?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In order to shed light on the insect part involved in the forest decline associated with Atlas cedar (Cedrus atlantica (Endl.) Carriere.), an etiological study was carried out on eight representative stations in the Aures mountains located in Eastern Algeria. 1,728 woodlogs was prospected, representing three levels of tree’s health status (healthy, decaying, and moribund) and four heights (base, medium, crown, and branches) over three years (2010, 2013 and 2016). From this large-scale investigation, it was found that beetles caused the most important damages on Atlas cedar trees, 22 species of which belong mainly to the Scolytidae (Cryphalus numidicus, Scolytus numidicus, Crypturgus cedri, Phloeosinus cedri, and Hylastes batnensis); the Buprestidae (Melanophila marmottani and Anthaxia martini) as well as the xylomycophage Ciidae (Cis corioli) were found to be the most significant decline agents who impacted most of the phytosanitary status of Cedrus atlantica. The thorough examination of the infestation level and the manner of how these insects affected each stage of decline (taking into account the various specimens collected at different high levels) showed an intra and inter-specific heterogeneity, as well as a succession of the xylophagous entomofauna when comparing various stations.
Introduction
Mediterranean woodlands represent a quite fragile natural environment, already deeply disturbed (Quezel and Barbero Citation1990). The disturbance is even more pronounced on the southern edge of forest ecosystems, where several authors have reported a worrying state for some forest stands such as the formations of Tetraclinis articulata Mast., Juniperus thurifera L. and Cedrus atlantica (Endl.) Carriere. (Quezel and Barbero Citation1990; Bentouati and Bariteau Citation2006; Hammi et al. Citation2007; Roche Citation2009; Beghami Citation2012).
Endemic to the Maghreb Mountains, Atlas cedar is one of the noble trees of the of the Maghreb (Morocco and Algeria) forest. However, this typical forest species has shown in the last few years a worrying sanitary state that is threatening its durability in the region (Quezel Citation1998; Harfouche and Nedjahi Citation2003).
The decline of the cedar forests in the Maghreb areas started at the end of the 20th century. In Algeria, cedar rapid degradation is noteworthy, especially in the southern part of Aures and Belezma forests, where decline—expressed through dead standing trees—can reach 95% incidence in some stands. This state of facts expresses probably the fragility of the Atlas cedar in its natural habitats (Bentouati Citation2008; Beghami Citation2010).
Several authors pinpointed the preponderant effect of drought, which is an eliciting factor that triggers outbreaks in Aures forests (Boudy Citation1950; Landmann Citation1994; Touchan et al. Citation2008, Citation2011, Citation2014; Allen et al. Citation2010; Beghami Citation2010; Williams et al. Citation2013). However, the good relative phytosanitary state of some water demanding woody species, such as the common yew (Taxus baccata L.) brings other factors into play and causes collective and synchronous senescence of Atlas cedar trees which are known for their plasticity (Beghami Citation2012).
In this context, Manion (Citation1991), M’hirit (Citation2008), and Mouna (Citation2009) indicate that cedar dieback is a complex phenomenon where various spatio-temporal (biotic and/or abiotic) factors interact. These interdependencies make difficult to identify and prioritize these factors.
Bark and wood boring (BWB) insects considered as one of the main biotic factors inciting and aggravating decline process that often leads to tree death. Once the decline of trees has begun, a loss in vigor follows, resulting in increased susceptibility to secondary pests and pathogens agents (Wargo Citation1995; Sallé et al., Citation2014).
Indeed, these remarkable insects present special challenges to researchers worldwide, since their effects are usually indirectly visible on hosts as they are difficult to rear them outside of their normal environments; they affect both biogeophysical and biogeochemical processes, disrupt the physiological processes of hosts, inoculate fungal diseases and transmit nematodes to attack weakened forest species (Gebhardt et al. Citation2004; Dajoz Citation2007; Eckhardt et al. Citation2007; Jacobi et al. Citation2007; Lieutier Citation2007; M’hirit Citation2008; Togashi et al., Citation2008; Wermelinger et al. Citation2008; Nageleisen et al. Citation2010; Edburg et al. Citation2012; Sallé et al. Citation2014; Raffa et al. Citation2016).
Besides, data collected on the ecology of xylophagous insects (lato sensu) indicate selective trophic specificity toward the different tissues of their hosts, inducing a spatio-temporal distribution characteristic of each insect over the attacked species (Lieutier Citation2007; Paine and Lieutier Citation2016). Even though the majority of these taxa develop on host tissues of dead or recently wounded trees that exhibit low moisture content, some taxa regularly kill heavily defended healthy trees by means of mass attack (Wood Citation1982; Paine et al., Citation1997).
The irreversible damages caused by these insects are more intense on forest ecosystems that are weakened by the combined actions of abiotic factors (intense and prolonged episodic droughts and skeletal soils), and biotic parameters like the defoliator attacks—such as processionary caterpillar—or the actions of anthropozoogenic agents (Lieutier Citation2007; M’hirit Citation2008; Gandh and Herms Citation2010; Sallé et al. Citation2014).
Some early research has been devoted to the inventory of bark beetles and woodborer insects in association with the Atlas cedar population in the northern cedar forests of Algeria (De Peyerimhoff Citation1915, Citation1919, Citation1930; Villiers Citation1946; Balachowsky Citation1963, Citation1969; Chararas et al. Citation1968; Khemici Citation2001).
However, no studies have previously been carried out in the southern cedar forests of Algeria, thus several questions remain unanswered. In this context, the scientific community would search for wood-boring taxa that are closely associated with the Atlas cedar in Aures forest, in order to know how these taxa are involved on the decline process. The degree of the pathological impairment would to be quantified and the logical attack suction according to the relationship between the density of each wood-boring taxa with the prevalence of cedar trees explained.
