Abstract
The concentration of atmospheric CO2 is increasing largely owing to human activities. Although it is well established that elevated CO2 (eCO2) stimulates plant growth and primary productivity, the effect of eCO2 on soil microbial communities remains poorly understood. In this study, we aimed to examine the effects of eCO2 on the taxonomical diversity, composition, and structure of rhizosphere microbial communities of Korean red pine (Pinus densiflora) through next-generation sequencing (NGS) of 16S rRNA genes. Three bacterial phyla, Proteobacteria, Acidobacteria, and Actinobacteria, were found to be dominant in all samples. Species richness estimates (Chao1 and ACE) and diversity indices (Shannon and Simpson) for the three sampling chambers were the highest from the eCO2 (approximately 1.4× ambient, ≈ 560 ppm). These findings suggested that elevated atmospheric CO2 affected the bacterial community composition in the rhizosphere of Korean red pine (Pinus densiflora).
Introduction
The effect of climate change and global warming on the natural forest ecosystem as well as the trees in the ecosystem are very important in relation to global climatic changes, as they play a key role in the global carbon balance and carbon sequestration (Ncipha and Sivakumar Citation2019). Carbon dioxide (CO2) concentration started at around 280 parts per million (ppm) in pre-industrial times, but it now exceeds 400 ppm and is expected to reach 600–800 ppm by the end of the century (Quirk et al. Citation2019).
To understand the physiological response and determine the future consequences of the increasing atmospheric CO2 concentration on trees, long-term field experiments are necessary to improve our understanding of the responses of trees (Morin et al. Citation2018). Several trees, including Pinus radiata (Tissue et al. Citation2001), P. ponderosa (Pushnik et al. Citation1999), Norway spruce (Bader et al. Citation2016), populus (Yadav et al. Citation2019), P. sylvestris (Jach and Ceulemans Citation1999), Picea sitchensis (Laitat and Boussard Citation1995), and Picea abies (Janouš et al. Citation2000), have been shown to control CO2 concentrations in open-top chambers (OTCs), which provide moderately precise environmental controls in relatively natural, field-like conditions.
It is well documented that elevated CO2 (eCO2) induces an initial stimulation of plant growth (Jifon and Wolfe Citation2005), and studies on soil microbial community around plant roots under eCO2 have been performed with crop plants, as their rhizosphere contains a diverse and complex microbial structure and a community critical for the health of plants and the processing of soil organic matters (OMs) (Nie et al. Citation2015; Wang et al. Citation2017). Despite the abundance and the importance of soil microorganisms for key forest ecosystem functions, the effects of eCO2 on rhizosphere microbial communities in trees have not been well characterized.
Korean red pine (P. densiflora) is one of the most economically and culturally important tree species in Korea and widely distributed throughout Korea, Japan, and northeastern China (Kang et al. Citation2015). However, the growth and habitats of conifer trees, including pine tree, have been reduced owing to climate change and global warming (Battles et al. Citation2008; Jo et al. Citation2019). It is well known that beneficial plant-associated microorganisms promote plant growth and enhance plant resistance to various environmental stresses, including eCO2, drought, warming, diseases, and insects (Lau et al. Citation2017; Yadav et al. Citation2018). The symbiotic relationship between fungi and pine roots has been well studied (Dhyani et al. Citation2019), but there is limited information available regarding soil microbes in the rhizospheres of Korean red pine. Therefore, the use of culture-independent molecular techniques has led to a new understanding of bacterial diversity, as improvements in sequencing technology have allowed for more robust estimates of microbial diversity in natural environments.
In this study, we used 16S rRNA gene-based pyrosequencing technique to investigate changes in soil microbial community structure under eCO2 in an OTC experiment, which was established in South Korea. Korean pine trees were exposed to different concentrations of CO2 in OTCs for 9 years. The objective of this study was to evaluate the rhizosphere microbial diversity of Korean red pine and to examine how CO2 affects changes in microbial community.
Materials and methods
OTC system
The OTC system was established for long-term studies to monitor the effect of climate change on Korean red pine and its community in Korea (37°15′N, 136°57′E; 49 m above sea level (a.s.l.)). The OTC facility consisted of three decagon chambers (10 m in diameter by 10 m in height) with controlled gas concentration. For this experiments, seedlings of 1-year old were planted on each OTC and investigations started 4 years after planting. The experiment consisted of three treatments: two eCO2 levels (approximately 1.4× (560 ppm) and 1.8× (680 ppm) ambient CO2) and one control chamber (ambient CO2, 380 ppm). Differences in temperature and relative humidity between the three OTC chambers were not detected.
