?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A large number of mangrove species are growing in the Sundarbans Reserve Forests (SRF), Bangladesh, yet little is known about their phenology. The aim of the present study was to understand the phonological patterns such as leaf emergence, leaf fall, flower buds, flowers, and propagules maturation, in the mangrove Bruguiera sexangula using litterfall data. This study was conducted at Karamjol and Ghagramari areas of SRF, Bangladesh and using litterfall data over two (2) years. Leaf and stipule litterfall occurred throughout the years, with distinct seasonal patterns. New leaf production was significantly correlated with monthly day length. Mean total litterfall was 18.75 Mg ha−1 yr−1, with the largest component being vegetative organs (68.4%). Flowers and propagules litterfall were highest in summer and rainy season, respectively. Kendall’s coefficient of concordance, W, revealed that flower and propagule were significantly concordant during the study years. Flowers and propagules litterfall were significantly influenced by monthly day length and rainfall, respectively. The development of propagule could be affected by the climate during the development and the number of flowers etc. The average development period from flowers to propagules was around 4 months. B. sexangula did not show any correlation between leaf emergence and reproductive organs production.
Introduction
Mangrove forests are important contributors of nutrients to coastal ecosystems. Litterfall is one of the main components of net primary production (Sukardjo et al. Citation2013) and the input of materials and energy into inter-tidal systems (Mackey and Smail Citation1995). The study on litter dynamics and phenology will be an effective method to understand the ecosystem dynamics (Rani et al. Citation2016). Studies of species phenology are also especially important for an understating of mangrove production, ecology, and trophodynamics (Duke et al. Citation1984). A large dataset for litterfall production in different mangrove stands around the world is available (Saenger and Snedaker Citation1993) but there is very few information available on the litterfall production of the Sundarbans mangrove forests, Bangladesh. It is therefore very possible that the Sundarbans-the world’s largest mangrove was ignored for estimating the global inventories of the trends and patterns for the productivity and carbon sinks of the mangroves.
Litterfall also indicates phonological events in mangrove species (Leach and Burgin Citation1985; Duke Citation1990; Clarke Citation1994) when the time lag between the formation and shedding of plant organs is known (Mehlig Citation2006). Descriptive studies of plant phenology are fundamental to understand the resource base of other populations, communities, or ecosystems (Bullock and Solis-Magallanes Citation1990). It is also important to understand the mangrove phenology for understanding both the contribution of mangroves to near-shore productivity and plant-animal interactions within the community itself (Coupland et al. Citation2005). In addition, fallen leaves and reproductive organs are often an important food source for a wide variety of animals, including insects and crabs (Robertson et al. Citation1992). Phonological events in mangrove communities have been suggested to be influenced by local or regional environmental conditions, particularly day length, air temperature, rainfall, salinity, and tidal inundation (Saenger and Moverley Citation1985; Naido Citation1989; Duke Citation1990; Fernandes Citation1999; Coupland et al. Citation2005; Kamruzzaman et al. Citation2013).
Bruguiera sexangula (Lour.) Poir. is widely distributed from Sundarbans mangrove forests, Bangladesh, India (Naskar and Mandal Citation1999), Sri Lanka (Jayatissa et al. Citation2002), South East Asia- Indonesia and Malaysia (Sun and Lo Citation2011; Sukardjo et al. Citation2013), Hainan Island, China (Lu and Lin Citation1990) to Queensland, Australia (Sun and Lo Citation2011). No previous studies have examined the vegetative and reproductive phenology of B. sexangula growing at the Sundarbans mangrove forests, though a very few studies have investigated about phenophases of five mangrove species (Rahman and Islam Citation2015) and litterfall production and decomposition at Hainan Island, China (Lu and Lin Citation1990). Therefore, the aims of the present study are to investigate the vegetative and reproductive phenology of B. sexangula, growing in the Sundarbans mangrove forests and to identify how climatic factors affect the vegetative and reproductive organ’s litterfall of B. sexangula.
