Abstract
The study of vegetative and reproductive phenology through litterfall collection of mangrove species is vital for mangrove management and restoration in climate change situation. This study was carried out to investigate vegetative and reproductive phenological pattern of Heritiera fomes using litterfall data in the Sundarbans, Bangladesh for a period of 24 months. Leaf and stipule litterfall released throughout the year with a distinct periodic pattern. Vegetative litterfall (981.9–1211.3 g/sq m/yr) contributed 59.3% of total litterfall (1791.1–1907.3 g/m/yr). The order of vegetative litterfall was leaf > branch > stipule. Peak flowering and mature fruit litterfall were noticed in April (1.52 times of mean monthly flower production) and July (1.56 times of mean monthly production), respectively. Analysis of variance (ANOVA) showed significant difference (p < 0.05) among monthly litterfall productions, whereas t-test showed no significant difference (p > 0.05) of litterfall production between two successive years during study periods. Vegetative and flower litterfall were significantly influenced by maximum wind speed, mean monthly temperature and day length. On the contrary, mature fruits were significantly correlated with rainfall.
1. Introduction
Mangrove forests are the most productive ecosystems and important contributors of nutrients to the marine and estuarine communities in tropical and subtropical coastal regions (Lugo and Snedaker Citation1975; Nagarajan et al. Citation2008). Mangroves are securely limited to the coastal ecosystems. Mangroves deal recreational potentials, community subsistence, and a sustainable food supply for herbivores, detritivores, and aquatic communities (as mangroves are primary producer) (Alongi Citation1996). Studies of mangrove leaf litterfall have been concentrated on ecological roles in nutrient dynamics, mangrove restoration (Azad et al. Citation2019), and possible interactions between biotic and abiotic factors for many years (Wafar et al. Citation1997). Litterfall not only provides nutrients to the vegetation but also provides energy to the soil and aquatic fauna and micro-organisms (Kathiresan and Bingham Citation2001). Litterfall is the principle component of net primary production in marine ecosystem (Hoque et al. Citation2015; Kamruzzaman, Ahmed, et al. Citation2017; Sarno et al. Citation2018). Litterfall is a sign of the phenophases of a species (Clarke Citation1994; Duke Citation1990). The information on litter dynamics and phenological events of species is essential for understanding of ecological functioning and management of mangrove ecosystem (Duke et al. Citation1984; Rani et al. Citation2016). Phenological studies examine the relationship between plant process (growth and development) and ecosystem components (biotic and abiotic factors) (Kikuzawa and Ackerly Citation1999). Plants differ extensively in their phenological behavior because of morphological traits related to resource gaining and maintenance (Bertiller et al. Citation1991). Phenological studies on vegetative traits development in mangrove species, e.g., recruitment and loss quantify leaf turnover and leaf longevity (Duke et al. Citation1984). Reproductive phenology is important as mangroves propagate only through sexual reproduction system; hence, its survival, existence and recovery completely depend on propagule production, dispersal and establishment (Azad and Matin Citation2012; Tomlinson Citation1986). Phenology of mangrove species may be investigated by direct observation of sample trees and indirect observation through litterfall collection and measurements. Indirect observation is very important for new leaf formation in mangrove vegetation as the occurrence of stipules is a reliable indicator of new leaf formation (Mehlig Citation2006; Sharma et al. Citation2012). Phenology is considered one of the most important and dependable bio-indicator for climate change. Due to climate change, various organisms modify their lifestyles and thereby life cycles. Mainly plants are the most appropriate organisms to study climate change on phenology. Usually plants are sessile and may survive in their habitat which led them to show high plasticity of phenophases, such as bud burst phenology, flushing of leaves (Azad Citation2012), flowering, and fruiting (Peel et al. Citation2019).
Many researchers documented vegetative and reproductive phenology of different species through the indirect observation of litterfall measurement in different mangrove stands around the world (Saenger and Moverley Citation1985; Coupland et al. Citation2005; Mehlig Citation2006; Sharma et al. Citation2012; Kamruzzaman, Sharma, and Hagihara Citation2013; Kamruzzaman et al. Citation2016; Kamruzzaman et al. Citation2019). But there is still lack of information on the vegetative and reproductive phenology of mangrove species of the Sundarbans, Bangladesh. Therefore, the Sundarbans, the largest continuous mangrove forest in the world cannot be ignored for measuring litterfall of phenological events of different species. Phenological events of mangrove communities are influenced by regional environmental factors, mainly day length, temperature, rainfall, tidal inundation and salinity (Saenger and Moverley Citation1985; Fernandes Citation1999; Coupland et al. Citation2005; Kamruzzaman et al. Citation2012; Sharma et al. Citation2012; Kamruzzaman Sharma, and Hagihara Citation2013; Kamruzzaman et al. Citation2013a; Kamruzzaman, Osawa, et al. Citation2017).