Growth measurements
The heights and root diameters of Korean red pine were annually measured at the end of the growing season after the trees were planted.
Chemical analyses of plant leaf and soil
The collected leaves samples were freeze-dried and ground, and carbon and nitrogen concentrations were determined by elemental analyzers (Thermo Scientific, Flash 2000, Rodano, Italy). Phosphorus concentration was pulverized sample after wet digestion (Kalra and Maynard, Citation1991), and then it was analyzed using ICP (Perkin Elmer Optima 5300, Waltham, MA). Three replicates of rhizosphere soils and 500 g of fresh sunlit leaves of pine trees were collected from the three Korean red pines for each treatment. The collected soil samples were filtered to 2 mm after air-dried, and physicochemical analysis was performed according to the methods of Soil and Plant Analysis I (National Institute of Forest Science Citation2014a) and II (National Institute of Forest Science Citation2014b) by National Institute of Forest Science. Soil acidity and electrical conductivity (EC) were measured using pH and conductivity meter after shaking with distilled water at a ratio of soil and water of 1:5 (s). OM content in the soil was measured by oxidizing OM with potassium dichromate to measure organic carbon content, and multiplied by the conversion factor (1.724) to quantify the OM content (Tyurin method). Total nitrogen (TN) was completely decomposed by adding concentrated sulfuric acid and catalyst to the soil sample, collected in boric acid after Kjeldahl distillation, and then it was titrated with NH4+ using 0.1 N sulfuric acid to quantify TN (%) in the soil. Available phosophate (mg/kg) was analyzed using a UV spectrophotometer after leaching phosphoric acid from soil by the Bray I method and color-developing with ascorbic acid. Cation exchange capacity was measured by adsorbing the soil surface with NH4+ ions using ammonium acetate, and then titrating NH4+ through Kjeldahl distillation. Cations (K+, Na+, Ca2+, Mg2+) were analyzed for each concentration by measuring the solution leached with ammonium acetate with an atomic absorption spectrophotometer (AAS).
PCR amplification and Illumina MiSeq sequencing
Three replicates of rhizosphere soil DNA from Korean red pine in the three different CO2 concentration treatment chambers were extracted using a Power Soil DNA extraction kit (Mobio, Carlsbad, CA). Pyrosequencing was conducted in accordance with a previous research (Lee and Eom Citation2016). PCR reaction was carried out using primers targeting the V1–V3 regions of the 16S rRNA gene from the extracted soil DNA. The primers used for bacterial amplification were 27 F (5 (CCTATCCCCTGTGT-GCCTTGGCAGTC-TCAG-AC-GAGTTTGATCMTGG-CTCAG-3CAG-33T518R (5′-CCATCTCATCCCTG-CGTGTCTCCGAC-TCAG-X-AC-WTTACCGCGGCT-GCTGG-3′). Sequencing was performed at Chun lab, Inc. (Seoul, Korea) using a 454 GS FLX titanium next-generation sequencing (NGS) system (Roche, Branford, CT) according to the manufacturer’s instructions and previously described procedures (Chun et al. Citation2010).
Sequencing data analysis
Sequencing data were analyzed as previously described (Chun et al. Citation2010; Lee and Eom Citation2016). For NGS analysis, the obtained reads were subjected to quality check and trimming of low-quality scores (defined as average scores of <25) by Trimmomatic version 0.32 (Bolger et al. Citation2014). The complete hierarchical taxonomic classification of each read was assessed using a BLASTN search against the EzTaxon-e database (http://eztaxon-e.ezbiocloud.net) containing 16S rRNA gene sequences of type strains that had valid published names and representative species-level phylotypes (Kim et al. Citation2012). The richness and diversity of pine rhizosphere microbial communities were determined by Chao1 estimation and Shannon diversity index at the 3% distance. The overall phylogenetic distance among the treatments was estimated using Fast UniFrac (Hamady et al. Citation2010). To compare operational taxonomic units (OTUs) between samples, shared OTUs were obtained with the CL community program’s Taxon XOR by CD-HIT analysis (Chunlab Inc., Seoul, South Korea).