Materials and methods
Study site
The study was carried out in Karamjol and Ghagramari areas of SRF, Bangladesh, over 2 years from March 2016 to February 2018. This area receives regular tidal inundation through the River Passur. The mean annual rainfall in the region with 80% probably varies from about 2000 mm in the east and 1600 mm in the west. The study area was designated in the Oligohaline zone of SRF under the tropical region. Bangladesh has three distinct seasons: the pre-monsoon hot season from March through May, rainy monsoon season which lasts from June through October, and a cool dry winter season from November through February. However, March may also be considered as the spring season, and the period from mid-October through mid-November may be called the autumn (Banglapedia Citation2014). Higher temperature (26–34 °C) occur from March to June and lower temperature (12–25 °C) from December to February. The monthly mean temperature range was calculated from daily average temperature. The annual relative humidity varies from 70 to 80% (Rahman and Asaduzzaman Citation2010). Six mangrove species grow at the study site: Heritiera fomes Buch-Ham. in the family Malvaceae, Excoecaria agallocha L. in Euphorbiaceae, Bruguiera sexangula (Lour.) Poir. in Rhizophoraceae, Aglaia cucullata (Roxb.) Pellegr. and Xylocarpus mekongensis J. Koenig are in Meliaceae, and Avicennia officinalis L. in Avicenniaceae. We established four plots (20 m × 20 m) in B. sexangula stands at these two locations to represent the full tidal range at which the species occurred though we did not find any tidal effects on litterfall production. We covered 1600 m2 within the four plots in our study area, where the distance of all plots 200 m away from the river shore to avoid destruction of plots from the breakdown of river embankment and damages due to the high velocity of the wind. All trees in the study plots were numbered, and height (H) and stem diameter at breast height (DBH) were measured in March 2016. The mean height and mean DBH of B. sexangula were 9.9 ± 2.9 m and 14.3 ± 5.0 cm, respectively, as of 2016.
Climate data
Meteorological data were collected from the Bangladesh Meteorological Department, Dhaka, Bangladesh, from March 2016 to February 2018 (i.e. over the time period during which the fieldwork was conducted). The observatory station is in Mongla, Bagerhat, Bangladesh and it is approximately 8 km far from the study area. The temperature fluctuated approximately 14 °C from the coldest month to the hottest month, and mean of the mean annual air temperature during the study period was 30.8 ± 0.5 °C. The mean of monthly day length hour was 274.7 ± 1.3 hr month−1. Rainfall varied throughout the year and during winter months it almost 0 mm month−1. The highest rainfall ranged from 0 mm month−1 in December 2018 to 409.3 mm month−1 in August 2017 during the study period, and the mean of annual rainfall was 1636.4 ± 314.9 mm yr−1. The mean monthly air relative humidity was 61.0 ± 1.1%. Monthly maximum wind speed varied from the lowest 11.9 km hr−1 in November 2017 to the highest 30.2 km hr−1 in April 2016.
Litter collection
Litterfall was collected using 1 mm mesh litter traps with the collection area of 0.42 m2. Each plot (20 m × 20 m) having six litter traps and each trap was placed > 1 m above the ground surface to avoid tidal inundation. We placed 24 litter traps within the 4 plots. The litter traps were emptied monthly; the collected litterfall was kept in a cotton bag and carried to the laboratory where it was separated into leaves, stipules, branches, flower buds, flowers, and propagules. Individual litterfall components were dried at 80 °C for 48 h, desiccated at room temperature, and then weighed using a digital balance (EK-600H, A & D Co., Ltd., Tokyo, Japan).