In this regard, the present study aims to highlight the main xylophagous taxa associated with cedar dieback, in order to understand their importance and indicate the dynamics of their infestation on Atlas cedars. The study was out in 2010–2013 and 2016. In addition, we have also tried to identify any affinities occurring with regards to sampling stations, seasonal activity, height distribution, and wood moisture. Finally, we investigated attacks rate and emergence densities of key bark and wood-boring species in the cedar forest region of Aures.
Materials and methods
Study area
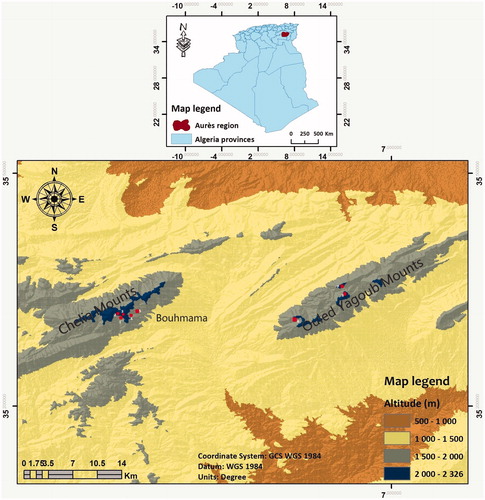
The present study was carried out on two cedars areas (Chelia and Ouled Yagoub mounts, 7,000 ha and 3,000 ha respectively) in the Aures forest -eastern Algeria- (Supplemental Figure S1) . The cedar trees have almost the same age (55 years in the Chelia mounts and 65 years in the Ouled Yagoub mounts), grew on lower cretaceous sandstones associated with dolomites (Abdessemed Citation1981).
In these areas, we have selected eight plots of one hectare (Martikainen et al. Citation1999; Gering and Crist Citation2000). Four plots were exposed north (two ones in Chelia and Ouled Yagoub mounts), the rest of the plots were exposed south (south-facing) (three in the Chelia mounts and one plot in the Ouled Yagoub mounts), all plots are characterized by a subhumid climate (; ). Although, observation prevalence of trees shows different dieback degrees that are clearly pronounced on Ouled Yagoub mounts.
Table 1. Location of sampling stations.
The chosen plots, host pure cedars in association with Rananculetum spicatii (Cedro atlanticae – Rananculetum spicatii) (Abdessemed Citation1981), to provide accurate results on xylophagous entomofauna of Atlas cedar.
Sampling logs
To study the incidence of the xylophagous insects in the decline of Atlas cedar forests, three classes of trees representing three categories of dieback were chosen according to needle-drying degrees (Benhalima Citation2004; Rouault et al. Citation2006; Samalens Citation2009; Beghami Citation2010).
Healthy trees (D0) are related to subjects that show a good physiological state with normal foliage, the dying subjects (D50) shows 50% needle loss from the crown. Last, dead trees (D100) presents individuals with 100% dry leaves.
The choice of the latter classes is justified by the absence of a significant difference between the emerging entomofauna in classes that vary between 50 and 80% needle loss (Benhalima Citation2006; Wermelinger et al. Citation2008; Beghami Citation2010; Talbi and Bouhraoua Citation2015).
In each station, a tree was removed from each dieback blocks, in sum, 24 trees were sampled at all eight stations for each decline level over each year.
The trees were split into three blocks of 5 meters long: top, mid, crown, and branches (Kelseya and Joseph 2001; Johansson et al. Citation2007). On each high class, two sections of trunks of an overall lateral area of 42 dm2 (corresponding to logs that of an average of 0.45 m × 0.3 m) have been collected and placed in hatchers (Leather Citation2005; Manly Citation2015).
This operation has been performed over three periods: End of January, May, and September, during three sampling years (2010, 2013, and 2016), in order to follow the seasonal infestations process of the BWB species (Okland Citation1996; Kelsey and Joseph Citation2001; Benhalima Citation2004; Johansson et al. Citation2007). These sampling periods were defined according to preliminary investigations (Beghami Citation2010) that were carried out by the monitoring of the emergence of the principal wood-boring species by the means of a multidirectional window and yellow traps.
Monitoring of diachronic emergences
1,728 roundwood logs are sampled throughout three tree sampling campaign 2010–2013 and 2016. These campaigns were carried out in order to monitor the bioecology of the main BWB species, and observe the influence of biotic and/or abiotic stationary conditions on the BWB insects infestation process overtime (incoming studies), succession of the main beetles attacks, and unreeling the pattern of the prevalence of healthy, dead and dying trees according to the density of insect attacks.
The sampled logs were then put into hatchers bearing similarity to the “cylinder extraction and photo-eclectors” (Okland Citation1996) or “totholz eklektor” (Albrrecht Citation1990; Schmitt Citation1992). The harvesting principle is based on the attraction that insects have for light (Speight Citation2005; Wermelinger et al. Citation2008; Shimoda and Honda Citation2013), the hatchers are cardboard boxes lined inside with a dark plastic film with a collection device annexed to the outside.
In order to follow the emergence chronology of the main xylophagous species, daily monitoring of the collected insects is carried out during one year after each campaign, since the sampling of the logs until the cessation of insects’ emergence.
The logs were then husked after a period of immersion in water to soften tree bark so as to study the frequency and density of infestation in relation with the bark thickness.
The gathered insects were identified and inventoried according to their taxonomic characteristics. Eight major species whose biological cycle had been controlled were set aside to study their contribution to the Atlas cedar decline phenomenon and thus became the key species of interest in this study.
The infestation frequency (FA%) of different wood-boring taxa is calculated according to the formula: where Ni is the number of attacked logs by each specific species and Nt is the total number of logs observed in each station (Benhalima Citation2004; Starzyk et al. Citation2008).
The average emergence of the most frequent wood-boring species is explained by both dieback and height classes. Furthermore, the seasonal activity of these insects is noted and averaged over the three sampling campaigns.
In the aftermath, the average infestation density (Id) of the three major BWB insects was expressed as the number of average mating chambers per 1 dm2 for all stations per year. (Grodzki et al. Citation2004).