Results
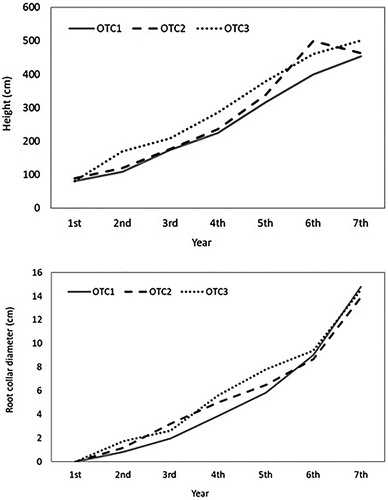
The annual growth in height and diameter of Korean red pine is presented in . The results showed that plant growth tend to increase with increasing CO2 concentration owing to the CO2 fertilization effect caused by high concentrations of CO2. However, the difference in growth was not significant.
Figure 1. Cumulative heights and root diameters of Korean red pine from three different CO2 concentration treatment chambers. OTC1: Elevated CO2 level as 1.4x (approximately 560 ppm), OTC2: elevated CO2 level as 1.8x (approximately 680 ppm), and OTC3: ambient CO2 level (approximately 380 ppm).
NGS of 27 rhizosphere soil samples from Korean red pine resulted in approximately over 13,569 reads per sample, representing 40 phyla, 150 classes, and 500 genera of bacteria and archaea, as well as 2045–2874 OTUs at a similarity level of 97%. The numbers of observed OTUs from the rhizosphere of pine trees were significantly different among different CO2 concentrations in the OTC chambers. The rarefraction curves calculated with QIIME pipeline at 97% similarity also showed different OTU richness patterns for all three chambers ().
Figure 2. Rarefaction curves for bacterial operational taxonomic units (OTUs; 97% sequence similarity) in each sampling chamber associated with the rhizosphere of pine tree. In the rarefaction curves, the number of OTUs increased with the number of sequencing reads. OTC1: elevated CO2 level as 1.4x (approximately 560 ppm), OTC2: elevated CO2 level as 1.8x (approximately 680 ppm), and OTC3: ambient CO2 level (approximately 380 ppm).
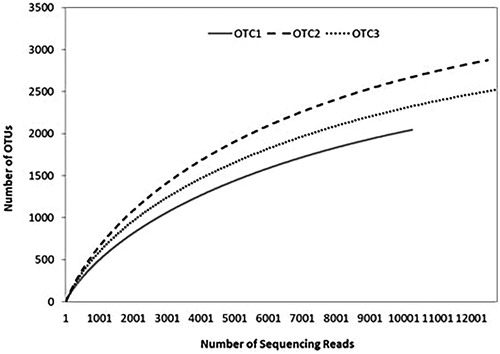
OTU richness estimations, such as Chao 1, indicated that samples collected from control chamber OTC1 (ambient CO2) contained the lowest number of bacteria. However, exposure to eCO2 altered the microbial populations and diversity. Comprehensive OTU richness analysis results ( and ) revealed that the bacterial community of OTC2 (CO2 × 1.4) was the most diverse. Rarefaction analysis of the OTUs also indicated that bacterial diversity and richness significantly increased as CO2 concentration increased (p < .01). In particular, bacterial diversity and richness from OTC2 (approximately 1.4× ambient, ≈560 ppm) were the highest.
Table 1. Comparison of the estimated OTU richness and diversity indexes in the rhizosphere of Korean red pine according to CO2 concentration.
Shannon index analysis revealed that the values of bacterial diversities in each chamber were 6.22 in OTC1, 7.20 in OTC2, and 6.88 in OTC3, which were consistent with the results of rarefaction analysis and the rank-abundance curves ().
Each bacterial 16S rRNA gene sequence was taxonomically assigned from the phylum level to the species level using the Ribosomal Database Project Classifier. The proportion of unclassified bacteria was no more than 0.1%. A total of 44 phyla were identified in all three chambers. Particularly, three bacterial phyla, namely Proteobacteria, Acidobacteria, and Actinobacteria, were the most dominant in all three chambers (). The three dominant phyla accounted for 69.1%, whereas other phyla accounted for 30.9%. The relative abundance was more than 37.2% for Proteobacteria, 17.6% for Acidobacteria, and 14.3% for Actinobacteria. The following phyla were also observed: Planctomycetes (5.5%), Chloroflexi (5.3%), Bacteroidetes (4.8%), Verrucomicrobia (4.6%), Gemmatimonadetes (1.8%), Firmicutes (1.2%), Cyanobacteria (1.3%), Chlamydiae (2.5%), Nitrospirae (1.8%), Armatimonadetes (1.2%), and others (1.7%), with less than 1% composition for each phylum ().