Statistical analysis
Kendall’s coefficient of concordance, W, was used to evaluate the degree of similarity in monthly change during the study period for each litterfall component. Litterfall data were ranked by monthly mass for every year, and W was calculated for monthly litterfall: where Ri is the sum of rank is (i = 1, 2,…., n) of monthly litterfall in each year, n (=12) is the number of months in a year, and m (=2) is the number of years. When W = 1.0, the monthly changes of the litterfall are concordant among years, while when W = 0.0, the monthly changes are completely different among years. χ2
test with n − 1 degree of freedom was used to determine the significance of the W value (Saito et al. Citation2003).
We performed stepwise multiple regression analysis to determine the effects of five environmental factors—temperature, maximum wind speed, day length, rainfall, and humidity—on litterfall of leaves, stipules, branches, flower buds, flowers, and propagules, using MA-MACRO/MRA software (ver. 3.0, Practical Business Education Institute, Tokyo, Japan). The stepwise multiple regression was continued until the adjusted R2 value showed a decreasing trend. A criterion value of F value > 2.0 was set o determine the minimum significance of a variable to be included in the equation. In order to avoid multicollinearity between the variables, we selected variables meeting the criterion of tolerance >0.1 (Shiga et al. Citation2004).
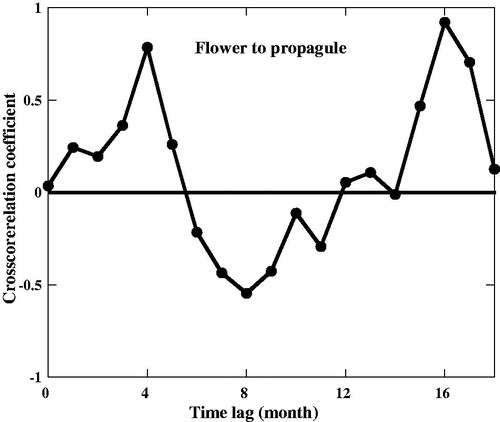
The crosscorrelation coefficient (rxy) was calculated to identify the time lag l in maturation period between reproductive organs using their time series data: where l (= 0, 1, 2,…) is the time lag; N (=24) is the total number of months in time series; Xj and Yj+l are the reproductive components respectively of the jth month and (j + l)th month;
1,N−1 is the mean value of an element from the first month to the (N − l)th month;
1+l,N is the mean value of the other element from the (1 + l)th month to the Nth month.
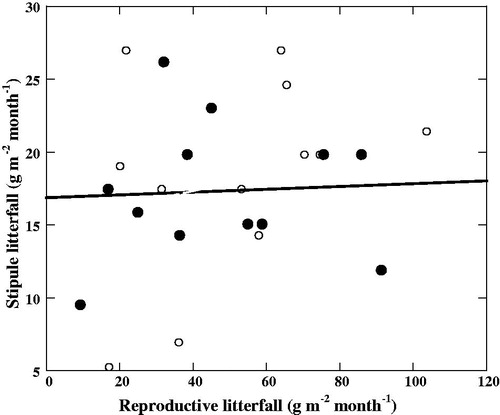
The simple regression method was used to understand the effect of reproductive organ litterfall on the vegetative organ litterfall. The annual mass of stipules and reproductive components of litterfall was calculated by summing the monthly data of each plot.
Result
Vegetative phenology
shows the monthly patterns of vegetative (leaf, stipule, and branch) organ litterfall in the Bruguiera sexangula. Leaf litterfall () and stipule litterfall () occurred continuously throughout the year, and each showed a clear seasonal trend. Leaf litterfall was highest in summer or hot season (March–May) followed by rainy season (June–October) and lowest in winter (November–February). Leaf litterfall peaks appeared two times in a year firstly in summer and the second one was in late autumn (October) (). Stipule litterfall, which is an indicator of new leaf production (Sheue et al. Citation2005), was also highest in summer season (March–May). Lowest stipule litterfall was also found in the winter (November–February) (). Like leaf litterfall stipule litterfall also showed higher in the rainy season (June–October) especially second peak appeared in late autumn (October). Kendall’s coefficient of concordance, W, revealed that the monthly leaf litterfall and stipule litterfall were concordant during the study period but they were not significant (). Branch litterfall, including small twigs, bark, and large branches, showed no clear seasonal trend (; ).