Finally, the infestation rate of the most forgotten insects is calculated according to the formulas: Where
and Ta (%) is the infestation rate; Sai is the attacked surface that is associated with each species; Lgm: is the length of the maternal galleries, lgm: is the width of the maternal galleries, Lgl: is the length of larval galleries, lgl: is the width of larval galleries and Ngl: is the number of larval galleries (Benhalima Citation2004). These parameters are measured by imageJ—annexed to SmartRoot plugin—software after picture calibration.
Statistical analyses
In the present study, we have performed a χ2 test to highlight the effect of height and dieback classes on the cumulative emergence of the main adult woodborers species followed by Cramer’s Phi in order to evaluate the strength of the association. In addition, a correlation study based on the calculation of Spearman’s ρ coefficient was carried out in order to assess the relationship between bark thickness and attacks intensity, expressed in terms of the number of total maternal galleries associated with the principal bark and wood-boring species.
Furthermore, an ANOVA analysis (p < 0.05) followed by a Student Newman, Keuls Post-Hoc test, was performed in order to study the variation between the attacked area and the infestation density of the three main species over the three sampling campaigns.
Finally, potential links between the main woodborers species and the prevalence of different tree health classes at different sampling sites are established by means of factorial correspondence analysis (FCA). Agglomerative hierarchical clustering has been generated to distinguish similarities between stations.
Statistical tests were performed using SPSS Version 22.0 (IBM®, Corp, USA) software after testing for homoscedasticity and normality of the residual.
Results
During the 3 sampling instances, 23,879 specimens were identified and grouped into 23 xylophagous species belonging to 2 systematic orders. Beetles represent nearly 99% of the emerging species that are classified into five families, the Curculionidae and Buprestidae with 10 and 6 species respectively, Urocerus augur (Siricidae; Hymenoptera) is the only one xylophagous species that does not belong to beetles ().
Table 2. Attacks frequency of Cedrus atlantica log by the main bark beetle and other wood-boring species.
The majority of the sampled xylophagous taxa on Atlas cedar logs showed a high infestation frequency (FA%). A maximum of 62.96% and 54.17% was recorded for Cryphalus numidicus Eichhoff, 1878 respectively at the 1st and 7th stations of Chelia and Ouled Yagoub montains. While a minimum of 4.17% noted for Urocerus augur at the 1st and 5th stations in Chelia Highlands and 5.09% in the 8th station in Ouled Yagoub mountains.
However, Cis corioli, Cryphalus numidicus, Crypturgus cedri, and Melanophila marmottani record the highest average (FA%) with 43.06, 43.86%, 55.15% and 39.58% respectively.
Data analysis of key BWB insects obtained after χ2 test () on contingency table regarding the emergence of adults of the main woodborers species according to height and decline classes indicates that C. numidicus and S. numidicus attacks all parts of C. atlantica trees in D50 class with a value of Cramer’s Phi between 0.40 and 0.50. While C. cedri settles on dead trees in D100 class with Cramer’s Phi between 0.41 and 0.53). Phloeosinus cedri infects the branches and top parts of trees of D0 class with Cramer’s Phi from 0.43 to 0.55, although it’s noted on the middle parts and the branches of D50 trees class.
Table 3. Cramer’ Phi after χ2 test on contingency table regarding the emergence of adults of the main woodborers species according to the height and decline classes.
Buprestidae, M. marmottani and A. martini reported significantly on subjects in class D50 on all prospected parts (Cramer’s Phi between 0.42 and 0.61). The longhorn beetle C. cedri was found on trees of class D50 with a clear preference for tops and branches. Whereas, the Ciidae Cis corioli is clearly noticed on dead trees where it settles on all parts of the tree.
Seasonal activity of main species () indicates that C. numidicus, S. numidicus, M. martinii and Ciidae Cis corioli are active from spring to early autumn. On the other hand, P. cedri was observed during late summer and early autumn. C. cedri has been noticed at late spring to early summer; M. martinii and Cis corioli emerge in spring and their spreads are in early summer (maximum activity).
Figure 2. Seasonal average emergence of the main BWB of Atlas cedar during the three sampling campaigns.
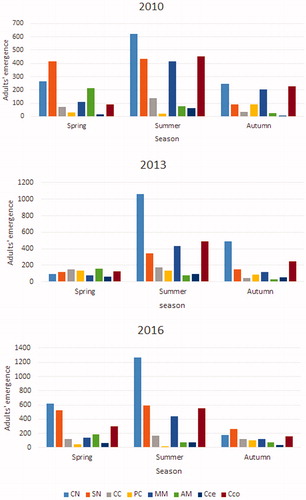
Cryphalus numidicus is the most active species over all seasons with a maximum of 5.97, 15.33, and 6.5 adults/log that were respectively obtained in spring; summer and autumn on the medium and basal stratum ridges.
In addition, Scolytus numidicus and Crypturgus cedri have shown a maximum activity in summer with an estimated abundance of 5.55 and 2.36 adults/logs. Whereas, Phloeosinus cedri was found to be active in late summer-early Autumn with 2.4 adults/logs.
Melanophila marmottani is active on summer with maximum mean emergence of 3.93 adults/logs. However, Anthaxia martini occurs in earlier spring, with intense activity on the ridges of the middle stratum.
The Cerambycidae, Callidium cedri, was found on summer with 1.55 adults/log. The Ciidae, Cisdygma corioli mainly occurs in summer where we record a mean of 4.77 adults in the log bases and 4.16 adults in branches of D100.