Figure 3. Similarity of phylogenetic diversity at the phylum level (97% sequence similarity) for bacterial communities. The hierarchical relationship was obtained via the unweighted pair group method with arithmetic mean (UPGMA) clustering dendrogram. OTC1: elevated CO2 level as 1.4x (approximately 560 ppm), OTC2: elevated CO2 level as 1.8x (approximately 680 ppm), and OTC3: ambient CO2 level (approximately 380 ppm).
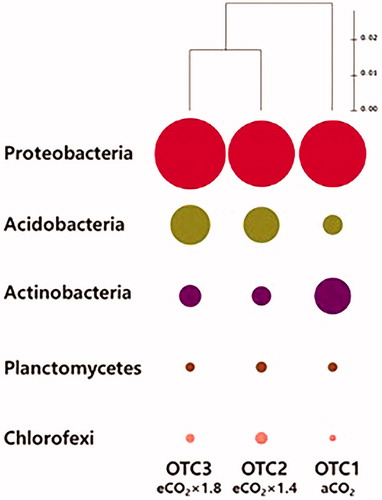
Figure 4. Relative distribution (%) of the main bacteria taxa detected in the rhizosphere of Korean red pine tree according to CO2 concentration. OTC1: elevated CO2 level as 1.4x (approximately 560 ppm), OTC2: elevated CO2 level as 1.8x (approximately 680 ppm), and OTC3: ambient CO2 level (approximately 380 ppm).
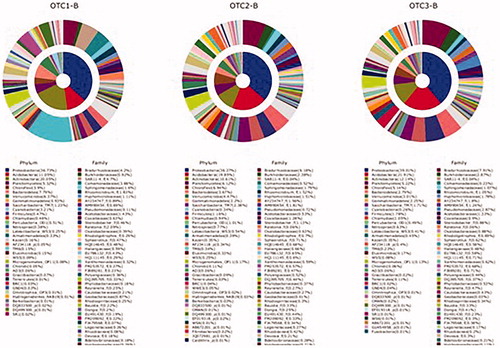
We compared the constituent taxa of rhizosphere soil bacterial communities and their rank orders among all chambers. At the phylum level, Proteobacteria was the most abundant phyla in the rhizosphere soil of pine tree. The proportion of Proteobacteria, Gemmatimonadetes, and Firmicutes significantly increased with eCO2 as follows: Proteobacteria: 36.6% in OTC1, 37.2% in OTC2, and 38.9% in OTC3; Gemmatimonadetes: 0.9% in OTC1, 2.2% in OTC2, and 2.2% in OTC3; Firmicutes: 0.5% in OTC1, 1.2% in OTC2, and 1.8% in OTC3.
At the class level, Alpha-proteobacteria (18.7%), Beta-proteobacteria (10.3%), Actinobacteria (9.7%), Solibacteres (4.7%), Verrucomicrobiae (4.3%), and Gammaproteobacteria (4.0%) were observed. Interestingly, the proportion of Actinobacteria significantly decreased as CO2 concentration increased. The proportions were 16% in OTC1, 6% in OTC2, and 7% in OTC3.
In the family level, the proportions of Bradyrhizobiaceae (18.3%), Acidobacteriaceae (11.3%), and Planctomycetaceae (11%) significantly increased in the eCO2. The proportions were was 4.2% in OTC1, 6.2% in OTC2, and 7.9. The proportion of Acidobacteriaceae (11.3%) also increased as CO2 concentration increased.
In the genus level, the proportion of Acidobacteria significantly increased with increasing CO2: 11% in OTC1, 19.8% in OTC2, and 21.9% in OTC3. However, those of Actinobacteria, Cyanobacteria, and Bacteroidetes decreased as follows: Actinobacteria: 20.1% in OTC1, 10.6% in OTC2, and 12.1% in OTC3; Cyanobacteria: 3.2% in OTC1, 0.3% in OTC2, and 0.3% in OTC3; Bacteroidetes: 7.8% in OTC1, 3.7% in OTC2, and 2.8% in OTC3.