Figure 1. Phenograms of the litterfall of the vegetative organs of Bruguiera sexangula in the Sundarbans. Vertical bars represent standard errors of the means.
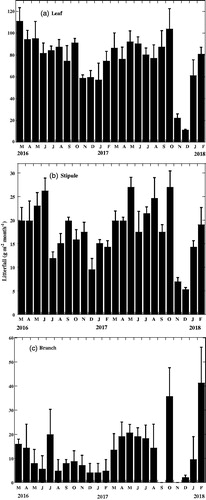
Table 1. Kendall’s coefficient of concordance, W, showing agreement in the monthly changes in litterfall components of Bruguiera sexangula during the study period.
represents the results from the stepwise multiple regression analyses for variation in vegetative and reproductive organ litterfall of B. sexangula with variation in environmental factors. Leaf litterfall was significantly influenced by monthly mean air temperature, whereas leaf initiation or stipule litterfall was significantly correlated with monthly day length. Branch litterfall was not correlated with any environmental factors.
Table 2. Adjusted R2 values from the stepwise multiple regression analysis of vegetative and reproductive litterfall components from Bruguiera sexangula in relation to environmental factors.
summarizes the amount of vegetative and reproductive organ litterfall components and their contributions to the total litterfall. Leaves were the dominant element of litterfall during the study period. The mean total litterfall amount was 1875.17 ± 14.44 g m−2 yr−1 (mean ± SE), of which leaves were the largest contributor, with an estimated amount of 919.48 ± 50.36 g m−2 yr−1. presents the amounts of stipule, branch, and the sum of vegetative organs litterfall and their contribution to the total litterfall.
Table 3. Annual amounts of vegetative and reproductive litterfall components of Bruguiera sexangula during the study period.
Reproductive phenology
The reproductive cycle of B. sexangula had a regular monthly periodicity (). Litterfall of flower buds, including both immature and mature buds, was highest in January (winter season) and in the rainy season (September–October). Flower bud litterfall was lowest in the months of November and December i.e. in late autumn (). Kendall’s W value showed that monthly flower bud litterfall was strongly and significantly concordant during the study period (). Flower litterfall was observed throughout the year but was highest in March-April and lowest in September–October (). Unfertilized flowers aborted and fell quickly from the trees, and some pollinated flowers also fell. Kendall’s W value revealed that the monthly trends in flower litterfall were concordant during the study period but it was not significant (). Propagule litterfall including both mature propagule and immature propagule was found throughout the year, but the abundance of mature propagules was in July i.e., in the rainy season and lowest in the month of December i.e., in the winter season (). Kendall’s W value also showed that monthly propagule litterfall was strongly and significantly concordant during the study period ().
Figure 2. Phenograms of the litterfall of the reproductive organs components of Bruguiera sexangula in the Sundarbans. Vertical bars represent standard errors of the means.
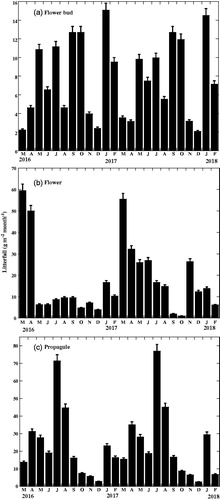
As shown, in , reproductive organ (including flower buds, flowers, and propagules) litterfall showed a clear monthly trend, with its highest peak in the month of July i.e., in the rainy season and lowest in December i.e., in the winter season. Kendall’s W value suggested that monthly trends of reproductive litterfall were strongly and significantly concordant during the study period (). indicates that flower litterfall of B. sexangula was significantly correlated with monthly day length, whereas propagules litterfall was significantly correlated with monthly maximum wind speed and monthly rainfall rather than other climatic factors. However, flower bud litterfall showed no correlation with any climatic factors.