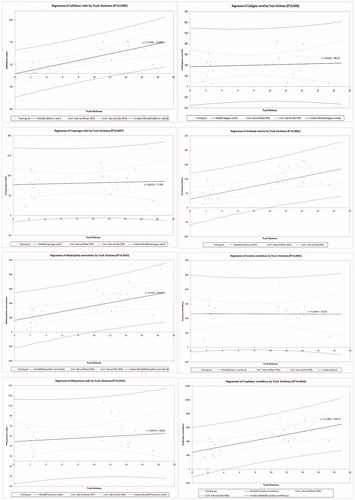
Correlation analysis between bark thickness and adult emergence of the main woodborers species () indicates that the activity of C. numidicus, P. cedri. Callidium cedri, M. marmottani, and A. Martini is positively correlated with bark thickness with coefficients values equal to 0.68, 0.15, 0.74, 0.63, and 0.69 respectively; C cedri and C. corioli seem to be indifferent to bark thickness; while bark thickness seems to be negatively correlated with S. numidicus attacks.
An evaluation of the infestation rate and infestation density on the three most forgotten BWB species () indicates the presence of a significant difference between C. numidicus that are most active species with infestation rates from 30.71% to 47.56%. and Id between 10.83 and 11.19 compared to, S. numidicus and M. marmottani who showed respectively an infestation rates from 1.90% to 2.05% and 1.11% to 3.38% and an infestation density from 2.14 to 6.21 from S. numidicus and between 2.17 and 4.2 for M. marmottani.
Table 4. Cambial surface infested of the three main species of woodborers associated with Atlas cedar.
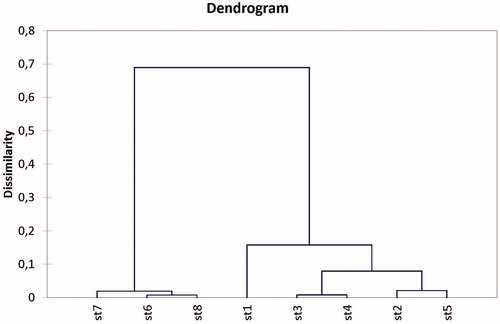
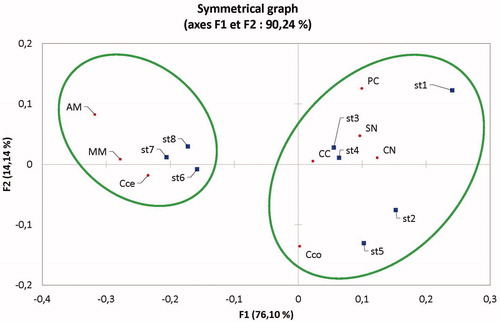
In the analytical part of this study, we have performed a factorial correspondence analysis () that showed a prominent dominance of the Scolytidae (bark beetle) in the Chelia massifs. On the other hand, the Buprestidae species (jewel beetles) were more abundant and associated with the Ouled Yagoub Massifs where the cedar wood seems to be more painful. The agglomerative hierarchical clustering study has confirmed this trend by showing similarity of abundances between the 2nd and the 5th station; the 3rd and 4th station along with the 1st one and finally the 6th and the 8th station along with the 7th plot ().
Figure 4. Factorial correspondence analysis (CFA) showing the distribution of the main Bark beetle and woodborer insects in relation to the sampling sites (CN: Cryphalus numidicus; SN: Scolytus numidicus; CC: Crypturgus cedri; PC: Phloeosinus cedri; MM: Melanophila marmottani; AM: Anthaxia martini; Cce: Callidium cedri; Cco: Cis corioli).
Discussion
Bark and wood-boring insects are one of the most dangerous pests in forest ecosystems. Indeed, some species can kill huge numbers of trees over large areas during intermittent outbreaks (Wargo Citation1995; Johnson and Miyanishi Citation2007; Raffa et al. Citation2008).
Xylophagous insects that infest conifers in the Mediterranean basin show a high level of endemism since it has been shown that most of the wood-boring insects are secondary species that attack those subjects who exhibit physiological weakness and ailing health because of constraining ecosystems.
From 23 wood-boring species that have been inventoried in this study, The Scolytidae species, are the most prominent with 8 numbered species. Among these species, three were found to be the most important according to their endemism characteristics, specificity, and virulence of attack (Supplemental Figures S2).
Cryphalus numidicus is an endemic species to the Mediterranean region, reported on Abies numidica de Lannoy ex Carrière, Abies pinsapo Boissier, Pinus halepensis Miller and Cedrus atlantica Endlicher in northern Europe and northern Africa (De Peyerimhoff Citation1919; Barbey Citation1925; Chararas Citation1963, Citation1974; Mouna Citation1994; Knížek, Citation1994; Paine and Lieutier Citation2016).
On the other hand, Scolytus numidicus and Crypturgus cedri are oligophagous and endemic species to Algeria and Morocco forests, these two species are exclusively reported on Cedrus atlantica (De Peyerimhoff Citation1919; Mouna and Graf Citation1994; Fabre et al. Citation1999; Paine and Lieutier Citation2016).
Phloeosinus cedri, is also an endemic and oligophagous species of Cedrus atlantica (De Peyerimhoff Citation1919; Paine and Lieutier Citation2016).
Melanophila (Phaenops) marmottani and Anthaxia martini are the main species of the Buprestidae family which originated from Atlantic-Mediterranean area, these species are found on several Abies, Cedrus and Cupressus forests (Théry Citation1928; Kocher Citation1969; Fabre et al. Citation1999; Benhalima Citation2004; Mouna Citation2009; Ilmen and Benjelloun Citation2013; Nichane and Khelil Citation2017).
The Cerambycidae, Callidium cedri is recorded only in North Africa on cedar forest (Villiers Citation1946; Benhalima Citation2006).
At last, Cis corioli is the unique Ciidae that found to be an endemic species from Algeria, and was observed for the first time by De Peyerimhoff on cedar trees (De Peyerimhoff Citation1915; Jelínek Citation2008; Beghami Citation2010).