Foliar chemical analysis revealed that the total organic carbon, nitrogen, and total phosphate content tended to decrease with increasing CO2. The total organic carbon and phosphate content in the leaves tended to decrease with increasing CO2, although the decrease was not significant (). Rhizosphere soil chemical analysis showed that soil acidity, phosphoric acid, and CEC significantly decreased in the CO2-treated groups. Soil OM, potassium, sodium, and magnesium content were not significant among three treatments ().
Table 2. Chemical properties of Korean red pine leaves according to CO2 concentration.
Table 3. Soil chemical properties in the rhizosphere of Korean red pine according to CO2 concentration.
Discussion
We evaluated microbial communities in the rhizosphere of Korean red pine and compared them among different concentrations of CO2 in OTCs with Illumina MiSeq high-throughput sequencing. This was the first study to describe microbial community composition in the rhizosphere of Korean red pine. The microbial community plays an important role in the soil biochemical cycle. In addition, many environmental factors, such as soil OM chemistry, plant species, soil pH, and environmental factors, can affect rhizosphere microbial diversity. In the case of rhizosphere, the community has a very close relationship with plant growth and health, as the root exudate composition can affect rhizospheric microbial communities (An et al. Citation2020). In the phylum level, three bacterial phyla, including Proteobacteria, Acidobacteria, and Actinobacteria, accounted for a high proportion of pine rhizosphere soil. In particular, Proteobacteria occupied the highest proportions in the rhizosphere. In general, Proteobacteria is a major group (phylum) of soil bacteria, and its abundance in the soil promotes various substrates to thrive and thus plays a very important role in nitrogen fixation, substrate degradation, and biochemical cycles, especially in soil ecosystems (Malisorn et al. Citation2020). Acidobacteria are also widely distributed and abundant in soils, but their ecological roles are poorly understood (He et al. Citation2012). Most phylotypes were detected at both ambient CO2 and eCO2, with only a few detected at only ambient CO2 or only eCO2. At the phylum level, the number of OTUs was 4740 (36.6%) in OTC1, 5822 (36.2%) in OTC2, and 6194 (38.9%) in OTC3 for Proteobacteria, a phylum with the highest number of detectable OTUs, followed by Acidobacteria (17.6%) and Actinobacteria (14.3%). Firmicutes and Bacteroidetes are involved in fermentation. However, in samples from the eCO2-treated groups, the proportion of Firmicutes and Bacteroides was only 1.2 and 4.8%, respectively.
Members of the Alpha, Beta, and Gamma sub-phyla of Proteobacteria are considered eutrophs, and they are more prevalent where resource availability is high, such as in rhizosphere soils. Generally, elevated atmospheric CO2 stimulates the flow of organic C into the soil system (Kuzyakov et al. Citation2019). In addition, CO2 increases root production and exudation. Moreover, eCO2 stimulates photosynthesis, leading to increased carbon uptake and assimilation, thereby increasing plant growth and microbial biomass (Prior et al. Citation2011). There is little evidence that atmospheric CO2 enrichment will increase total soil OM content because greater C flow into soil stimulates the soil food web, often leading to equivalent increases in soil CO2 efflux (Pritchard Citation2011).
Bradyrhizobiaceae (18.3%) is a family of Rhizobiales order in the Alphaproteobacteria class. It includes plant-associated bacteria, such as Bradyrhizobium, a genus of rhizobia that could fix nitrogen (Larimer et al. Citation2004). However, nitrogen was significantly reduced under eCO2. These phenomena were consistent with reports from previous studies that when CO2 concentration in the air increases, the TN content of the leaves decreases. Although three trees in the treatment groups were not sufficient to show significant differences, there was no decrease in productivity parameters, such as height and root collar diameter, due to nitrogen reduction. Growth is expected to decrease as the period of exposure to high-concentration CO2 increases. Annual growth analysis in this study revealed that exposure to CO2 initially had a significant effect on plant growth owing to the fertilizing effect of CO2, but it is believed that the effect of high-concentration CO2 will not last long. For this reason, it is expected that the diversity and abundance of microorganisms and various indices were the highest in OTC2 (approximately 1.4× ambient, ≈560 ppm).