Figure 3. Monthly amounts of vegetative, reproductive, and total litterfall of Bruguiera sexangula in the Sundarbans.
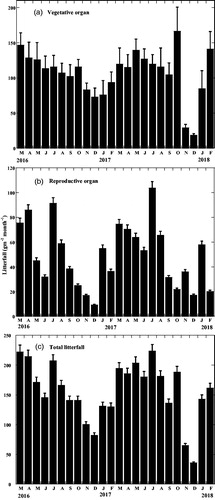
The mean total reproductive organs litterfall was 592.57 ± 23.23 g m−2 yr−1, which contributed 31.6% to the total litterfall (). shows the detailed amounts of the flower bud, flower, and propagule litterfall, respectively, and their percentages of contribution to the total litterfall. As shown in , the development phase from flowers to mature propagules took 4 months. shows that there was no relationship between stipule litterfall and reproductive litterfall. Litterfall of stipules of B. sexangula did not show any correlation with the litterfall of reproductive organs, because, in B. sexangula, leaf recruitment and flowering occurred throughout the year () and propagules were also found for more than half of the year ().
Discussion
Both leaf and stipule litterfall of B. sexangula were highest in summer and rainy season and lowest in the winter season. Our results are supported by the findings of Allen and Duke (Citation2006), who reported that peak leaf litterfall in B. gymnorrhiza another species of the same genus in the northern hemisphere occurred from April–September and peak stipule litterfall from May–September. Our results also coincide with the findings of Duke et al. (Citation1984), who reported that marked seasonal periodicity in both leaf and stipule litterfall for B. gymnorrhiza in northern Australia, with a peak in the summer wet season. The leaf fall of B. sexangula community in the Sundarbans mangrove forest showed a low yield during the winter season, but it had a tendency to increase from February just after ending the winter or beginning of spring season. In tropical mangrove areas, unimodal, bimodal, and trimodal patterns have been observed in the species of the same family and it is very species specific (Wium-Andersen and Christensen Citation1978; Wium-Andersen Citation1981; Leach and Burgin Citation1985). Tropical climate may be determined the multimodal peaks of leaf and stipule litterfall in the mangrove forests.
The data presented in the present study demonstrate that new leaf production and leaf litterfall of B. sexangula were significantly correlated with monthly day length and monthly mean temperature, in agreement with Gill and Tomlinson’s (Citation1971) finding that leaf productions of R. mangle L. in Florida, USA, was most common in summer when solar radiation and temperature levels were highest. Similarly, Gwada et al. (Citation2000) found that air temperature was the strongest environmental factor influencing leaf production in the species of the family Rhizophorace at Sashiki on Okinawa Island, Japan.
The present study showed that the leaves of B. sexangula were dominant (49.1%) in the total litterfall. Lu and Lin (Citation1990) documented that the contribution of leaf litterfall of B. sexangula on the mangroves on Hainan Island, China to the total litterfall was 68.9%, which are comparable to our findings. Our result is also comparable with the findings of Wafar et al. (Citation1997) who reported that the percentage of leaf litterfall to total litterfall in the mangrove species Rhizophora apiculata Blume., Rhizophora mucronata Lam., Sonneratia alba J. E. Smith and Avicennia officinalis L. were 61.2, 61, 57.7 and 43% respectively on the west coast of India. Our result also similar to the findings of Rani et al. (Citation2016) who reported that percentage contribution of leaf litterfall to total litterfall of mixed mangrove species was 48% on the south west coast of India.