Heritiera fomes Buch-Ham is distributed in Bangladesh, India (West Bengal and Orissa), Myanmar, Malaysia, and Thailand. In Bangladesh, it is widely distributed in the Sundarbans, the largest continuous mangrove forest in the world, Chakaria Sundarbans, and in coastal areas of Satkhira, Khulna, Bagerhat and Cox’s Bazar districts. Leaves of this species are simple, alternate and short petiolate. Flowers are unisexual and fruits are indehiscent and one seeded. No previous studies have investigated on vegetative and reproductive phenological events of H. fomes at the Sundarbans mangrove forests, though Kamruzzaman et al. (Citation2019) investigated vegetative and reproductive phenology of Bruguiera. sexangula at the Sundarbans. Rahman and Islam (Citation2015) investigated about phenophases of five mangrove species at the Sundarbans. Therefore, the objectives of the study are to examine the vegetative and reproductive phenological events of H. fomes, and to explore the influences of environmental variables on the vegetative and reproductive organ litterfall of H. fomes.
2. Materials and methods
2.1. Study site
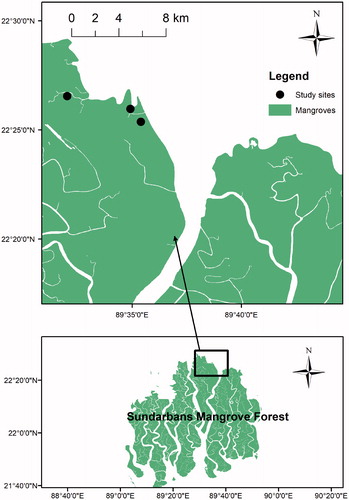
The study was conducted from March in 2016 to February in 2018 at Dhangmari, Karamjol, and Ghagramari areas of the Sundarbans Reserve Forest (SRF), Bangladesh () which lies within the limit of the latitudes between 21°31′and 22°30′N and longitudes between 89°00′ and 89°55′E in the southwest part of Bangladesh. This mangrove forest is a highly diverse and productive ecosystem having 123 plant species (among them 33 true mangrove species) from 22 families maintaining 30 genera (Iftekhar and Islam Citation2004; Iftekhar and Saenger Citation2008; Rahman et al. Citation2015; Azad et al. Citation2020). Species colonization based on salinity is an important factor in the Sundarbans mangrove forest, Bangladesh. This part has three distinct salinity zones: oligohaline/low salinity (<2 ds/m), mesohaline/medium salinity (2–4 ds/m) and polyhaline/high salinity (>4 ds/m) zones (Siddiqi Citation2001). The study sites were located in the oligohaline zone and received regular tidal inundation via the River Passur. The soils of the study sites were medium to hard clay. The topography of the sites was flat.
2.2. Forest structure
Six mangrove species were found at the study plots: Aglaia cucullata (synonym Amoora cucullata) (Roxb.) Pellergr (family: Meliaceae), Avicennia officinalis L. (family: Avicenniaceae), Bruguiera sexangula (Lour) Poir. (family: Rhizophoraceae), Excoecaria agallocha L. (family: Euphorbiaceae), H. fomes Buch-Ham (family: Malvaceae) and Xylocarpus mekongensis J. Koenig (family: Meliaceae). Stand structural characteristics of the study sites were measured. Diameter at breast height (DBH) and height of all the trees (height >1.5 m) in the study sites were measured and numbered in March 2016 (Kamruzzaman et al. Citation2018). Density of the study sites (mean ± SE) was 2629 ± 530 ha−1, DBH (mean ± SE) was 8.7 ± 1.5 cm and height (mean ± SE) was 8.9 ± 1.4 m. The order of importance value in the study sites was H. fomes > E. agallocha > A. officinalis > X. mekongensis > B. sexangula > A. cucullata (Kamruzzaman et al. Citation2018). Shannon–Weiner diversity index and Pielou evenness index were 1.1 ± 0.2 and 0.71 ± 0.09, respectively (Kamruzzaman et al. Citation2018). Mean above-ground biomass and below-ground biomass were 153.7 Mg/ha (ranged from 51.5–314.6 Mg/ha) and 82.3 Mg/ha (ranged from 12.9 to 141.3 Mg/ha), respectively, (Kamruzzaman et al. Citation2018). The mean above-ground carbon, below-ground carbon were found 76.8 ± 20.4 and 41.1 ± 9.8 Mg/ha, respectively (Kamruzzaman et al. Citation2018).
2.3. Climate data
Meteorological data during the study period (from March 2016 to February 2018) was collected from the meteorological department, Dhaka, Bangladesh. The weather observation station is very close (5–8 km) to our study sites. Annual mean temperature during the study period was 30.8 ± 0.50 °C. The temperature fluctuation between the coldest and the warmest months is approximately 14 °C. Annual mean rainfall during the study period was 1636.4 ± 314.9 mm per year. Most of the rainfall (about 80%) appeared during the rainy season. The highest rainfall was 409.3 mm in August 2017 and the lowest precipitation was 0 mm in December 2018. Mean monthly day length during the study period was 274 ± 1.3 hours per month from March 2016 to February 2018. Mean monthly wind speed and relative humidity were 17.66 ± 4.5 km per hour and 61.0 ± 1.1%, respectively, during the study period. In general, the climate conditions of the study sites are characterized by reasonably high temperature and humidity throughout the year and well distributed rainfall during the monsoon. The average temperature of this areas ranges from 18 to 22 °C during winter (November–February) and rises up to 27 to 31 °C rest of the year. The mean annual rainfall varies from 1600 mm to 2000 mm (Chowdhury et al. Citation2016).
