlorine dioxide fumigation. Sci Hortic. 178:138–144.
- Du J-H, Fu M-R, Li M-M, Xia W. 2007. Effects of chlorine dioxide gas on postharvest physiology and storage quality of green bell pepper (Capsicum frutescens L. Var. Longrum). Agric Sci China. 6(2):214–219.
- Du J, Han Y, Linton RH. 2003. Efficacy of chlorine dioxide gas in reducing Escherichia coli O157:H7 on apple surfaces. Food Microbiol. 20(5):583–591.
- Fisk CL, McDaniel MR, Strik BC, Zhao Y. 2006. Physicochemical, sensory, and nutritive qualities of hardy kiwifruit (Actinidia arguta ‘Ananasnaya’) as affected by harvest maturity and storage. J Food Sci. 71(3):S204–S210.
- Fisk CL, Silver AM, Strik BC, Zhao Y. 2008. Postharvest quality of hardy kiwifruit (Actinidia arguta ‘Ananasnaya’) associated with packaging and storage conditions. Postharvest. Biol Technol. 47(3):338–345.
- Gates DJ. 1998. Chlorine dioxide handbook (Water disinfection series). Colorado (CO): American Water Works Association. p. 186.
- Gomez-Lopez VM, Ragaert P, Jeyachchandran V, Debevere J, Devlieghere F. 2008. Shelf-life of minimally processed lettuce and cabbage treated with gaseous chlorine dioxide and cysteine. Int J Food Microbiol. 121(1):74–83.
- Guo Q, Lv X, Xu F, Zhang Y, Wang J, Lin H, Wu B. 2013. Chlorine dioxide treatment decreases respiration and ethylene synthesis in fresh-cut ‘Hami’ melon fruit. Int J Food Sci Technol. 48(9):1775–1782.
- Guo Q, Wu B, Peng X, Wang J, Li Q, Jin J, Ha Y. 2014. Effects of chlorine dioxide treatment on respiration rate and ethylene synthesis of postharvest tomato fruit. Postharvest Biol Technol. 93:9–14.
- Guroo I, Wani SA, Ahmad M, Mir SA, Masoodi FA. 2017. A review of production and processing of kiwifruit. J Food Process Technol. 8:699.
- Han Y, Linton RH, Nielsen SS, Nelson PE. 2001. Reduction of Listeria monocytogenes on green peppers (Capsicum annuum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7 degrees C. J Food Prot. 64(11):1730–1738.
- Jayasena V, Cameron I. 2007. °Brix/acid ratio as a predictor of consumer acceptability of crimson seedless table grapes. J Food Qual. 31(6):736–750.
- Kaur S, Smith DJ, Morgan MT. 2015. Chloroxyanion residue quantification in cantaloupes treated with chlorine dioxide gas. J Food Prot. 78(9):1708–1718.
- Kaya M, Česonienė L, Daubaras R, Leskauskaitė D, Zabulionė D. 2016. Chitosan coating of red kiwifruit (Actinidia melanandra) for extending of the shelf life. Int J Biol Macromol. 85:355–360.
- Kim GH, Kim DR, Park S-Y, Lee YS, Jung JS, Koh YJ. 2017. Incidence rates of major diseases of kiwiberry in 2015 and 2016. Plant Pathol J. 33(4):434–439.
- Kingsley DH, Perez-Perez RE, Niemira BA, Fan X. 2018. Evaluation of gaseous chlorine dioxide for the inactivation of Tulane virus on blueberries. International Journal of Food microbiological safety and quality of foods: a review. J Food Prot. 62(9):1071–1087.
- Krupa T, Latocha P, Liwińska A. 2011. Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta its hybrid) during storage. Sci Hortic. 130(2):410–417.
- Latocha P, Krupa T. 2008. The mineral composition of new genotypes of hardy kiwifruit (Actinidia Lindl.) bred at SGGW. Horticult Landsc Architect. 29:105–110.
- Latocha P, Krupa T, Wołosiak R, Worobiej E, Wilczak J. 2010. Antioxidant activity and chemical difference in fruit of different Actinidia sp. Int J Food Sci Nutr. 61(4):381–394.
- Latocha P, Krupa T, Jankowski P, Radzanowska J. 2014. Changes in postharvest physicochemical and sensory characteristics of hardy kiwifruit (Actinidia arguta and its hybrid) after cold storage under normal versus controlled atmosphere. Postharvest Biol Technol. 88:21–33.
- Lee Y, Burgess G, Rubino M, Auras R. 2015. Reaction and diffusion of chlorine dioxide gas under dark and light conditions at different temperatures. J Food Eng. 144:20–28.
- Leontowicz H, Leontowicz M, Latocha P, Jesion I, Park Y-S, Katrich E, Barasch D, Nemirovski A, Gorinstein S. 2016. Bioactivity and nutritional properties of hardy kiwifruit Actinidia arguta in comparision with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan. Food Chem. 196:281–291.
