 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Deteriorated seeds can be improved by invigoration treatment, including gamma-ray irradiation by Cobalt-60 (60Co). The purpose of this study was to determine the proper dose of gamma-ray irradiation (60Co) to increase the decreased vigor of suren (Toona sureni (Blume) Merr.) seeds. This study uses a factorial completely randomized design (CRD) with aging and irradiation as the treatments. Aging factors consisted of 0, 2, and 4 days. Irradiation factor consisted of 7 levels, namely 0, 5, 10, 20, 40, 80, and 100 Gy, repeated four times. The parameters observed were the electrical conductivity, germination percentage, germination speed, and seedling height. The results showed that Seed aging treatment significantly affected Suren seed germination with 75% (2 days aging) and 56% (4 days aging) germination. The 40 Gy dose of gamma-ray irradiation could increase germination percentage and germination speed of low suren seed vigor increasing 51.42% and 60.21%, respectively. Simultaneously, the cell membrane damage of stored suren increased with gamma-ray irradiation treatment of more than 40 Gy based on electrical conductivity. In seeds with high germination, such as seeds without aging and seed aging for two days with a germination percentage of 80% and 75%, respectively, the invigoration treatment was not effective. Invigoration treatment was effective in increasing the deteriorated or low viability and vigor of the suren seeds.
Introduction
Suren (Toona sureni (Blume) Merr.), which belongs to the family Meliaceae, is a short-cycle plant widely planted in the forest community, especially in Java and Sumatra (Djam'an and Sudrajat Citation2017). Suren wood, which has high economic value, is widely used as planks in residential buildings, plywood, containers, furniture, interior spaces, and handicrafts (Suryanto Citation2013). Other plant parts such as leaves, seeds and tree bark can be used as a vegetable insecticide (Asmaliyah, Citation2013), food, medicinal and cosmetic ingredients (Chen Citation2012; Wu et al. Citation2014). The availability of quality suren seeds constrains suren cultivation because suren seeds have an intermediate character that can only be stored relatively short.
According to Zanzibar (Citation2010), the physiological deterioration of suren seeds stored in air-conditioned rooms within five months of storage reached 59.3%, based on seed germination capacity. Sudrajat and Nurhasybi (Citation2017) also reported a decrease in the germination of five seedlot of suren after four months of storage, with germination percentages ranging from 15–39%. Suren seed deterioration is deterioration in the seed's physiological quality, which can cause a complete change in the physical, physiological and chemical traits, which results in decreased viability of the seed (Sudrajat and Nurhasybi, Citation2017). The deterioration of seeds can be viewed from the physical, physiological, and biochemical aspects (McDonald, Citation1999). In suren seed, fat and protein contents positively correlate with the germination percentage and germination speed (Sudrajat and Nurhasybi, Citation2017). Biochemical indications in seeds that have deteriorated are changes in enzyme activity, protein synthesis, respiration rate changes, changes in food reserves, changes in membranes, chromosomal damage, DNA degradation, and accumulation of toxic substances in the seed affected on decreasing of seed viability and vigor (Shaban, Citation2013; Siregar et al. Citation2020).
To storing seeds, important information that must be known is the storage capacity of seeds or the seeds' ability to be stored so that the storage time can be estimated. Seeds with a high vigor storage capacity were able to maintain their viability well during storage. On the other hand, seeds with a low vigor storage capacity deteriorated rapidly. For the quality of the distributed seed management to be maintained, information is needed about the presumed storage capacity so that the seeds are not stored more than their ability to maintain viability (Rosida et al. Citation2015). Based on several studies, suren seed storage is only optimal for under 4–5 months and after that period the seeds will experience serious deterioration in germination (Zanzibar Citation2010; Sudrajat and Nurhasybi, Citation2017; Djam'an and Sudrajat Citation2017).
Efforts to improve the quality of seeds that have deteriorated can be carried out using invigoration treatment. Seed navigation is a treatment carried out to improve the physiology and biochemistry of seeds related to synchronization, speed, and an increased ability of seeds to germinate (Sutariati et al. Citation2014). One of the invigoration methods used is low gamma-ray irradiation doses (Piri et al. Citation2011, Iglesias-Andreu et al. Citation2012, Zanzibar et al. Citation2015, Zanzibar and Sudrajat, Citation2016).