2.4. Litter collection
We collected litterfall by using 1-mm litter traps having an open mouth area of 0.42 m2. The opening of the litter trap is relatively small but is sufficiently big to trap litters for the study of vegetative and reproductive phenology of mangrove species. Peel et al. (Citation2019) carried out a study on phenology and floral synchrony of Rhizophora mangle along a natural salinity gradient using litter traps with an open mouth area of 0.16 m2. Sharma et al. (Citation2011) conducted vegetative and reproductive phenology and litterfall production of Rhizophora stylosa in Okinawa Island, Japan using litter traps with an open area of 0.19 m2. Kamruzzaman et al. (Citation2012) carried out a phenological study on litterfall of three subtropical mangrove species in the family Rhizophoraceae using litter traps with an open area of 0.20 m2. Twenty four litter traps were placed at 1 m above the ground to avoid tidal water in 6 plots (two plots in each site, plot size 20 m × 20 m). Litter traps were tightly attached to three neighboring trees to avoid wash away with high tide. Litterfall was collected monthly in a separate cotton bag and marked. Litterfall was separated into leaves, stipules, branches, flower buds, flowers, immature fruits and mature fruits in the laboratory. The separated vegetative and reproductive organs were dried at 80 °C for 48 h and desiccated at room temperature. A digital balance was used for taking weight of each component of vegetative and reproductive organs. Vegetative and reproductive litterfall biomass of H. fomes were estimated directly through measurement of separated oven-dried components using digital balance reported by Kamruzzaman et al. (Citation2018).
2.5. Statistical analysis
Analysis of variance (ANOVA), t-test, principal component analysis (PCA) and Pearson correlation coefficient were carried out for data analysis. ANOVA was carried out to determine the significant difference in monthly litterfall during the study period by using Statistica (version 10) software. We conducted t-test at 95% confidence level to identify the statistical significance of litterfall release between two successive years. PCA was conducted to determine key components which are correlated with other underlying variables of vegetative and reproductive litterfall release and environmental variables. PCA was conducted by using past software (version 2.12). Prior to PCA, the data were log-transferred for normalizing each variable of different measurable scales, so that to equalize variables’ contribution to the ordination plot. Pearson’s correlation coefficient (r) was determined to specify the strength and direction of the linear relationship between litterfall release and environmental variables.
3. Result
3.1. Vegetative litterfall and phenological observation
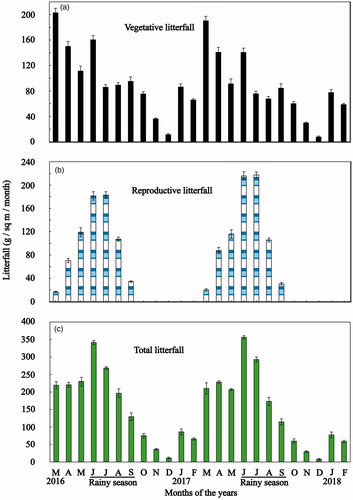
shows the summary of annual amount of vegetative and reproductive organs’ litterfall release during the study period. Mean of the two years vegetative and total litterfall (sum of vegetative and reproductive litterfall) amount were 1096.63 (981.9–1211.3) and 1849.21 (1791.1–1907.3) g/sq m/yr. (mean with 95% confidence limit), respectively. Vegetative litterfall contributed 59.30% of total litterfall production. Among the vegetative litterfall production, leaf litterfall contributed maximum followed by branch and stipule litterfall ().
Table 1. Mean annual mass (g/sq m/yr) of vegetative and reproductive litterfall release of Heritiera fomes in the Sundarbans mangrove forest during study period.
Although H. fomes produced leaf (), stipule () and branch () litterfall throughout the year having a clear seasonal trend, release of leaf litterfall is maximum in summer season (from March to May, peak in March), continued to rainy season (from June to September), and minimum in dry season (November to February, the lowest in November and December but little increased in January and February). During the peak leaf litterfall release in March, the production was 2.17 times of mean monthly production during the study periods. ANOVA showed significant difference (p < 0.05) among monthly leaf litterfall productions. But t-test showed no significant difference (p > 0.05) of leaf litterfall production between two successive years during study periods.
Figure 2. Phenograms of vegetative organs (a) leaf litterfall; (b) stipule litterfall; and (c) branch litterfall for Heritiera fomes during the study period. Vertical bars represent standard error.
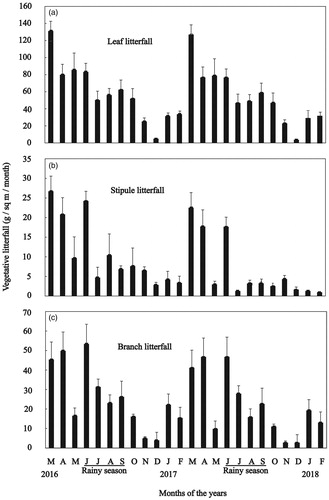
Like leaf litterfall release of H. fomes, stipule litterfall also produced throughout the year () having a clear trend, as occurrence of stipules is an indicator of leaf production. Release of stipule litterfall is maximum in summer season (from March to May, peak in March), followed by rainy season (from June to October), and decreasing in dry season (November to February). During the peak (in March) stipule litterfall release, the production was 2.26 times of mean monthly production throughout the study periods. ANOVA showed a significant difference (p < 0.05) among monthly stipule litterfall productions. But t-test showed no significant difference (p > 0.05) of stipule litterfall production between two successive years during study periods.