- Lim S, Han SH, Kim J, Lee HJ, Lee JG, Lee EJ. 2016. Inhibition of hardy kiwifruit (Actinidia aruguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food Chem. 190:150–157.
- Milošević T, Miloševi N. 2012. Main physical and chemical traits of fresh fruits of promising plum hybrids (Prunus domestica L.) from Cacak (Western Serbia). Rom Biotechnol Lett. 17(3):7358–7365.
- Nam H, Seo HS, Bang J, Kim H, Beuchat LR, Ryu JH. 2014. Efficacy of gaseous chlorine dioxide in inactivating Bacillus cereus spores attached to and in a biofilm on stainless steel. Int J Food Microbiol. 188:122–127.
- Napolitano MJ, Green BJ, Nicoson JS, Margerum DW. 2005. Chlorine dioxide oxidations of tyrosine, N-acetyltyrosine, and dopa. Chem Res Toxicol. 18(3):501–508.
- Nishiyama I, Yamashita Y, Yamanaka M, Shimohashi A, Fukuda T, Oota T. 2004. Varietal difference in vitamin C content in the fruit of kiwifruit and other Actinidia species. J Agric Food Chem. 52(17):5472–5475.
- Park SH, Kim WJ, Kang DH. 2018. Effect of relative humidity on inactivation of foodborne pathogens using chlorine dioxide gas and its residues on tomatoes. Lett Appl Microbiol. 67(2):154–160.
- Popa I, Hanson EJ, Todd ECD, Schilder AC, Ryser ET. 2007. Efficacy of chlorine dioxide gas sachets for enhancing the microbiological quality and safety of blueberries. J Food Prot. 70(9):2084–2088.
- Praeger U, Herppich WB, Hassenberg K. 2016. Aqueous chlorine dioxide treatment of the horticultural produce: effects on microbial safety and produce quality-A review. Crit Rev Food Sci Nutr. 58:1–16.
- Remorini D, Landi M, Tardelli F, Lugani A, Massai R, Graziani G, Fogliano V, Guidi L. 2015. Effect of chlorine dioxide and ascorbic acid on enzymatic browning and shelf life of fresh-cut red delicious and granny smith apples. J Food Process Preserv. 39(6):2925–2934.
- Robertson JA, Meredith FI, Senter SS, Okie WR, Norton JD. 1992. Physical, chemical and sensory characteristics of Japanese-style plums growing in Georgia and Alabama. J Sci Food Agric. 60(3):339–347.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299:152–178.
- Smith DJ, Ernst W, Herges GR. 2015. Chloroxyanion residues in cantaloupe and tomatoes after chlorine dioxide gas sanitation. J Agric Food Chem. 63(43):9640–9649.
- Strik BC, Hummer KE. 2006. Ananasnaya’ Hardy Kiwifruit. J Am Pomol Soc. 60(3):106–112.
- Sun X, Bai J, Ference C, Wang Z, Zhang Y, Narciso J, Zhou K. 2014. Antimicrobial activity of controlled-release chlorine dioxide gas on fresh blueberries. J Food Prot. 77(7):1127–1132.
- Sun X, Zhou B, Luo Y, Ference C, Baldwin E, Harrison K, Bai J. 2017. Effect of controlled-release chlorine dioxide on the quality and safety of cherry/grape tomatoes. Food Control. 82:26–30.
- Sy KV, McWatters KH, Beuchat LR. 2005. Efficacy of gaseous chlorine dioxide as a sanitizer for killing Salmonella, yeasts, and molds on blueberries, strawberries, and raspberries. J Food Prot. 68(6):1165–1175.
- Trinetta V, Linton RH, Morgan MT. 2013. Use of chlorine dioxide gas for the postharvest control of Alternaria alternata and Stemphylium vesicarium on Roma tomatoes. J Sci Food Agric. 93(13):3330–3333.
- Wang YZ, Wu J, Ma DW, Ding JD. 2011. Preparation of a cross-linked gelatin/bacteriorhodopsin film and its photochromic properties. Sci China Chem. 54(2):405–409.
- Wu VCH, Kim B. 2007. Effect of a simple chlorine dioxide method for controlling five foodborne pathogens, yeast and molds on blueberries. Food Microbiol. 24:974–800.
- Wu VCH, Rioux A. 2010. A simple instrument-free gaseous chlorine dioxide method for microbial decontamination of potatoes during storage. Food Microbiol. 27(1):179–184.
- Zheng F, Zheng W, Li L, Pan S, Liu M, Zhang W, Liu H, Zhu C. 2017. Chitosan controls postharvest decay and elicits defense response in kiwifruit. Food Bioprocess Technol. 10:1937–1945.