The present study found that flowering abundance occurred in March–May i.e. in the summer season and propagules dropping was highest in July i.e., in the rainy season (). During summer and rainy season, temperature, day length, humidity, rainfall, and maximum wind speed were at their maximum. In the Sundarbans mangrove forest, reproductive organs were associated with monthly day length (flower) or monthly maximum wind speed and monthly rainfall (propagules), in agreement with Borchert (Citation2019) found that expansion of dormant flower buds may be triggered by increasing exposure to sunlight or the first heavy rains of the wet season. Duke (Citation1990) also found that climatic factors such as day length and temperature have a strong influence on Avicennia marina flowering in Australia, Papua New Guinea, and New Zealand. It may be concluded that monthly day length, monthly rainfall, and monthly maximum wind speed are the environmental factors that control the seasonality of reproductive organs and its dispersion for natural regeneration of B. sexangula in the Sundarbans mangrove forest.
Reproductive organ litterfall of the present B. sexangula contributed 31.6% of the total litterfall. Our results agree with Sukardjo et al. (Citation2013), who reported that reproductive organs of B. sexangula contributed 27.1% to the total litterfall accumulation in Bornean mangrove forests. The present contribution of the reproductive organ to the total litterfall was lower than the 41% found by Kamruzzaman et al. (Citation2013) for B. gymnorrhiza in sub-tropical mangroves on Okinawa Island, Japan. The proportion of reproductive organs to the total litterfall of B. sexangula in the Sundarbans mangrove forest was higher than for mixed species in a tropical mangrove stand on the southwest coast of India (28.7%; Rani et al. Citation2016), and for R. apiculata (21.8%) and R. mucronata (23.2%) (Wafar et al. Citation1997).
This is the very first study to calculate the maturation period of reproductive organs of B. sexangula in the Sundarbans mangrove forests, Bangladesh. The development phase of flowers to mature propagules took 4 months (), similarly to the findings of Samo et al. (Citation2018), who reported that B. sexangula in the mangroves of South Sumatra needed around 5 months from flowers to mature propagules and 7 months 2 days from bud initiation to mature propagules. Our results also similar to the findings of Nagarajan et al. (Citation2010), who reported that B. cylindrica in the mangroves of Kerala, India need 3–4 months for maturation period and in case of B. sexangula and B. gymnorrhiza took 6–8 months for mature propagules production. The developmental phase of the flower to mature propagules was 3–4 month B. cylindrica in southern Thailand (Wium-Andersen and Christensen Citation1978). Another study on B. gymnorrhiza in Okinawa Island, Japan found that it took 8 months for mature propagules production (Kamruzzaman et al. Citation2013). Our result is supported by the findings of Duke et al. (Citation1984), who reported that B. parviflora another species of the same genus in north-eastern Australia needed 4–5 months for maturation period. In comparison with already reported data of species belonging to the same genus, it appears that B. sexangula growing in the Sundarbans mangrove forests; Bangladesh has a relatively short maturation period for its reproductive organs.
The mean total litterfall of B. sexangula observed in the present study (18.75 Mg ha−1 yr−1) was higher than that in the Hainan Island, China (11.04–12.55 Mg ha−1 yr−1; Lu and Lin Citation1990), and lower than that in Bornean mangrove forests (24.2 Mg ha−1 yr−1; Sukardjo et al. Citation2013). Our result showed higher mean total litterfall than Shunula and Whittick (Citation1999), who reported 16 Mg ha−1 yr−1 for B. gymnorrhiza on Unguja Island, Zanzibar, Tanzania. Total litterfall was also higher than those recorded in B. gymnorrhiza stands at Manko wetland, Okinawa Island, Japan (10.1 Mg ha−1 yr−1; Kamruzzaman et al. Citation2013) and on Irimote Island, Okinawa, Japan (8.8 Mg ha−1 yr−1; Kishimoto et al. Citation1987). Our result was also higher than to the 16.57 Mg ha−1 yr−1 for mixed species in a tropical mangrove stand on the southwest coast of India (Rani et al. Citation2016). Compared to the previous results, the results of the present study may indicate that productivity of this mangrove species is relatively high in the Sundarbans mangrove forests, Bangladesh with an exception of the results of Bornean mangrove, because the most obvious reason for high litterfall in southeastern Borneo is the humid equatorial climate (Sukardjo et al. Citation2013).