Low doses of gamma irradiation can stimulate early seed germination by improving enzyme activities, increasing cell division, increasing seed germination and seedling growth (Piri et al., Citation2011; Iglesias-Andreu et al., Citation2012; Araújo et al., Citation2016). The use of irradiation to improve seed vigor has been widely used in agricultural crop types (Piri et al. Citation2011), but in forest tree species, especially tropical species, are still very limited (Zanzibar and Sudrajat Citation2009; Iglesias-Andreu et al. Citation2012). In connection with the problems mentioned above, research was carried out on the invigoration of suren (T. sureni) seeds with gamma-ray irradiation. The purpose of this study was to determine the optimal dose of gamma-ray irradiation (60Co) to increase the decreased vigor of suren (T. sureni) seeds.
Materials and Methods
Materials
Seeds were collected from the identified seed stand of suren at Kebon Kalapa Village, Cibugel Sub-District, Sumedang District, West Java Province (6°93′26″ S, 107°77′13″ E, altitude 780 m asl) in March-April. Seed collection is done by climbing and picking physiologically mature seed, marked with green-brown fruit color. The seed processing was carried out at the Forest Tree Seed Technology Research and Development Institute, Bogor, West Java.
Seed aging and irradiation treatments
The aging treatment was carried out at the Laboratory Tree Seed Testing, Forest Tree Seed Technology Research and Development Institute, Bogor, West Java. Seed aging is carried out to obtain various seed viability and vigor for further use in seed invigoration tests using gamma-ray irradiation. The aging method was done by placing the seeds in a spindle container placed on a shelf in a plastic box filled with water. The plastic crates are tightly closed so that they have a relative humidity of 100%, then put in an incubator at 27 °C for two days and four days. Group of seeds with two days of aging are considered medium vigor seeds, four days of aging are considered low vigor seeds, whereas without aging are the high vigor seeds. The seeds were wrapped in straw paper and covered with transparent plastic, then irradiated with gamma-rays using a 4000 A gamma chamber, Irpasena, India. Irradiation uses 7 (seven) doses, namely 0, 5, 10, 20, 40, 80 and 100 Gray (Gy). Each dose of irradiation was used as much as 50 seeds, and each dose was repeated four times. The seed irradiation was carried out at the Center for Application of Isotope and Radiation Technology, National Nuclear Energy Agency, Jakarta.
Seed testing
Germination was carried out in a greenhouse using sand as a medium. The number of seeds sown for each treatment combination was 50, and each of them was using four times repetition. The number of normal seedlings that grow every two days was counted (ISTA, Citation2012; Sudrajat et al. Citation2015). Normal seedling criteria were shown by the appearance of two fresh growing cotyledons. The final calculation (on day 24th) is done since germination has not been found for four consecutive days (Xu et al. Citation2016).
Normal seedlings were transplanted in polybags with mixed soil and sand (1: 1, v/v) and placed in a nursery with 65% shade. The seedling height was measured on the 40th day after transplanting. Seedling maintenance in the nursery includes watering the seedlings twice a day every morning and evening, and clean the weeds. This study used a factorial completely randomized design (CRD) with two factors: aging time and the dose of gamma-ray irradiation. The aging factor consisted of 3 levels, namely 0, 2, and 4 days, while the irradiation dose consisted of 7 levels, namely 0, 5, 10, 20, 40, 80 and 100 Gy. Each combination is repeated four times. The parameters observed were the electrical conductivity, germination percentage, germination speed, and seedling height.
The method of measuring electrical conductivity is by washing the seeds in running water, then drying the seeds for 18 hours on straw paper with a seed moisture content of 14% (Zanzibar Citation2016). Furthermore, the seeds are immersed in ion-free water (aquabidest) for 24 hours (50 ml aquabidest for 25 g of seed). The electrical conductivity value of the seed immersion was measured using the Denver Instrumental Conductivity Meter Type 30. Each treatment combination was repeated four times, each experimental unit consisting of 50 seeds. The actual conductivity value is calculated according to the formula:
(1)
(1)
where Nkond is the actual conductivity value, Ncb is the fluid conductivity value of the test seed, Nct is the fluid conductivity value without test seed, B is the test seed weight (gram), and μSg −1 is the micromhos per gram (unit).