Branch litterfall of H. fomes also noticed throughout the year () with a peak release in June. Branch litterfall didn’t show clear seasonality. Branch litterfall appeared maximum in March and April then decreased in May, and increased again in June. It followed a decreasing trend until December after June and then little increased in January and February. During the peak branch litterfall release in June 2016 (53.24 ± 3.46 g/sq m), the production was 2.11 times of mean monthly production (25.23 ± 13.12 g/sq m) during the study periods but in April and June 2017, the production was 1.97 (46.63 ± 3.32 g/sq m) and 1.98 (46.65 ± 3.46 g/sq m) times of mean monthly production, respectively (). ANOVA showed a significant difference (p < 0.05) among monthly branch litterfall productions. But t-test showed no significant difference (p > 0.05) of branch litterfall production between two successive years during study periods.
showed the summary of the annual amount of vegetative and reproductive organs’ litterfall release during the study period. Mean of the two years vegetative and total litterfall (sum of vegetative and reproductive litterfall) amount were 1096.63 (981.9–1211.3) and 1849.21 (1791.1–1907.3) g/sq m/yr. (mean with 95% confidence limit), respectively. Vegetative litterfall contributed 59.30% of total litterfall production. Among the vegetative litterfall production, leaf litterfall contributed maximum followed by branch and stipule litterfall ().
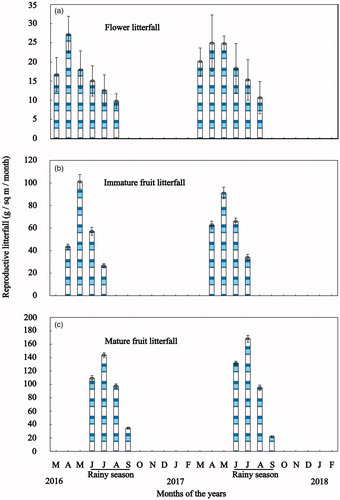
3.2. Reproductive litterfall and phenological observation
Although H. fomes produced vegetative organs throughout the year, it produced reproductive organs for a specific period (). Flower litterfall was noticed from March to August. The peak flowering was noticed in April and then reducing the flowering trend. No flowering was noticed from September to February (). In April (peak flowering season), the production was 1.52 times of mean monthly flower production during the study period. ANOVA showed a significant difference (p < 0.05) among monthly flower litterfall productions. But t-test showed no significant difference (p > 0.05) of flower litterfall between two successive years during study periods. Fruit litterfall was released from April to September, in which immature fruit litterfall noticed from April to June in both years whereas mature fruits observed from June to September. The maximum immature and mature fruit litterfall noticed in May and July, respectively (). During the peak of immature (May) and mature (July) fruit litterfall, the production was 1.59 and 1.56 times of mean monthly production, respectively (). ANOVA showed a significant difference (p < 0.05) among monthly immature litterfall productions throughout the study period. ANOVA also showed a significant difference (p < 0.05) among monthly mature litterfall productions throughout the study period. But t-test showed no significant difference (p > 0.05) of immature and mature litterfall production between two successive years during study periods. Vegetative, reproductive, and total litterfall showed in . The summary of the annual amount of vegetative and reproductive organs’ litterfall showed in during the study period. Mean of the two years reproductive and total litterfall (sum of vegetative and reproductive litterfall) amount were 752.58 (694.4–810.7) and 1849.21 (1791.1–1907.3) g/sq m/yr. (mean with 95% confidence limit), respectively. Reproductive litterfall contributed 40.70% of total litterfall production. The order of reproductive litterfall production was mature fruit > immature fruit > flower litterfall ().
3.3. Relationship between phenological events and environmental variables
PCA of vegetative and reproductive litterfall release with environmental variables showed the ordination plot in which PC 1 (first principal component) and PC 2 (second principal component) explained 63.1 and 10.27% of the variance, respectively, with a big difference in eigenvalues between PC 1 (6.84) and PC 2 (1.28) (). The ordination explained that the environmental variable mainly maximum wind speed was very strongly correlated with PC1. The ordination also explained that mean monthly temperature and day length were strongly correlated with PC1 which satisfied the results of the Pearson correlation coefficient. Pearson correlation coefficient showed mature fruit fall was significantly correlated with monthly mean rainfall and relative humidity. Whereas flower and immature fruit fall were strongly and significantly correlated with monthly mean temperature, maximum wind speed, and day length. Pearson correlation coefficient also showed vegetative litterfall (leaf, stipule, and branch) were significantly correlated with monthly mean temperature, maximum wind speed, and day length but the correlations were not so strong ().
Figure 5. Principal component ordination of vegetative and reproductive litterfall release of Heritiera fomes with environmental factors. The horizontal axis of the ordination plot shows the first principal component (PC 1) with an eigenvalue of 6.84, explaining 63.1% of variance and the vertical axis shows the second principal component (PC 2) with an eigenvalue of 1.28, explaining 10.27% of variance. The dots represent the months of the study period, in which the litterfall and metrological data collected. The dots within the circle represent rainy season.