B. sexangula did not exhibit any correlation between leaf production and reproductive organs production, in contrast to the findings of Wium-Andersen and Christensen (Citation1978), who reported that four mangrove species showed a depression tendency in leafing rates when the production of flowers and fruits was at maximum. In B. sexangula, reproductive organs produced throughout the year and leaf recruitment also occurred the entire year, and the peaks of leaf recruitment and reproductive organs production did not coincide each other. It is therefore assumed that growth of reproductive organs does not affect the vegetative organs production.
Acknowledgements
We are very much grateful to Mr. Kalayan Basak his cordial help during field data collection. We are also grateful to Forest Department of Bangladesh for giving permission to establish plot in the Sundarbans Reserve Forest.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Allen JA, Duke NC. 2006. Bruguiera gymnorrhiza (large-leafed mangrove), ver. 2.1. In: Elevitch CR, editor. Species profiles for Pacific Island Agroforestry. Holualoa, Hawaii: Permanent Agriculture Resources (PAR). http://www.traditionaltree.org
- Banglapedia. 2014. Season. [accessed on 2019 Feb 1] http://en.banglapedia.org/index.php?title=Season.
- Borchert R. 2019. The phenology of tropical trees. [accessed 2019 Feb 8] http://www.borchert.faculty.ku.edu/.
- Bullock SH, Solis-Magallanes JA. 1990. Phenology of canopy trees of a tropical deciduous forest in Mexico. Bitropica. 22(1):22–35.
- Clarke PJ. 1994. Base-line studies of temperate mangrove growth and reproduction; demographic and litterfall measures of leafing and flowering. Aust J Bot. 42(1):37–48.
- Coupland GT, Paling EI, McGuinness KA. 2005. Vegetative and reproductive phenologies of four mangrove species from northern Australia. Aust J Bot. 53(2):109–117.
- Duke N. 1990. Phenological trends with latitude in the mangrove tree Avicennia marina. J Ecol. 78(1):113–133.
- Duke NC, Bunt JS, Williams WT. 1984. Observations on the floral and vegetative phenologies of north-eastern Australian mangroves. Aust J Bot. 32(1):87–99.
- Fernandes MEB. 1999. Phenological patterns of Rhizophora L., Avicennia L. and Lagunculara Gaertn.f. in Amazonian mangrove swamps. Hydrobiologia. 413:53–62.
- Gill AM, Tomlinson PB. 1971. Studies on the growth of red mangrove (Rhizophora mangle L.). 3. Phenology of the shoot. Biotropica. 3(2):109–124.
- Gwada P, Makoto T, Uezu Y. 2000. Leaf phenological traits in the mangrove Kandelia candel (L.) Druce. Aquat Bot. 68(1):1–14.
- Jayatissa LP, Dahdouh-Guebas F, Koedam N. 2002. A review of the floral composition and distribution of mangroves in Sri Lanka. Bot J Linean Soc. 138(1):29–43.
- Kamruzzaman M, Sharma S, Kamara M, Hagihara A. 2013. Vegetative and reproductive phenology of the mangrove Bruguiera gymnorrhiza (L.) Lam. on Okinawa Island, Japan. Trees. 27(3):619–628.
- Kishimoto T, Miyajima H, Nakasuga T, Baba S. 1987. Zonation of mangrove forest (III) Litterfall and its seasonal change. Trans Mtg Jap for Soc. 98:301–302.
- Leach GJ, Burgin S. 1985. Litter production and seasonality of mangroves in Papua New Guinea. Aquat Bot. 23(3):215–224.
- Lu C, Lin P. 1990. Studies on litterfall and decomposition of Bruguiera sexangula (Lour.) Poir, community on Hinan Island, China. Bull Mar Sci. 47:139–148.
- Mackey A, Smail G. 1995. Spatial and temporal variation in litter fall of Avicennia marina (Forssk.) Vierh. in the Brisbane River, Queensland, Australia. Aquat Bot. 52(1–2):133–142.