The germination is calculated using a formula (ISTA Citation2012):
(2)
(2)
where GP is the germination percentage, ∑NS is the number of normal seedlings, and ∑TS is the number of germinated seeds.
The germination speed was calculated based on the ratio of the percentage of normal seedlings per observation day. The calculation formula is:
(3)
(3)
where GS is the germination speed, N is the percentage of normal sprouts, t is the observation time, and tn is the end time of observation.
The seedlings' height at 40 days after transplanting was measured from the stem’s base to the highest seedling (cm) to estimate each treatment's vigor.
Data analysis
Data were analyzed using multivariate analysis of variance (ANOVA) to test the effect of seed aging and gamma-ray irradiation on electrical conductivity, germination percentage, germination speed, and seedling height. If there is a significant difference at the 5% significant level, then a further test is carried out with the Duncan Multiple Range Test (DMRT). LD50 value was calculated by using the curve expert application.
Results and Discussion
Results
Seed aging treatment significantly affected the electrical conductivity, germination speed and seedling height. Gamma-ray irradiation had a very significant effect on germination percentage, germination speed, and seedling height. The interaction of seed aging treatment and gamma-ray irradiation significantly affected all observed parameters, i.e., electrical conductivity, germination percentage, germination speed, and seedling height ().
Table 1. Multivariate analysis of variance summary of aging and gamma-ray irradiation treatment various effects on suren seeds on germination percentage, germination speed, seed height, and electrical conductivity.
The electrical conductivity of suren seeds ranged from 0.17 μSg−1 − 0.34 μSg−1. In the group of seeds that did not undergo aging (fresh seeds), the lowest conductivity value was obtained at the irradiation dose of 100 Gy, significantly different from without irradiation (control) and irradiation at a dose of 5 Gy − 40 Gy. At 2-days aging, the lowest electrical conductivity value was shown in the seeds irradiated at a dose of 10 Gy, significantly different from the irradiation dose of 80 Gy and 100 Gy. For four days of aging, the lowest electrical conductivity value was obtained at 20 Gy and only significantly different from the dose of 100 Gy. In general, the lowest electrical conductivity was obtained at two and four days of aging with irradiation doses below 40 Gy. On aging up to four days, the seeds irradiated below ≤ 40 Gy will result in a low electrical conductivity value.
The germination percentage (GP) of suren seeds due to scouring and irradiation was between 23.00% − 81.00%. The highest GP was obtained in fresh seeds in control (not irradiated), and it was not significantly different from seeds irradiated at 5 Gy, 10 Gy and 20 Gy. The lowest GP was obtained from the seeds without scouring treatment and irradiated with 80 Gy. In the seeds with aging for two days, the irradiation dose did not give a different response with a range of 63.50% −76.50%. The highest GP on aging for four days was obtained at a dose of 40 Gy and only significantly different from the treatment without irradiation. The highest GP was obtained from fresh seeds that were not irradiated, while the lowest was obtained from fresh seeds irradiated with 80 Gy.
The results of the Linear Fit curve analysis () show that the LD50 value due to gamma ray irradiation is 61.52 Gy with the equation Y = (75.81 − 0.92X)/(1 − 0.0055X − 7.53×2) (Se = 13, 97; r = 0.88). This shows that the radiation dose of 61.52 Gy can cause 50% mortality of fresh suren seeds. The LD50 value due to gamma-ray irradiation for suren seeds under scouring for 2 days and 4 days was 217.9 Gy; and 110.55 Gy. This value shows that gamma-ray irradiation at a dose of 5–100 Gy LD50 is still relatively low for suren seeds with two and four days of aging, so it has not caused physiological damage and a lethal dose has not been obtained. The resulting equation models are Y = 1/(0.0129 + 0.0007 × 0.28) (Se = 4.69; r = 0.76) and Y = 1/(0.013 + 2.87E-10 × 3.61) (Se = 4.31; r = 0.91) ().
Figure 1. Germination percentage of suren seeds from gamma-ray irradiation doses 0, 20.40, 60, 80 and 100 Gy, which have undergone aging treatment for 0 days (a), 2 days (b)and four days (c).