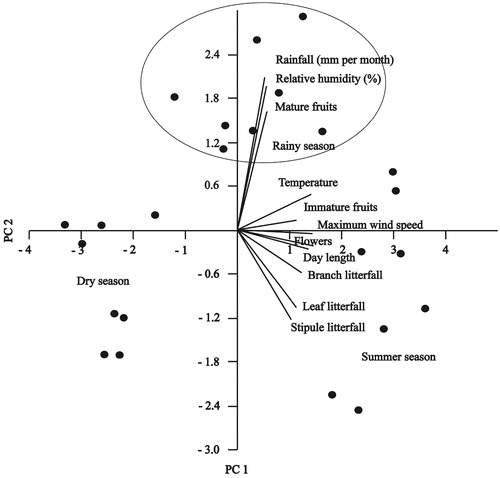
Table 2. Pearson’s correlation coefficient between litterfall release of Heritiera fomes and environmental variables.
4. Discussion
Heritiera fomes is an evergreen mangrove species in the Sundarbans. Leaf and stipule litterfall of this were maximum in summer and rainy season and minimum in dry season. Seasonal patterns of leaf recruitment are highly correlated with those of stipule litterfall (Sharma et al. Citation2014). The balance between leaf fall and leaf gain is important in the maintenance of crown foliage dynamics, which regulate the primary productivity of the forests (Reich et al. Citation1991). In general, evergreen mangrove species flash maximum leaves in summer and rainy season than those in winter (Leach and Burgin Citation1985; Reich et al. Citation1991; Sharma et al. Citation2014). R. stylosa flushed leaves 38.1% in summer of the annual total, whereas only 15.8% flushed in winter (Sharma et al. Citation2014). Similar results were also reported by Kamruzzaman et al. (Citation2019) in Bruguiera sexangula, Allen and Duke (Citation2006) in B. gymnorrhiza, Duke et al. (Citation1984) in Rhizophora apiculata, R. stylosa and B. gymnorrhiza. Litterfall is the heaviest in summer months may be due to reduce transpiration through leaf shedding and in the wet season when freshwater supply increases nutrient input (Wafar et al. Citation1997). Duke et al. (Citation1984) explained that wet season enhanced new leaf production due to consequences of low salinity. Leach and Burgin (Citation1985) argued that lowering of salinity reduced stress, thus mangrove species produce new leaves and shedding leaves. Mangrove communities accumulate salt during the lifetime and leaves become thicker due to deposition of salt. Thus mangrove species loss senescing leaves which are rich in salt to eliminate excessive salt accumulation (Tomlinson Citation1986; Zheng et al. Citation1999).
In tropical mangrove forest, leaf production may not maintain a uniform trend (Wium-Andersen and Christensen Citation1978; Wium-Andersen Citation1981). Several researchers noticed unimodal in B. sexangula (Kamruzzaman et al. Citation2019), bimodal in R. stylosa (Duke et al. Citation1984), and trimodal in R. stylosa (Leach and Burgin Citation1985), in R. apiculata (Duke et al. Citation1984) leaf production trends in tropical mangrove forest. Deviations from these leaf production patterns may result from habitat-specific stresses, for example, aridity and poor soil (Saenger and Snedaker Citation1993); nature of tidal inundation (Wafar et al. Citation1997), and changes of salinity (Leach and Burgin Citation1985). Litterfall production pattern may also result from deviation of climatic factors like day length (Duke Citation1990), air temperature (Saenger and Moverley Citation1985; Duke Citation1990), rainfall (Curtis and McIntosh Citation1951) and wind speed (sometimes storms) (Woodroffe and Moss Citation1984). This irregular leaf production pattern could also be due to some endogenous rhythm within the tree (Williams et al. Citation1981). Leaf fall pattern of the present study was unimodal may be due to regular inundation, no evidence storm during the study and favorable climatic condition. New leaves produced all the year round, suggesting that climatic factors were favorable for leaf emergence throughout year and thus stress did not appear to limit leaf production (Leach and Burgin Citation1985).
The PCA ordination of this study showed maximum wind speed, temperature and day length were significantly correlated with PC1 which explained 63.1% of variance. The correlation between vegetative litterfall (leaf, stipule, and branch) and climatic variables were significant (p < 0.05) but not so strong. The Pearson correlation coefficient showed maximum wind speed was significantly correlated with leaf (p < 0.01), stipple (p < 0.05), and branch fall (p < 0.01). Similarly monthly mean temperature influenced leaf (p < 0.01), stipple (p < 0.05) and branch fall (p < 0.01). Day length also influenced leaf (p < 0.01), stipple (p < 0.05) and branch fall (p < 0.01). Kamruzzaman et al. (Citation2019) noticed that leaf production of B. sexangula in the Sundarbans mangrove forest was also strongly and significantly correlated with monthly day length and monthly mean temperature. Gill and Tomlinson (Citation1971) also documented that leaf production of R. mangle in Florida, USA was maximum in summer. Gwada et al. (Citation2000) mentioned that leaf production of Rhizophorace family species were strongly correlated with air temperature at Sashiki on Okinawa Island, Japan. In the summer months temperature was high, thus trees shed maximum leaf to reduce transpiration (Wafar et al. Citation1997). The contribution of leaf litterfall was maximum to the total vegetative organ production (Wafar et al. Citation1997; Rani et al. Citation2016; Kamruzzaman et al. Citation2019) which was similar to our findings.