Our current findings indicated that the composition and variation of microbial communities of endogenous bacteria or fungi in Korean red pine in different eCO2 conditions. These studies will help elucidate the interactions between microorganisms and pine trees in a more comprehensive manner, overcome challenge associated with climate change, and promote health.
Conclusion
By comparing the characteristics of Korean red pine rhizosphere bacterial community among different concentrations of CO2 by Illumina MiSeq sequencing, we showed that there were differences in the dominant rhizosphere bacterial taxonomic composition and community functions among the different concentrations of CO2. Moreover, CO2 concentrations higher than 560 ppm were shown to be beneficial for plant growth in the early stages, but damaging in the long term. The richness of the rhizosphere bacterial communities and the abundance of beneficial microbes, such as Acidobacteria, Gemmatimonadetes, and Firmicutes, increased as CO2 concentration increased. Overall, our results revealed atmospheric CO2 concentration as the main factor influencing bacterial community composition and diversity in the rhizosphere of the Korean red pine. In addition, our results suggested that the compositional structure of bacterial communities in the rhizosphere of pine tree could be determined by conditions associated with CO2 in the air, as climate change due to increased atmospheric CO2 affects physiological mechanisms, such as photosynthesis, and this usually leads to an increase in growth. Thus, our study confirmed a strong positive correlation between CO2 concentration and composition of rhizosphere microbes, providing a strategy to cope with climate change, which causes destruction of the habitat of conifer trees, through improvement of soil bacterial communities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- An J, Chang H, Han SH, Khamzina A, Son Y. 2020. Changes in basic soil properties and enzyme activities along an afforestation series on the dry Arial Sea Bed, Kazakhstan. For Sci Technol. 16:26–31.
- Bader MKF, Mildner M, Baumann C, Leuzinger S, Körner C. 2016. Photosynthetic enhancement and diurnal stem and soil carbon fluxes in a mature Norway spruce stand under elevated CO2. Environ Exp Bot. 124:110–119.
- Battles JJ, Robards T, Das A, Waring K, Gilless JK, Biging G, Schurr F. 2008. Climate change impacts on forest growth and tree mortality: a data-driven modeling study in the mixed-conifer forest of the Sierra Nevada, California. Clim Change. 87(S1):193–213.
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120.
- Chun J, Kim KY, Lee JH, Choi Y. 2010. The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol. 10(1):101.
- Dhyani A, Jain R, Pandey A. 2019. Contribution of root-associated microbial communities on soil quality of oak and pine forests in the Himalayan ecosystem. Trop Ecol. 60(2):271–280.
- Hamady M, Lozupone C, Knight R. 2010. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 4(1):17–27.
- He Z, Piceno Y, Deng Y, Xu M, Lu Z, DeSantis T, Andersen G, Hobbie SE, Reich PB, Zhou J. 2012. The phylogenetic composition and structure of soil microbial communities shifts in response to elevated carbon dioxide. ISME J. 6(2):259–272.
- Jach ME, Ceulemans R. 1999. Effects of elevated atmospheric CO2 on phenology, growth and crown structure of Scots pine (Pinus sylvestris) seedlings after two years of exposure in the field. Tree Physiol. 19(4–5):289–300.
- Janouš D, Pokorný R, Brossaud J, Marek M. 2000. Long-term effects of elevated CO2 on woody tissues respiration of Norway spruce studied in open-top chambers. Biologia Plant. 43(1):41–46.
- Jifon JL, Wolfe DW. 2005. High temperature-induced sink limitation alters growth and photosynthetic acclimation to elevated CO2 in bean (Phaseolus vulgaris L.). JASHS. 130(4):515–520.
- Jo H, Chang H, An J, Cho MS, Son Y. 2019. Species specific physiological responses of Pinus densiflora and Larix Kaempferi seedlings to open-field experimental warming and precipitation manipulation. For Sci Technol. 15(1):44–50.
- Kalra Y, Maynard DG. 1991. Methods manual for forest soil and plant analysis. Forestry Canada, Northwest Region, Northern Forestry Centre. Information report, NOR-X-319E, Edmonton, Alberta, p. 116.
- Kang JW, Kim HT, Lee WY, Choi MN, Park EJ. 2015. Identification of a potential metabolic marker, inositol, for the inherently fast growth trait by stems of Pinus densiflora via a retrospective approach. Can J For Res. 45(6):770–775.
- Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, et al. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 62(Pt_3):716–721.
- Kuzyakov Y, Horwath WR, Dorodnikov M, Blagodatskaya E. 2019. Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: no changes in pools, but increased fluxes and accelerated cycles. Soil Biol Biochem. 128:66–78.
- Laitat E, Boussard H. 1995. Comparative response on gas exchange of Picea spp. exposed to increased atmospheric CO2 in open top chambers at two test sites. J Biogeogr. 22(2/3):241–248.
- Larimer FW, Chain P, Hauser L, Lamerdin J, Malfatti S, Do L, Land ML, Pelletier DA, Beatty JT, Lang AS, Tabita FR, et al. 2004. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat Biotechnol. 22(1):55–61.
- Lau JA, Lennon JT, Heath KD. 2017. Trees harness the power of microbes to survive climate change. Proc Natl Acad Sci USA. 114(42):11009–11011.
- Lee SY, Eom YB. 2016. Analysis of microbial composition associated with freshwater and seawater. Biomed Sci Lett. 22(4):150–159.
- Malisorn K, Chanchampa S, Kanchanasin P, Tanasupawat S. 2020. Identification and plant growth-promoting activities of proteobacteria isolated from root nodules and rhizospheric soils. Curr J Appl Sci Technol. 20:479–493.
- Morin X, Fahse L, Jactel H, Scherer-Lorenzen M, García-Valdés R, Bugmann H. 2018. Long-term response of forest productivity to climate change is mostly driven by change in tree species composition. Sci Rep. 8(1):5627.
- National Institute of Forest Science. 2014a. Laboratory guide for conducting soil tests and plant analysis I- soil physical properties. Seoul, Korea: National Institute of Forest Science; p. 6–16.
- National Institute of Forest Science. 2014b. Laboratory guide for conducting soil tests and plant analysis II- soil chemical properties. Seoul, Korea: National Institute of Forest Science; p. 6–256.
- Ncipha XG, Sivakumar V. 2019. Natural carbon sequestration by forestry. Sustainable agriculture reviews; p. 73–92. Cham: Springer.
- Nie M, Bell C, Wallenstein MD, Pendall E. 2015. Increased plant productivity and decreased microbial respiratory C loss by plant growth-promoting rhizobacteria under elevated CO2. Sci Rep. 5:9212.
- Prior SA, Runion GB, Marble SC, Rogers HH, Gilliam CH, Torbert HA. 2011. A review of elevated atmospheric CO2 effects on plant growth and water relations: implications for horticulture. HortScience. 46(2):158–162.
- Pritchard S. 2011. Soil organisms and global climate change. Plant Pathol. 60(1):82–99.
- Pushnik JC, Garcia-Ibilcieta D, Bauer S, Anderson PD, Bell J, Houpis JL. 1999. Biochemical responses and altered genetic expression patterns in Ponderosa pine (Pinus ponderosa Doug ex P. Laws) grown under elevated CO2. In: Sheppard LJ, Cape JN, editors. Forest growth responses to the pollution climate of the 21st century. Springer-Dordrecht; 413–422.
- Quirk J, Bellasio C, Johnson DA, Beerling DJ. 2019. Response of photosynthesis, growth and water relations of a savannah-adapted tree and grass grown across high to low CO2. Ann Bot. 124(1):77–90.
- Tissue DT, Griffin KL, Turnbull MH, Whitehead D. 2001. Canopy position and needle age affect photosynthetic response in field-grown Pinus radiata after five years of exposure to elevated carbon dioxide partial pressure. Tree Physiol. 21(12–13):915–923.
- Wang P, Marsh EL, Ainsworth EA, Leakey AD, Sheflin AM, Schachtman DP. 2017. Shifts in microbial communities in soil, rhizosphere and roots of two major crop systems under elevated CO2 and O3. Sci Rep. 7(1):1–12.
- Yadav SK, Singh H, Ginwal H, Barthwal S. 2019. Elevated CO2 Enhanced Growth and Physiological Process of Populus deltoides Bartr. Ex Marsh Indian For. 145(1):23–27.
- Yadav AN, Verma P, Kumar V, Sangwan P, Mishra S, Panjiar N, Gupta VK, Saxena AK. 2018. New and future developments in microbial biotechnology and bioengineering. Penicillium system properties and applications; p. 3–18. Amsterdam: Elsvier.