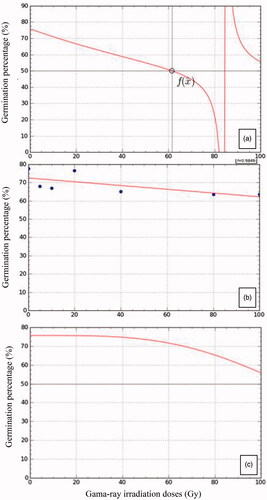
Based on the germination speed (GS) (), it was obvious that in the seeds with aging for four days, the 40 Gy irradiation gave the best GS value (6.04% etmal−1), which was not significantly different from those irradiated with a dose of 5, 10, 20, and 80 Gy. At two-days aging, the highest GS of 5.35% etmal−1 was obtained after irradiated the seeds at 20 Gy. It was not significantly different from all treatments of irradiation doses. Whereas for fresh seeds, the fresh seeds irradiated with 80 Gy have the lowest GS, namely 1.40% etmal−1, which was significantly different from other treatments. Overall, scavenging for four days then irradiated with 40 Gy gave the best growth rate of 6.04% etmal−1. The lowest growth rate was obtained from fresh seeds irradiated with 80 Gy, namely 1.40% etmal−1.
Table 2. The average of germination percentage, germination speed, seedling height, and electrical conductivity of suren seed treated with aging and gamma-ray irradiation.
As seen from the seeds' height (), in the group of seeds that did not undergo aging, then irradiated with a dose of 5 Gy, would give the best seedling height, namely 4.50 cm. Meanwhile, the seeds did not undergo aging, and then irradiated with 80 Gy would result in the lowest seedling height reaching 1.06 cm, different from other treatments. Aging for two days had the best seedling height of 4.59 cm at a 5 Gy irradiation dose, which was not significantly different when irradiated at 0, 10, and 20 Gy, while the lowest seed height was found in seeds irradiated by 80 Gy, namely 2.07 cm, significantly different from other treatments. When the aging time was increased to 4 days, the best seedling height was 4.31 cm, which was obtained in unirradiated seeds, but it was not significantly different from the use of doses of 5, 10, 20 and 40 Gy, while the lowest seedling height was 2.03 cm, after the seeds were irradiated at a dose of 80 Gy which was not significantly different from the use of 100 Gy.
Overall, the seeds without aging (fresh seeds) which combined with 5 Gy irradiation treatments, gave the best seedling height, namely 4.50 cm, which is not significantly different from seeds that undergo two days of aging using 5 Gy and 10 Gy irradiation doses. Whereas the seeds did not undergo aging and then irradiated with 80 Gy would result in the lowest seedling height, reaching 1.06 cm, different from other treatments.
Discussion
Seed aging treatment for four days was caused suren seed physiological traits to decline, as seen from a decrease in viability (germination percentage) and seed vigor (germination speed) by 35.18% and 30.70%, respectively. Seed aging treatment increased the respiration rate, and the heat generated decreases the energy contained in the seeds during the germination process. The heat generated from the respiration process also spurs the metabolic process during the storage period, resulting in decreased carbohydrate content, protein and lipids, while free amino acids and free fatty acids increase (Begum et al. Citation2013). As in cotton seeds, protein denaturation occurs (Kapoor et al. Citation2010).
Another symptom of the deterioration of seeds is damage and decreased stability of the cell membrane, reflected in an increase in electrical conductivity. High conductivity values indicate a poor membrane structure (Pujiastuti and Sudrajat Citation2017) and will impact the seed germination process (Suita Citation2013). An increase in the electrical conductivity value may reflect the low seed viability, characterized by a decrease in germination. However, in this study, the seeds' conductivity value resulting from two days of rapid processing (0.16 μ Sg−1) was relatively lower than that of fresh seeds (0.33 μ Sg−1). These results indicate a slowdown or even a delay in the metabolic processes in the 2-day-grown Suren seeds. Likewise, the viability and seed vigor's response was relatively not significantly different between 2-day aging seeds and fresh seeds.