The flowering of H. fomes in the Sundarbans was during March to August with a peak in April (Spring), which was very similar to the findings of Rahman and Islam (Citation2015) and Duke et al. (Citation1984). Rahman and Islam (Citation2015) documented that flowering time of most of the mangrove species in the Sundarbans was in the spring. Duke et al. (Citation1984) noticed that most of the mangrove species in Australia flowered during spring to summer. In contrast, Saenger and Moverley (Citation1985) and Fernandes (Citation1999) noticed that most of the mangrove species flowered during summer season. Climatic factors during spring and summer may be favored flowering of mangrove species. Our results from the Pearson correlation coefficient revealed that maximum wind speed, temperature and day length were strongly correlated with Flowering of H. fomes. PCA ordination () also explained that maximum wind speed, temperature and day length were strongly correlated with PC1. Flowering of H. fomes of our study was found for a specific period which was similar to the findings of de Lima Nadia et al. (Citation2012) (Avicennia schaueriana and Laguncularia racemosa), Rahman and Islam (Citation2015) (Ceriops decandra, Excoecaria agallocha and H. fomes) and Kamruzzaman, Sharma, and Hagihara (Citation2013) (Kandelia obovata). In contrast, Kamruzzaman et al. (Citation2019) (B. sexangula), Kamruzzaman et al. (Citation2013a) (R. stylosa) and Mehlig (Citation2006) (R. mangle) noticed continuous flowering throughout the year in different mangrove forests. Thus flowering of different mangrove species was very much seasonal, annual and species specific (de Lima Nadia et al. Citation2012).
Heritiera fomes produced fruits from April to September, in which mature fruits observed from June to September (wet season) with a peak in July. Saenger and Snedaker (Citation1993) documented that wet season (June and July) was the best time for mangrove seed collection from the Sundarbans, Bangladesh. They also mentioned that mature seeds and propagules of most of the mangrove species released during rainy season. Mehlig (Citation2006) documented that the propagule releasing time of R. mangle was restricted to the wet season which provided better environment for propagule establishment and encouraged their survival. Duke et al. (Citation1984) also mentioned that the seeds (propagules) of most of the mangrove species matured during wet season. Seeds and propagules of most of the mangrove species in the Sundarbans dispersed by water bodies, so it is expected that wet season is appropriate for seed (propagule) releasing time. Van der Stocken et al. (Citation2017) reported that flowering appeared during dry season, so that seed dispersal coincided with high water level. They noticed that release of mature fruits (propagule) has strong positive significant correlation with rainfall which was very much similar to our results (). They also noticed that 72% of the reported data propagule release during the rainy season.
The production of reproductive organs by H. fomes in the Sundarbans contributed 40.69% of total litter fall production ( and ). The contribution of this species was higher than B. gymnorrhiza (13.1%) in Okinawa Island, Japan (Mokolensang and Tokuyama Citation1998). The contribution of reproductive organ litterfall of H. fomes was also higher than R. stylosa (21.5%) (Woodroffe and Moss Citation1984); R. apiculata (21.8%) (Wafar et al. Citation1997); K. obovata (18.7) (Wafar et al. Citation1997) and B. gymnorrhiza (37.5%) (Kamruzzaman et al. Citation2013b). Thus the litterfall productivity of H. fomes in the Sundarbans was relatively high ().
5. Conclusion
Heritiera fomes produced vegetative organs throughout the year whereas reproductive organs produced for a specific period. The contribution of vegetative litterfall to total litterfall was higher than reproductive organs litterfall. The order of vegetative organ litter fall was leaf > branch > stipule, whereas the contribution of mature fruits litterfall to reproductive organ litterfall was higher than the others. This study confirmed seasonality of reproductive organ development of H. fomes in the Sundarbans. Our study also confirmed that maximum wind speed, temperature and day length were significantly correlated with leaf, stipules, branch, flower and immature fruit litterfall release of H. fomes. On the contrary, rainfall was strongly correlated with mature fruit litterfall release. Thus reproductive organ production of H. fomes was very much seasonal and annual. The information provided by this study will be useful to understand the ecological significance of seed release of this species to researchers and policy makers in mangrove management, conservation and restoration.
Acknowledgments
The authors are grateful to Bangladesh Forest Department for their valuable support during field work and all the staff and field personnel for assistance with logistics and data collection. The authors are also grateful to late Professor Akira Osawa, Kyoto University, Japan for his contribution to prepare the manuscript. We are very much grateful to Mr. Kalayan Basak, and Mr. Shamim Ahmed for their cordial help during field data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allen JA, Duke NC. 2006. Bruguiera gymnorrhiza (large-leafed mangrove). In: Elevitch, CR, editor. Species profiles for Pacific Island agroforestry permanent agriculture resources (PAR), Hōlualoa, Hawai’i.
- Alongi DM. 1996. The dynamics of benthic nutrient pools and fluxes in tropical mangrove forests. J Marin Res. 54(1):123–148.
- Azad M, Matin M. 2012. Climate change and change in species composition in the Sundarbans mangrove forest, Bangladesh. Meeting on Mangrove Ecology, Functioning and Management (MMM3); Jul 2–6, 2012; Galle, Sri Lanka. p. 34.
- Azad MS. 2012. Observations on bud burst phenology in a field trial established with Poplar (Populus spp.). For Stud China. 14(4):251–259.