In this paper, the taxa inventoried show a high frequency of attacks on all sampling stations that can probably be attributed to the fact that weakened trees release more allelochemical substances (e.g. α-pinene, δ-3-carene) that attract xylophagous taxa. In this context, several physicochemical processes that belong to the natural defenses process of the host trees (active and/or passive) are weakened and consequently, trees lose its ability of repelling woodborer infestations (Wood Citation1982; Byers Citation2007; Lieutier et al. Citation2004; Vega and Hofstetter Citation2015; Paine and Lieutier Citation2016).
The study of the BWB activity according to height and decline classes demonstrated that the attacks of C. numidicus depend on wood moisture content, where it was recorded frequently on all parts of weakened trees (class D50). S. numidicus attacks all parts of the cedars that belong to D50 class; Whereas, P. cedri seems to be the only Scolytidae species able to attack all parts of healthy cedar in D0 class.
Melanophila (Phaenops) marmottani and Anthaxia martini attack trees of D50 and D100 class. A. martini operates on well-heated parts of the hosts. as well as C. cedri that operate on physiologically weakened (D50, D100).
At last, C. Corioli is significantly associated with all parts of D100 class of cedar.
The present study showed a positive correlation between the thickness of the trunk bark and attacks of S. numidicus, C. cedri, and A. martini. A negative correlation is recorded between S. numidicus activity and the thickness of Atlas cedar bark, while P. cedri, Crypturgus cedri, and C. corioli attacks seem to be indifferent to the thickness of the bark.
Data of seasonal monitoring of BWBs assigned to the atlas cedar indicates that C. numidicus have massive and intense attacks that can reach 3 generations per year (two sister broods in early spring and summer and the 2nd generation in mid-September, early November in the case of favorable weather conditions). Scolytus numidicus and Crypturgus cedri appear to be univoltine, adults are active in late spring/-early summer where we have exceptionally observed 2nd flight in 2010 and 2016 when we had mild winters. Crypturgus cedri is univoltine too and attack cedars that are in the situation of advanced decline in late spring and during summer.
Phloeosinus cedri has only one generation per year and exhibits intense activity in late summer and early fall.
The Buprestidae species M. marmottani and A. martini are univoltine, the first species are active from late spring to early fall, the second one has an intense activity in spring. Whereas, the Cerambycidae Callidium cedri seems to have one generation that operates in summer time.
Finally, C. corioli seems to be univoltine xylomycophagous species who operate from late spring to late summer.
A temporal succession of bark beetle and wood insect infestation has also been observed (Paine and Lieutier Citation2016) witnessing that BWB insect can be grouped according to their affinities to wood moisture (De Peyerimhoff Citation1919; Rungs Citation1940; Balachowsky Citation1969; Chararas Citation1963, Citation1974; Fabre et al. Citation1999; Benhalima Citation2004).
Cryphalus numidicus and P. cedri are “pioneer” species that established themselves on relatively healthy cedar after 4 months cutting (spring activity). Benhalima (Citation2004), Beghami (Citation2010), and Talbi and Bouhraoua (Citation2015) have shown that the two previously cited species are the first one to notice in the fresh wood and prefers middle and top parts of trees as well as the branches of small diameters.
Scolytus numidicus, C. cedri, M. marmottani, A. martini, and Callidium cedri are “weakness inducing” species that have a pronounced preference for drying or dry wood. This colonization pattern follows a dispersion gradient that depends on the size of the woodworm and the thickness of the bark. These findings are supported Fabre et al. (Citation1999), Benhalima (Citation2004), Mouna (Citation2009), Vega and Hofstetter (Citation2015), and Paine and Lieutier (Citation2016) who converge to the same findings in Morocco.
Analysis of infestation activity indicates that the cambiophagous species: C. numidicus; causes the most serious harm on D50 logs followed by S. numidicus and M. marmottani on weakened trees.
Since the insects studied are normally secondary, their attacks can directly succeed only in the case of large outbreaks, where they usually cause important damages on healthy trees, or when the damaged trees have physiological deficiencies (Vega and Hofstetter Citation2015; Paine and Lieutier Citation2016).
The correspondence factor analysis followed by the CAH analysis clearly indicates that the stations in the Chelia series are attacked by bark beetle species, particularly C. numidicus, P. Cedri, and S. numidicus; these data suggest a recent outbreak of these cedar forests. This hypothesis is consolidated by the good valence of healthy trees and an acceptable phytosanitary state compared to the Ouled Yagoub forest where Buprestidae and Cerambycidae species—known as dieback completion agents—are the most found, and where decline patterns range from 60% to 70%.
Stationary variations in infestation can be attributed to in situ conditions. This would include bark thickness, soil quality and nutrient availability that determine the physiology of the host tree, as well as the microclimates of each station, especially in summer when dry winds increase evapotranspiration and consequently disrupt the cedar water balance. The above factors are involved in the dispersion, selection, concentration, and woodborers taxa establishment (Wood Citation1982; Vega and Hofstetter Citation2015; Paine and Lieutier Citation2016).
The threat posed by BWB insects in all stations is not restricted to the damage inflicted to sap conducting channels. Indeed, the noxiousness of these insects extends to a set of other infestations such as the inoculation of ophiostomatoïd fungi (such as Ceratocystis, Ophiostoma, Thielaviopsis…) (Upcoming publications) and nematodes (Bursaphelenchus…) during their epidemic attacks.
Conclusion
Nearly 350 woodboring species are reported in Western Europe and North Africa, however, the main species living in Mediterranean conifers amount to only 42, which corresponds to about 11% of the western Palearctic fauna (Lieutier et al. Citation2007; Paine and Lieutier Citation2016).
Bark beetle and woodborer insects play an important part in the process of Atlas cedar decline in Aures forests. These areas trees, that are already submitted to stress, are prone to be more receptive to infestations from a wide range of cambiophagous taxa.
In this original study, we provided for the first time a list of BWB species associated with Atlas cedar in the forests of Chelia and Ouled Yagoub.
The gathered data showed that C. numidicus, S. numidicus and M. marmottani are the most active species in the eighth prospected stations.