- Mehlig U. 2006. Phenology of the red mangrove, Rhizophora mangle L., in the Caeté Estuary, Pará, equatorial Brazil. Aquat Bot. 84(2):158–164.
- Nagarajan B, Krishnamoorthy M, Warrier CSK. 2010. Conserving the endangered true mangroves of Kerala: some pragmatic solutions. Proceedings of 22nd Kerala Science congress, 28–31. January 2010, KFRI, Peechi :645–646.
- Naido G. 1989. Seasonal plant water relations in a South African mangrove swamp. Aquat Bot. 33:233–242.
- Naskar K, Mandal R. 1999. Ecology and biodiversity of Indian mangroves. Delhi, India: Daya Publishing House.
- Rahman MM, Islam SA. 2015. Phenophases of five mangrove species of the Sundarbans of Bangladesh. Int J Bus Soc Sci Res. 4:77–82.
- Rahman MR, Asaduzzaman M. 2010. Ecology of Sundarban, Bangladesh. J Sci Found. 8(1–2):35–47.
- Rani V, Sreelekshmi S, Preethy CM, BijoyNandon S. 2016. Phenology and litterfall dynamics structuring ecosystem productivity in a tropical mangrove stand on south west coast of India. Reg Stud Mar Sci. 8:400–407.
- Robertson AI, Alongi DM, Boto KG. 1992. Food chains and carbon fluxes. In: Robertson AI, Alongi DM, editots. Tropical mangrove ecosystems. Washington, DC: Amrican Geophysical Union; p. 239–329.
- Saenger P, Moverley J. 1985. Vegetative phenology of mangroves along the Queensland coastline. Proc Ecol Soc Aust. 13:257–265.
- Saenger P, Snedaker SC. 1993. Pantropical trends in mangrove aboveground biomass and annual litterfall. Oecologia. 96(3):293–299.
- Saito K, Okuno T, Asai A. 2003. Lectures on modern statistics. Tokyo, Japan: Practical Business Education Institute.
- Samo SR, Dahlan Z, Mumandar RMR. 2018. Reproductive phenology of Bruguiera sexangula (Lour.) Poir. In Berbakand Sembilang national park, South Sumatra. J Biol Res. 23:1–8.
- Sheue CR, Yong JWH, Yang YP. 2005. The Bruguiera (Rhizophoraceae) species in the mangroves of Singapore, especailly on the new record and the rediscovery. Taiwania. 50:251–260.
- Shiga T, Okuhara M, Nozawa M. 2004. Lectures on multiple regression analysis using Excel. Tokyo, Japan: Practical Business Education Institute.
- Shunula JP, Whittick A. 1999. Aspects of litter production in mangroves from Unguja Island, Zanzibar, Tanzania. Estuar Coast Shelf Sci. 49:51–54.
- Sukardjo S, Alongi DM, Kusmana C. 2013. Rapid litter production and accumulation in Bornean mangrove forests. Ecosphere. 4(7):art79–7.
- Sun M, Lo E. 2011. Genomic markers reveal introgressive hybridization in the Indo-West Pacific mangroves. A case study. PLoS ONE. 6(5):400–405.
- Wafar S, Untawale AG, Wafar M. 1997. Litter fall and energy flux in a mangrove ecosystem. Estua Coast Shelf Sci. 44(1):111–124.
- Wium-Andersen S. 1981. Seasonal growth of mangrove trees in southern Thailand. III. Phenology of Rhizophora mucronata Lamk. and Scyphiphora hydrophyllacea Gaertn. Aquat Bot. 10:371–376.
- Wium-Andersen S, Christensen B. 1978. Seasonal growth of mangrove trees in southern Thailand. II. Phenology of Bruguiera cylindrica, Ceriops tagal, Lumnitzera littorea and Avicennia marina. Aquat Bot. 5:383–390.