- Azad MS, Kamruzzaman M, Kanzaki M. 2020. Canopy gaps influence regeneration dynamics in cyclone affected mangrove stands in medium saline zone of the Sundarbans, Bangladesh. Acta Ecol Sin. https://doi.org/10.1016/j.chnaes.2020.03.002
- Azad MS, Kamruzzaman M, Osawa A. 2019. The influences of cyclone on abundance, species diversity and floristic composition in mangrove ecosystem in the Sundarbans, Bangladesh. Region Stud Marin Sci. 28:100621.
- Bertiller MB, Beeskow AM, Coronato F. 1991. Seasonal environmental variation and plant phenology in arid Patagonia (Argentina). J Arid Environ. 21(1):1–11.
- Chowdhury MQ, De Ridder M, Beeckman H. 2016. Climatic signals in tree rings of Heritiera fomes Buch.-Ham. in the Sundarbans, Bangladesh. PLOS One. 11(2):e0149788.
- Clarke P. 1994. Base-line studies of temperate mangrove growth and reproduction; demographic and litterfall measures of leafing and flowering. Aust J Bot. 42(1):37–48.
- Coupland GT, Paling EI, McGuinness KA. 2005. Vegetative and reproductive phenologies of four mangrove species from northern Australia. Aust J Bot. 53(2):109–117.
- Curtis JT, McIntosh RP. 1951. An upland forest continuum in the prairie forest border region of Wisconsin. Ecology. 32(3):476–496.
- de Lima Nadia T, Morellato LPC, Machado IC. 2012. Reproductive phenology of a northeast Brazilian mangrove community: environmental and biotic constraints. Flora-morphology, distribution. Funct Ecol Plants. 207(9):682–692.
- Duke N, Bunt J, Williams W. 1984. Observations on the floral and vegetative phenologies of north-eastern Australian mangroves. Aust J Bot. 32(1):87–99.
- Duke NC. 1990. Phenological trends with latitude in the Mangrove tree Avicennia Marina. J Ecol. 78(1):113–133.
- Fernandes MEB. 1999. Phenological patterns of Rhizophora L., Avicennia L. and Laguncularia Gaertn. f. in Amazonian mangrove swamps. Hydrobiologia. 413:53–62.
- Gill A, Tomlinson PB. 1971. Studies on the growth of red mangrove (Rhizophora mangle L.) 3. Phenology of the shoot. Biotropica. 3(2):109–124.
- Gwada P, Makoto T, Uezu Y. 2000. Leaf phenological traits in the mangrove Kandelia candel (L.) Druce. Aquat Bot. 68(1):1–14.
- Hoque MM, Mustafa Kamal AH, Idris MH, Haruna Ahmed O, Rafiqul Hoque ATM, Masum Billah M. 2015. Litterfall production in a tropical mangrove of Sarawak. Malaysia Zool Ecol. 25(2):157–165.
- Iftekhar M, Islam M. 2004. Managing mangroves in Bangladesh: a strategy analysis. J Coast Conserv. 10(1):139–146.
- Iftekhar M, Saenger P. 2008. Vegetation dynamics in the Bangladesh Sundarbans mangroves: a review of forest inventories. Wetlands Ecol Manage. 16(4):291–312.
- Kamruzzaman M, Ahmed S, Osawa A. 2017. Biomass and net primary productivity of mangrove communities along the Oligohaline zone of Sundarbans. For Ecosyst. 4(1):16.
- Kamruzzaman M, Ahmed S, Paul S, Rahman MM, Osawa A. 2018. Stand structure and carbon storage in the oligohaline zone of the Sundarbans mangrove forest. Bangladesh For Sci Technol. 14(1):23–28.
- Kamruzzaman M, Kamara M, Sharma S, Hagihara A. 2016. Stand structure, phenology and litterfall dynamics of a subtropical mangrove Bruguiera gymnorrhiza. J for Res. 27(3):513–523.
- Kamruzzaman M, Osawa A, Mouctar K, Sharma S. 2017. Comparative reproductive phenology of subtropical mangrove communities at Manko Wetland, Okinawa Island, Japan. J For Res. 22(2):118–125.
- Kamruzzaman M, Paul SK, Ahmed S, Azad S, Osawa A. 2019. Phenology and litterfall production of Bruguiera sexangula (Lour.) Poir. in the Sundarbans mangrove forests, Bangladesh. For Sci Technol. 15(3):165–172.
- Kamruzzaman M, Sharma S, Hagihara A. 2013. Vegetative and reproductive phenology of the mangrove Kandelia obovata. Plant Spec Biol. 28(2):118–129.
- Kamruzzaman M, Sharma S, Kamara M, Hagihara A. 2013a. Phenological traits of the mangrove Rhizophora stylosa Griff. at the northern limit of its biogeographical distribution. Wetlands Ecol Manage. 21(4):277–288.
- Kamruzzaman M, Sharma S, Kamara M, Hagihara A. 2013b. Vegetative and reproductive phenology of the mangrove Bruguiera gymnorrhiza (L.) Lam. on Okinawa Island. Japan Trees. 27(3):619–628.
- Kamruzzaman M, Sharma S, Rafiqul Hoque ATM, Hagihara A. 2012. Litterfall of three subtropical mangrove species in the family Rhizophoraceae. J Oceanogr. 68(6):841–850.