The monitoring of seasonal activity and the distribution of BWBs by height and decline class as well as the dynamics of attacks by the bark thickness allowed us to explain the process of cedars colonization.
The Attacks are initiated by both C. numidicus and P. cedri, the latter is considered to be the only species able to attack the crow and the mid of healthy cedars. Afterwards, C. numidicus impaired cedar defenses through massive attacks.
The remaining species attacks cedars of the D50 and D100 classes, increasing the decline of already weakened species.
Supplemental Material
Download JPEG Image (880.3 KB)Supplemental Material
Download JPEG Image (456.4 KB)Acknowledgments
The authors are grateful to all those who have contributed to the success of this work, especially Mr Cocquempot Christian, specialist in Animal Ecology and Agricultural Zoology at ENSA-INRA Montpellier (France); Mr Brustel Hervé from Purpan Engineering School – Toulouse (France); Mr Pablo Bercedo-Páramo, specialist in Buprestidae taxonomy (Spain); Mr Migeon Alain, from the Center of Biology and Population Management, INRA, Montpellier (France); Mr Pelletier Jean, expert in the taxonomy of Curculionidae, (France) for species identification. Mr A. Sallé from the University of Orléans for his advice on the choice of statistical tests. To Prof. A. Charif, Mr A. Aissi and Mr R. Berdja for reviewing the English version of this manuscript and to all the personnel of the forest administration of the of Batna and Khenchela provinces.
References
- Abdessemed K. 1981. Le cèdre de l’Atlas (Cedrus atlantica Manetti) dans le massif des Aurès et de Belezma: étude phytosociologique, problème de conservation et d’aménagement [Doct. Ing. thesis]. Faculty St Jerome, Marseille, France, p. 199.
- Alberrcht L. 1990. Grundlagen, Ziele und Methodik der Waldokologischen Forschung in Naturwaldreservaten. Naturwaldreservate in Bayern. Schriftenreihe. 1:1–220.
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, Mcdowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EHT, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage. 259(4):660–684.
- Balachowsky AS. 1963. Entomologie appliquée à l’agriculture. Vol. 2. Paris (France): Masson, 1391 p.
- Balachowsky AS. 1969. Les scolytes du cèdre dans le nord de l’Afrique. Ann Entomol Soc France. 5:647–655.
- Barbey A. 1925. Traité d’entomologie forestière à l’usage des sylviculteurs, des reboiseurs, des propriétaires de bois et des biologistes. Paris (France): Paris Berger-Levrault, 749 p.
- Beghami R. 2010. Contribution à l’étude des insectes associés au dépérissement du cèdre de l’Atlas (Cedrus atlantica) dans la région des Aurès: cas de la cédraie de Chélia [Magister thesis]. University Hadj Lakhdar, Batna, Algeria, 132 p.
- Beghami Y. 2012. Ecologie et dynamique de la végétation de l’Aurès: analyse spatio-temporelle et étude de la flore forestière et montagnarde [PhD thesis]. Biskra (Algeria): University Mohamed Khider, 193 p.
- Benhalima S. 2006. Les insectes xylophages et leur rôle dans le dépérissement du Cèdre de l'Atlas (Cedrus atlantica (Endl.) Carrière) dans le Haut et le Moyen Atlas (Maroc). Travaux de l'Institut Scientifique, Rabat: Série Zoologie. 46, 63p.
- Benhalima S. 2004. Les insectes xylophages et leur rôle dans le dépérissement du cèdre de l’Atlas (Cedrus atlantica (Endl) Carrière dans le haut et moyen Atlas (Maroc) [PhD thesis]. Agdal (Marocco): University Mohammed V, 107 p.
- Bentouati A. 2008. La situation du cèdre de l’Atlas dans les Aurès (Algérie). Forêt Méditerranéenne. 29:203–208.
- Bentouati A, Bariteau M. 2006. Réflexions sur le dépérissement du Cèdre de l’Atlas des Aurès (Algérie). Forêt Méditerranéenne. 27:317–322.
- Boudy P. 1950. Economie forestière nord-africaine: Monographie et traitements des essences forestières. France: Larousse, p. 878.
- Byers JA. 2007. Chemical ecology of bark beetles in a complex olfactory landscape. In: Lieutier F, Battisti A, Grégoire JC, Evans H, editors. Bark and wood boring insects in living trees in Europe, a synthesis. Netherlands: Springer, p. 89–134.
- Chararas C. 1963. Scolytides des Conifères. Encyclopédie Entomologique. Société Linnéenne de Lyon. 32:181–183.
- Chararas C. 1974. Recherches écophysiologiques sur certains Scolytidae spécifiques de Cedrus atlantica du Moyen Atlas. Travaux de la RCP, CNRS. 249:231–255.
- Chararas C, Juster M, Balmain N. 1968. Recherches sur le stimulus attractif de Cedrus libani Barr. vis-à-vis de Phloeosinus cedri Schedl. (Coléoptère: Scolytidae). Bull Soc Zool. 93:309–316.
- Dajoz R. 2007. Les insectes et la forêt: rôle et diversité des insectes dans le milieu forestier. Paris: TEC & DOC, p. 648.
- De Peyerimhoff P. 1915. Notes sur la biologie de quelques coléoptères phytophages du nord-africain. Ann Soc entomol. Fr. 84:19–61.
- De Peyerimhoff P. 1919. Notes sur la biologie de quelques coléoptères phytophages du nord-africain. Ann Soc entomol. Fr. 88:169–258.
- De Peyerimhoff P. 1930. Les Coléoptères attachés aux Conifères dans le nord de l’Afrique. Ann Soc entomol Fr. 102:359–412.
- Eckhardt LG, Weber AM, Menard RD, Jones JP, Hess NJ. 2007. Insect-fungal complex associated with loblolly pine decline in central Alabama. For Sci. 53:84–92.