The delay period for suren seeds' metabolism did not last long because at four days of aging; seed deterioration can be seen (based on electrical conductivity). Suren seeds' conductivity from 4 days of rapid aging was 0.23 μ Sg−1, which was relatively higher than the two days of aging. In line with Suryanto (Citation2013) report, the germination capacity of suren seeds increased by 22.95% after being stored for four weeks in a porous container at room temperature and then decreased to 40.98% at six weeks of shelf life.
This condition is probably related to the occurrence of after-ripening in suren seeds. After ripening, the seeds and fruit ripening process do not occur simultaneously, so the seeds do not germinate even though the fruit is ripe. This process is closely related to stopping the formation of protein and hormone reserves at the final stage of fruit ripening, but the dehydration process is still ongoing so that there is a balance between air humidity and moisture content of seeds and further stages of embryonic development (Mathad et al. Citation2016).
Gamma-ray irradiation is an effort to increase the vigor and viability of seeds that have deteriorated. Gamma-rays are ionizing radiation with strong penetrating power. It will form the free radicals when interacting with water and oxygen (Ikram et al. Citation2010). The free radicals produced are quite reactive and able to react with organic molecules (proteins, lipids and nucleic acids) (Lelang et al. Citation2015; Nurrachmamila and Saputro Citation2017), which in turn cause damage to the germ cell membrane (Khan Citation2003; Zanzibar Citation2011). At the right dose, irradiation will increase the viability of the seeds. The effect of gamma radiation on germination, morphology, anatomy, and physiochemical characteristics of plants is highly dependent on the level of irradiation (Maamoun et al. Citation2014).
Irradiation at doses above 40 Gy on fresh suren seeds (without aging) decreased the seeds' viability and vigor. Other results were in medium vigorous seeds (2 days aging); the use of irradiation up to 100 Gy still showed high seed viability. The use of irradiation on low vigor seeds (4 days aging) tends to increase germination percentage and germination speed, up to a dose of 40 Gy; this indicates an opportunity to increase the quality of stored seed vigor using an irradiation dose of 40 Gy, which is 51.43% for germination and 60.21% for growth speed. In this case, gamma-ray irradiation may increase germination inhibiting substances such as Surenon, Surenin and Surenolactone owned by suren, thereby increasing seed viability. Similar result reported by Suhartanto et al. (Citation2018) that dose of 40 Gy was able to improve seed vigor and seedling growth so that it can be applied to increase vigor of Neolamarckia cadamba seeds. As in the Magnolia champaca, irradiation using 10 Gy of the stored seeds will increase the germination capacity by 34.1% of the control (Zanzibar and Sudrajat Citation2016). Seeds with stronger dormancy such as Falcataria moluccana require higher doses to increase low seed viability and vigor, as reported by Yulianti et al. (Citation2016) who were able to increase seed germination from 40% to 80% with gamma ray irradiation at a dose of 60 Gy. The optimal irradiation dose for each species of plant is different, but the response to improve viability and vigor of seeds generally occurs at low doses (10–60 Gy), while the high gamma irradiation doses are generally used to create new diversity in the context of mutation breeding (Wang et al. Citation2016; Geng et al., Citation2019).
As with the response to seed viability, the effect of gamma-ray irradiation on the value of electrical conductivity will differ between fresh and aged seeds. Irradiation treatment at doses above 40 Gy on fresh seeds improves the cell membrane's condition based on the decreased electrical conductivity. On the other hand, for 2-day matured seeds, irradiation at doses above 40 Gy tends to worsen cell membrane condition, based on the increased electrical conductivity. The electrical conductivity values of the seeds with two days of the aging process with a dose of 80 Gy (0.34 μSg−1) and 100 Gy (0.29 μSg−1) were higher than two days of aging without irradiation (0.16 μSg−1). The treatment of four days aging and high dose irradiation (100 Gy) produced relatively the same electrical conductivity value (0.27 μSg−1) compared to four days of aging without irradiation treatment (0.23 μSg−1).