- Kathiresan K, Bingham BL. 2001. Biology of mangroves and mangrove ecosystems. Adv Marin Biol. 40:81–251.
- Kikuzawa K, Ackerly D. 1999. Significance of leaf longevity in plants. Plant Spec Biol. 14(1):39–45.
- Leach GJ, Burgin S. 1985. Litter production and seasonality of mangroves in Papua New Guinea. Aquat Bot . 23(3):215–224.
- Lugo AE, Snedaker SC. 1975. Properties of a mangrove forest in southern Florida. Gainesville (FL): Institute of Food and Agricultural Sciences, University of Florida.
- Mehlig U. 2006. Phenology of the red mangrove, Rhizophora mangle L., in the Caeté Estuary, Pará, equatorial Brazil. Aquat Bot. 84(2):158–164.
- Mokolensang JF, Tokuyama A. 1998. Litter production of mangrove forests at the Gesashi River. Bull Coll Sci Univ Ryukyus. 65:73–80.
- Nagarajan B, Pandiarajan C, Krishnamoorthy M, Sophia P. 2008. Reproductive fitness and success in mangroves: implication on conservation. Proceedings of Taal 12th World Lake Conference, Jaipur, Rajasthan, India. p. 29–33.
- Peel JR, Golubov J, Mandujano MC, López-Portillo J. 2019. Phenology and floral synchrony of Rhizophora mangle along a natural salinity gradient. Biotropica. 51(3):355–363.
- Rahman MM, Islam SA. 2015. Phenophase of five mangrove species of the Sundarbans of Bangladesh. IJBSC Res. 4:77–82.
- Rahman MM, Khan NIM, Hoque FAK, Ahmed I. 2015. Carbon stock in the Sundarbans mangrove forest: spatial variations in vegetation types and salinity zones. Wetlands Ecol Manage. 23(2):269–283.
- Rani V, Sreelekshmi S, Preethy CM, BijoyNandan S. 2016. Phenology and litterfall dynamics structuring Ecosystem productivity in a tropical mangrove stand on South West coast of India. Regional Stud Marine Sci. 8:400–407.
- Reich P, Uhl C, Walters M, Ellsworth D. 1991. Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia. 86(1):16–24.
- Saenger P, Moverley J. 1985. Vegetative phenology of mangroves along the Queensland coastline. Proc Ecol Soc Aust. 13:257–265.
- Saenger P, Snedaker SC. 1993. Pantropical trends in mangrove above-ground biomass and annual litterfall. Oecologia. 96(3):293–299.
- Sarno v, Suwignyo RA, Dahlan Z, Munandar M, Ridho MR. 2018. Reproductive phenology of Bruguiera sexangula (Lour.) Poir. In Berbakand Sembilang National Park, South Sumatra. J Biol Res. 23(2):62–69.
- Sharma S, Hoque AR, Analuddin K, Hagihara A. 2014. A model of seasonal foliage dynamics of the subtropical mangrove species Rhizophora stylosa Griff. growing at the northern limit of its distribution. Forest Ecosyst. 1(1):15.
- Sharma S, Kamruzzaman M, Hoque A, Analuddin K, Hagihara A. 2011. Vegetative and reproductive phenology, and litterfall production of Rhizophora stylosa in Okinawa Island. Japan. Int J Enviorn. 1:20–26.
- Sharma S, Kamruzzaman M, Rafiqul Hoque ATM, Hagihara A. 2012. Leaf phenological traits and leaf longevity of three mangrove species (Rhizophoraceae) on Okinawa Island. J Oceanogr. 68(6):831–840.
- Siddiqi NA. 2001. Mangrove forestry in Bangladesh. Chittagong, Bangladesh: Institute of Forestry & Environmental Sciences, University of Chittagong.
- Tomlinson PB. 1986. The botany of mangroves. London: Cambridge University Press.
- Van der Stocken T, López-Portillo J, Koedam N. 2017. Seasonal release of propagules in mangroves – assessment of current data. Aquat Bot. 138:92–99.
- Wafar S, Untawale AG, Wafar M. 1997. Litter fall and energy flux in a mangrove ecosystem. Estuarine Coastal Shelf Sci. 44(1):111–124.
- Williams WT, Bunt JS, Duke NC. 1981. Mangrove litter fall in North-Eastern Australia. II. Periodicity. Aust J Bot. 29(5):555–563.
- Wium-Andersen S. 1981. Seasonal growth of mangrove trees in Southern Thailand. III. Phenology of Rhizophora mucronata Lamk. and Scyphiphora hydrophyllacea Gaertn. Aquat Bot. 10:371–376.
- Wium-Andersen S, Christensen B. 1978. Seasonal growth of mangrove trees in southern Thailand. II. Phenology of Bruguiera cylindrica, Ceriops tagal, Lumnitzera littorea and Avicennia marina. Aquat Bot. 5:383–390.
- Woodroffe CD, Moss TJ. 1984. Litter fall beneath Rhizophora stylosa griff., Vaitupu, Tuvalu, South Pacific. Aquat Bot. 18(3):249–255.
- Zheng W-j, Wang W-q, Lin P. 1999. Dynamics of element contents during the development of hypocotyles and leaves of certain mangrove species. J Exp Mar Biol Ecol. 233(2):247–257.