- Edburg SL, Hicke JA, Brooks PD, Pendall EG, Ewers BE, Norton U, Gochis D, Gutmann ED, Meddens AJ. 2012. Cascading impacts of bark beetle-caused tree mortality on coupled biogeophysical and biogeochemical processes. Ecol Soc Am10(8):416–424.
- Fabre JP, Mouna M, Merle PD, Benhalima S. 1999. Le point sur certains ravageurs du cèdre de l’Atlas en Afrique du nord en France et en Europe. Forêt Méditerranéenne. 20:203–218.
- Gandh KJK, Herms DA. 2010. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions. 12(2):389–405.
- Gebhardt H, Begerow D, Oberwinkler F. 2004. Identification of the ambrosia fungus of Xyleborus monographus and X. dryographus (Coleoptera: Curculionidae, Scolytinae). Mycol Progress. 3(2):95–102.
- Gering JC, Crist TO. 2000. Patterns of beetle (Coleoptera) diversity in crowns of representative tree species in an old-growth temperate deciduous forest. Selbyana. 21:38–47.
- Grodzki W, McManus M, Knı́žek M, Meshkova V, Mihalciuc V, Novotny J, Turčani M, Slobodyan Y. 2004. Occurrence of spruce bark beetles in forest stands at different levels of air pollution stress. Environ Pollut. 130(1):73–83.
- Hammi S, Simonneau V, Alifriqui M, Auclair L, Montes N. 2007. Évolution des recouvrements forestiers et de l’occupation des sols entre 1964 et 2002 dans la haute vallée des Ait Bouguemez (Haut Atlas central, Maroc), Impact des modes de gestion. Sécheresse. 18:1–7.
- Harfouche A, Nedjahi A. 2003. Prospections écologiques et sylvicoles dans les cédraies du Bélézma et de l’Aurès à la recherche de peuplements semenciers et d’arbres plus. Rev For Fr. 55:113–122.
- Ilmen R, Benjelloun H. 2013. Les écosystèmes forestiers marocains à l’épreuve des changements climatiques. Forêt Méditerranéenne. 34:195–208.
- Jacobi WR, Koski RD, Harrington TC, Witcosky JJ. 2007. Association of Ophiostoma novo-ulmi with Scolytus schevyrewi (Scolytidae) in Colorado. Plant Dis. 91(3):245–247.
- Jelínek J. 2008. Family Ciidae. In: Löbl I, Smetana A, editors Catalogue of Palaearctic Coleoptera. 5. Apollo Books; p. 55–62.
- Johansson T, Hjältén J, Gibb H, Hilszczanski J, Stenlid J, Ball JP, Alinvi O, Danell K. 2007. Variable response of different functional groups of saproxylic beetles to substrate manipulation and forest management: Implications for conservation strategies. For Ecol Manage. 242(2–3):496–510.
- Johnson EA, Miyanishi K. 2007. Plant disturbance ecology the process and the response. London (UK): Elsevier Inc., 720 p.
- Kelsey RG, Joseph G. 2001. Attraction of Scolytus unispinosus bark beetles to ethanol in water-stressed Douglas-fir branches. For Ecol Manage. 144(1–3):229–238.
- Khemici M. 2001. Protection des cédraies en Algérie: inventaire des insectes ravageurs et réseaux d’avertissement et de lutte. In: Workshop on assessment of the scale of insect infestation in cedar forest in Lebanon and the Mediterranean region. Lebanon: American University of Bayreuth, p. 10–18.
- Knížek M. 1994. New species of Phloeosinus (Chapuis) from Cyprus (Coleoptera, Scolytidae). Folia Heyrovskyana. 2:124–127.
- KOCHER L. 1969. Catalogue commenté des coléoptères du Maroc. Feuillets rectificatifs. Travaux de l’Institut scientifique chérifien et de la Faculté des Sciences de Rabat.Jelínek J. 2008. family Ciidae. In: Löbl I, Smetana A, editors. Catalogue of Palaearctic Coleoptera. 5. Apollo Books; p. 55–62.
- Landmann G. 1994. Concepts, définitions et caractéristiques générales des dépérissements forestiers. Rev for Fr. 46(5):405–415.
- Leather SR. 2005. Insect sampling in forest ecosystems, Methods in ecology. Malden (MA): Blackwell Science Ltd, p. 303.
- Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF, editors. 2007. Bark and wood boring insects in living trees in Europe, a Synthesis. Springer, Netherlands, p. 569.
- Lieutier F, Yart A, Ye H, Sauvard D, Gallois V. 2004. Variations in growth and virulence of Leptographium wingfieldii Morelet, a fungus associated with the bark beetle Tomicus piniperda. Ann for Sci. 61(1):45–53.
- M’hirit O. 2008. Etude des causes de dépérissement de la cédraie du Moyen Atlas: Rapport de synthèse. FAO, p. 110.
- Manion PD. 1991. Tree disease concepts. Englewood Cliffs (NJ): Prentice-Hall, Inc., p. 399.
- Manly B. 2015. Introduction to ecological sampling. Boca Raton: CRC Press, p. 228.
- Martikainen P, Siitonen J, Kaila L, Punttila P, Rauh J. 1999. Bark beetles (Coleoptera, Scolytidae) and associated beetle species in mature managed and old-growth boreal forests in southern Finland. For Ecol Manage. 116(1–3):233–245.
- Mouna M. 1994. Etat des connaissances sur l’entomofaune du Cèdre de l’atlas (Cedrns atlantica Manetti) au Maroc. Ann Rech For Maroc. 27:513–526.
- Mouna M. 2009. Phaenops marmottani Fairmaire (Coleoptera: Buprestidae), xylophage primaire pour le cèdre de l’Atlas (Cedrus atlantica Man.). Bulletin de l’Institut Scientifique Maroc. 31:85–90.
- Mouna M, Graf P. 1994. Les ravageurs xylophages et sous-corticaux du cèdre. In: EL Hassani A, Hamdaoui M, Harrachi K, Messaoudi J, Mzirbi M, Stiki A, editors. Ravageurs et maladies des forêts du Maroc. Rabat (Marocco): DPVCTRF, p. 54–56.