A decrease in germination and seed growth capacity follows the electrical conductivity increment, as has been proven in R. apiulata propagules (Rohandi and Widyani, Citation2010), sorghum seeds (Fatonah and Rozen Citation2017), soybean seeds (Noviana et al. Citation2017) and A. mangium (Pujiastuti and Sudrajat Citation2017). In contrast to this study, the electrical conductivity increment was not followed by a decrease in seed germination or vice versa. The highest conductivity was indicated by fresh seeds irradiated with 5 Gy, which did not differ from doses of 0, 10, 20, 40.80 Gy and 2 and 4 days of aging seeds irradiated with 100 Gy. Meanwhile, the lowest germination percentage was found in fresh seeds irradiated with 80 Gy. The correlation analysis results also showed no significant correlation between the value of electrical conductivity and germination (R = − 0.04). Based on these results, in this study, the value of electrical conductivity cannot be used as a reference in assessing the potential viability of suren seeds. The electrical conductivity value with a narrow interval (0.17 μSg−1 − 0.34 μSg−1) is probably the answer to why it has not described the interval of suren seed viability values. Zanzibar (Citation2016) states that the larger the electrical conductivity value interval, the more vulnerable the seeds are; than if the value interval is narrow, especially for storage at high temperature and humidity. Immersion temperature conditions are relatively low for suren to be able to dissolve the electrolyte of suren seeds.
The seed group that had the lowest sensitivity was obtained from seeds that were pulverized for two days (LD50 = 217.9 Gy), then for four days (LD50 = 110.56 Gy), and the highest was in fresh seeds (61.52 Gy). Azizah (Citation2015) states that the lower the LD50 value of a plant, the higher the radiosensitivity level, and vice versa. Overall, seed aging and irradiation treatments cause the seed to have a low radiosensitivity level. LD50 in M. champaca seeds was obtained at irradiation doses higher than 30 Gy and below 40 Gy (Zanzibar and Sudrajat, Citation2016); while for Amorphophallus muelleri at a dose of 10 Gy − 20 Gy (Santosa et al. Citation2014). In general, invigoration treatment was very effective in increasing the viability and vigor, which had deteriorated as shown by the seeds that were treated with seed aging for four days (germination percentage 52.50%). In seeds with high germination such as seeds without aging and seed aging for 2 days with germination percentages of 80% and 75% respectively, the invigoration treatment was not effective.
Seedling height response is an approach to suren seed vigor. The use of gamma-ray irradiation at a dose of 40–100 Gy reduced the overall height growth of suren seedlings in both fresh and aging seeds. In almost all treatments, high seed vigor was obtained at low doses below 40 Gy.
Conclusions
Seed aging can reduce the viability and vigor of suren seeds along with the length of the aging treatment period. The use of gamma-ray irradiation at doses less than 40 Gy is effective to improve the low viability and vigor suren seeds with the germination percentage about 52.50%, but it is not effective for seeds with high viability (germination percentage > 75%). Gamma rays irradiation treatment on aging suren seeds (low viability and vigor seeds) can be applied to improve the quality of suren seeds that have experienced quality degradation.
Author contributions
All the authors have equal contributions as the main contributors.
Acknowledgments
The authors thank the Forest Tree Seed Technology Research and Development Institute for facilitating the research activities. The authors also thank Dwi Haryadi, who has helped carry out research activities in laboratories and greenhouses.
Disclosure statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Araújo SS, Paparella S, Dondi D, Bentivoglio A, Carbonera D, Balestrazzi A. 2016. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front Plant Sci. 7:1–12.
- Asmaliyah IB. 2013. Potency of rimau (Toona sp.) leaf in extract in suppressing the development of pest attacks on jabon (Anthocephalus cadamba) plants in the field. In Seminar Proceeding on Integration of Science and Technology in Policy and Management of Plantation Forest in Southen Sumatra (pp. 169–178.
- Azizah N. 2015. Determination of lethal dose 50 (LD50) gamma rays irradiation on the several Heliconia spp. cultivars. Faculty of Agriculture. Bogor Agricultural University. Bogor. https://repository.ipb.ac.id/handle/123456789/74611.
- Begum AJ, Jerlin R, Jayanthi M. 2013. Seed quality changes during storage of oil seeds- A review. Int J Sci Res. 2:1–2.
- Chen CM. 2012. Antioxidation activity and total phenolic contents of various Toona sinensis extracts. Afr J Biotechnol. 11(73):13831–13837.