- Nageleisen LM, Saintonge FX, Piou D. 2010. La santé des forêts: maladies, insectes, accidents climatiques: diagnostic et prévention. Paris: Institut pour le développement forestier, p. 608.
- Nichane M, Khelil MA. 2017. Occurrence et répartition verticale des xylophages dépérissant le cyprès vert (Cupressus sempervirens l.) dans les monts des Traras occidentaux (nord occidental Algérien). Lebanese Sci J. 18:186–203.
- Okland B. 1996. A comparison of three rnethods of trapping saproxylic beetles. Eur J Entarnal. 93:195–209.
- Paine T D, Raffa K F, Harrington T C. 1997. Interactions among scolytid bark beetles, , their associated fungi, Aand live host conifers. Annu Rev Entomol. 42(1):179–206.
- Paine TD, Lieutier F. 2016. Insects and diseases of Mediterranean forest systems. Switzerland: Springer International Publishing, p. 892.
- Quezel P. 1998. Cèdres et cédraies du pourtour méditerranéen : signification bioclimatique et phytogéographique. Forêt Méditerranéenne. 19(3):243–260.
- Quezel P, Barbero M. 1990. Les forêts méditerranéennes problèmes posés par leur signification historique, écologique et leur conservation. Acta Botánica Malacitana. 15:145–178.
- Raffa KF, Andersson MN, Schlyter F. 2016. Host selection by Bark Beetles. In: Tittiger C, Blomquist GJ, editors. Advances in insect physiology. 50. Elsevier; p. 1–74.
- Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH. 2008. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience. 58(6):501–517.
- Roche P. 2009. Forêts méditerranéennes au confluent de l’homme et du climat. Forêt Méditerranéenne. 30:304–306.
- Rouault G, Candau J-N, Lieutier F, Nageleisen L-M, Martin J-C, Warzée N. 2006. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann For Sci. 63(6):613–624.
- Rungs CC. 1940. Les ennemis du cèdre au Maroc. Soc Sci Nat Maroc. 5:14–16.
- Sallé A, Nageleisen L-M, Lieutier F. 2014. Bark and wood boring insects involved in oak declines in Europe: Current knowledge and future prospects in a context of climate change. For. Ecol. Manage. 328:79–93.
- Samalens JC. 2009. Stratégies d’échantillonnage des dommages forestiers à l’échelle du paysage – Application aux forêts cultivées de pin maritime (Pinus pinaster, Aït.) [PhD thesis]. France: University Bordeaux 1, 245 p.
- Schmitt M. 1992. Buchen-Totholz als Lebensraum für xylobionte Käfer. Waldhygiene. 19:97–191.
- Shimoda M, Honda KI. 2013. Insect reactions to light and its applications to pest management. Appl Entomol Zool. 48(4):413–421.
- Speight M. 2005. Sampling insects from trees: shoots, stems, and trunks. In: Leather SR, editor. Insect sampling in forest ecosystems. UK: Blackwell Science Ltd; p. 77–115.
- Starzyk JR, Bilecka K, Purgal M, Rotman K. 2008. Cambio- and xylophagous insects infesting Scots pine (Pinus sylvestris L.) cut off tree-tops and branches left in the forest after thinnings and final cuttings. Acta Scientiarum Polonorum. 7(1):59–74.
- Talbi Y, Bouhraoua RT. 2015. Complexe xylophage associé au dépérissement du cèdre de l’atlas au Bélezma (Algérie). Lebanese Sci J. 16:97–106.
- Théry A. 1928. Etudes sur les buprestes de l’Afrique du Nord. Mém Soc Sci Nat Maroc. 19:587.
- Togashi K, Taga Y, Iguchi K, Aikawa T. 2008. Bursaphelenchus mucronatus (Nematoda: Aphelenchoididae) vectored by Monochamus urussovi (Coleoptera: Cerambycidae) in Hokkaido, Japan. Journal of Forest Research. 13(2):127–131.
- Touchan R, Anchukaitis KJ, Meko DM, Attalah S, Baisan C, Aloui A. 2008. Long term context for recent drought in northwestern Africa. Geophys Res Lett. 35(13):5.
- Touchan R, Anchukaitis KJ, Meko DM, Sabir M, Attalah S, Aloui A. 2011. Spatiotemporal drought variability in northwestern Africa over the last nine centuries. Clim Dyn. 37(1–2):237–252.
- Touchan R, Mek DM, Anchukaitis KJ. 2014. Dendroclimatology in the eastern Mediterranean. Radiocarbon. 56(4):S61–S68.
- Vega FE. Hofstetter RW 2015. Bark beetles biology and ecology of native and invasive species. London (UK): Elsevier, p. 602.
- Villiers A. 1946. Coléoptères cérambycidés de l’Afrique du nord. Paris (France): Office de la recherche scientifique coloniale, p. 152.
- Wargo LG. 1995. Disturbance in forest ecosystems caused by pathogens and insects. In: Eskew LG, editor. Proceedings of the 1995 National Silviculture Workshop Mescalero, Forest Health Through Silviculture, 8-11 May 1995, New Mexico. Colorado: USDA Forest Service, Rocky Mountain Forest and Range and Experiment Station Fort Collins, p. 20–25.
- Wermelinger B, Rigling A, Schneider Mathis D, Dobbertin M. 2008. Assessing the role of bark and wood-boring insects in the decline of Scots pine (Pinus sylvestris) in the Swiss Rhone valley. Ecol Entomol. 33(2):239–249.
- Williams PA, Allen CD, Macalady AK, Griffin D, Woodhouse CA, Meko DM, Swetnam TW, Rauscher SA, Seager R, Grissino-Mayer HD, Dean JS, et al. 2013. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat Clim Change. 3(3):292–297.
- Wood DL. 1982. The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu Rev Entomol. 27(1):411–446.