- Djam'an D, Sudrajat DJ. 2017. Seed morpho-physiological variation of suren (Toona sinensis) from various growth sites in Sumatra and Java. J Pemuliaan Tanaman Hutan. 11(2):139–150.
- Fatonah K, Rozen N. 2017. Electrical conductivity for seed vigor test in sorghum (Sorghum bicolor L.). J Agroteknol Univ Andalas. 1(1):19–25.
- Geng X, Zhang Y, Wang L, Yang X. 2019. Pretreatment with high-dose gamma irradiation on seeds enhances the tolerance of sweet osmanthus seedlings to salinity stress. Forests. 10(5):406–411.
- Iglesias-Andreu LG, Octavio-Aguilar P, Bello-Bello J. 2012. Current importance and potential use of low doses of gamma radiation in forest species. In: Adrovic F, editor. Gamma radiation. Rijeka, Croatia: InTech Europe. p. 265–280.
- Ikram N, Dawar S, Abbas Z, Javed Z. 2010. Effect of (60cobalt) gamma rays on growth and root rot diseases in mungbean (Vigna radiata l.). Pak J Bot. 42(3):2165–2170.
- ISTA. 2012. International rules for seed testing. Edition 2012. International Seed Testing Association. Switzerland. https://www.seedtest.org/en/home.html.
- Kapoor N, Arya A, Asif Siddi M, Kumar H, Amir A. 2010. Physiological and biochemical changes during seed deterioration in aged seeds of rice (Oryza sativa L.). Am J Plant Physiol. 6(1):28–35.
- Khan MA. 2003. Halophyte seed germination: success and pitfalls. In: Hegazi HM, El-Shaer S, El-Demerdashe RA, Guirgis A, Abdel Salam Metwally FA, editors. International symposium on optimum resource utilization in salt affected ecosystems in arid and semi arid regions Cairo, Egypt: Desert Research Centre. p. 346–358.
- Lelang MA, Setiadi A., Fitria 2015. Study the effect of gamma irradiation on the performance of the plant seed from the chicken's comb (Celosia cristata L.) J Pertanian Konservasi Lahan Kering. 1(1):47–50.
- Maamoun MKM, El-Mahrouk ME, Dewir YH, Omran SA. 2014. Effect of radiation and chemical mutagens on seeds germination of black cumin (Nigella sativa L). J Agric Sci Technol. 10(5):1183–1199.
- Mathad RC, Patil SB., Basavegowda 2016. Impact of after-ripening in hot pepper seed development during post-anthesis physiological maturity. Indian J Hort. 73(4):607–610.
- McDonald MB. 1999. Seed deterioration: physiology, repair, and assessment. Seed Sci Technol. 27:177–237.
- Noviana I, Qadir A, Suwarno FC. 2017. Biochemical behaviour of soybean seed during control storage. J Agron Indonesia. 44(3):255–260.
- Nurrachmamila PL, Saputro TB. 2017. Germination analysis of radiated seed of (Oryza sativa L.) variety bahbutong. JSSITS. 6(2):2337–2342.
- Piri I, Babayan M, Tavassoli A, Javaheri M. 2011. The use of gamma irradiation in agriculture. Afr J Microbiol Res. 5(32):5806–5811.
- Pujiastuti E, Sudrajat DJ, Balai Penelitian dan Pengembangan Teknologi Perbenihan Tanaman Hutan 2017. Vigour test to predict seed germination and normal seedling emergence of Acacia mangium in nursery. J Perbenihan Tan Hutan. 5(2):81–94.
- Rohandi A, Widyani N. 2010. The effect of decreasing moisture content to the response of physiological and biochemical of Rhizophora apiculata Bl. propagules. Jurpenhuttanaman. 7(4):167–179.
- Rosida A, Sari M, Qadir A. 2015. Prediction of storability vigor of cabbage (Brassica oleracea L. var. capitata) seed using accelerated aging method with ethanol. J Hort Indonesia. 6(3):152–160.
- Santosa E, Pramono S, Mine Y, Sugiyama N. 2014. Gamma irradiation on growth and development of Amorphophallus muelleri Blume. J Agron Indones. 42(2):18–123.
- Shaban M. 2013. Review on physiological aspects of seed deterioration. Intl J Agric Crop Sci. 6 (11):627–631.
- Siregar IZ, Muharam KF, Purwanto YA, Sudrajat DJ. 2020. Seed germination characteristics in different storage time of Gmelina arborea treated with ultrafine bubbles priming. Biodiversitas. 21(10):4558–4564.
- Sudrajat DJ, Nurhasybi , Yulianti B. 2015. Forest Tree Seed Testing and Physical-physiological Quality Standard. Bogor, Indonesia: Forda Press. https://www.forda-mof.org/files/b_standar_mutu.pdf.
- Sudrajat DJ, Nurhasybi 2017. Seed storability of suren (Toona sinensis) in correlation with origin site and mopho-biochemical characteristics. In Proceding of 4th Silviculture National Seminar of “Proper Silviculture to Mitigate Climate Change towards Sustainable Forest and Bio-Economic Resources. pp 379-389. https://www.researchgate.net/publication/342702204_Daya_Simpan_Benih_Suren_Toona_sinensis_dalam_Hubungannya_dengan_Karakteristik_Tempat_Tumbuh_dan_Morfo-biokimia_Benih.
- Suhartanto R, Suharsi TK, Rustam E, Sudrajat DJ, Departemen Agronomi dan Hortikultura, Kampus Institut Pertanian Bogor 2018. The improving vigor of white jabon seeds after storage for 4.5 years using gamma ray irradiation. J Perbenihan Tan Hutan. 6(2):145–158.
- Suita E. 2013. The effect of seed aging to the weru (Albizia procera Benth.) seed viability. J Perbenihan Tan Hutan. 1(1):31–35.
- Suryanto H. 2013. Effects of storage of suren (Toona sureni) seeds on germination. JPKW. 2(1):26–40.
- Sutariati GAK, Zul’aiza ZA, Darsan S, Karsa LMA, Wangadi S, Mudi L. 2014. Seed invigoration of local upland rice seed to enhance vigour and overcome problems of postharvest physiological dormancy. Jurnal Agroteknos. 4(1):10–17.
- Wang S, Yang R, Shu C, Zhang XC. 2016. Screening for cold-resistant tomato under radiation mutagenesis and observation of the submicroscopic structure. Acta Physiol. Plant. 38:1–12.
- Wu J, Peng W, Yi J, Wu Y, Chen T, Wong K, Wu J. 2014. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J Ethnopharmacol. 154(1):198–205.
- Xu YG, Liu R, Sui N, Shi W, Wang L, Tian C, Song J. 2016. Changes in endogenous hormones and seed-coat phenolics during seed storage of two Suaeda salsa populations. Aust J Bot. 64(4):325–332.
- Yulianti , Putri PK, Zanzibar M., Danu 2016. The utilization gamma rays radition to improve the viability of sengon seed. Jurnal Hutan Tropis. 4(1):14–20.
- Zanzibar M, Megawati M, Pujiastuti E, Sudrajat DJ. 2015. Gamma irradiation (60Co) to increase the germination and seedling growth of tembesu (Fagraea fragrans Roxb. ). Jurpenhuttanaman. 12(3):165–174.
- Zanzibar M, Sudrajat DJ. 2009. Prospek teknologi radiasi sinar gamma dalam peningkatan mutu benih tanaman hutan (Prospect and application of gamma-rays irradiation technology for improving of forest tree seed and seedling quality). Info Benih. 13(1):158–163.
- Zanzibar M, Sudrajat DJ. 2016. Effect of gamma irradiation on seed germination, storage, and seedling growth of Magnolia champaca L. Indones J For Res. 3(2):95–106.
- Zanzibar M. 2010. Improving of physiological suren seed quality by priming technique. j Standard. 12(1):1–6.
- Zanzibar M. 2011. Efectivity of priming treatment and quick physiological seed quality assessment of tusam (Pinus merkusii Jungh et de Vriese). J Standard. 13(2):91–98.
- Zanzibar M. 2016. The rapid prediction of seed viability of forest trees: Principle, methods and aplication. Jakarta, Indonesia: Penebar Swadaya. http://benih-bogor.litbang.menlhk.go.id/assets/files/METODA_PENDUGAAN_VIABILITAS_BENIH_TANAMAN_HUTAN_-_23092016_-_SMALL.pdf.
