 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Gerunggang (Cratoxylum arborescens (Vahl) Blume) is a tree native to Indonesian peatlands that has great potential as an alternative fiber-producing species for the pulp and paper industry because it is adaptable and does not have the potential to disrupt local ecosystems. The species also contains important anti-cancer compounds for the pharmaceutical industry. These industries require large quantities of raw materials to meet their needs, which cannot be met by the supply of conventional plant seedlings. Tissue culture is one of the propagation biotechnologies that can be used as an alternative to provide more efficient germplasm. In this study, we developed a protocol for gerunggang propagation using tissue culture technique. The results showed that the combination of 1 mg/l 6-benzylamino purine (BAP) and 0.5 mg/l naphthalene acetic acid (NAA) in Murashige & Skoog media provided the fastest bud break time (2 weeks before the first subculture), with the highest shoot initiation (3.0 ± 0.2 cm), and after 4 weeks of incubation, the highest auxiliary shoot elongation (7.4 ± 0.1 cm). The significant effect of this hormone concentration also increases the nodule multiplication coefficient (NMC), which is 7.5. In vitro root response on the same propagation medium showed good growth with an average root length of 4.8 ± 0.2 cm. Acclimatization of plantlets was successful under ex vitro conditions and resulted in good growth after 2 and 3 months in the greenhouse. The advantage of applying the tissue culture method to gerunggang is that it can produce about seven times more seedlings from one explant in about 4 weeks. These results will be very useful for further research in the field and require a more intensive and comprehensive study.
1. Introduction
Plant tissue culture propagation has been used for more than 30 years (Haque et al. Citation2022). A single explant can be multiplied into several thousand plants in a short space of time when grown under controlled conditions, regardless of season or weather (Roosslee Citation2021). A simple method that many poor countries have already mastered is plant tissue culture. Its implementation requires only a clean working environment, a nursery, a greenhouse, and skilled labor. The plant tissue culture will continue to grow and have significant potential up to 2030 due to rapid advances in plant tissue culture techniques and the high demand for disease-free plants, hybrid plants, and others (Allied Market Research, Plant Tissue Culture Market, Global Opportunity Analysis and Industry Forecast, 2021–2030 Citation2022). Tissue engineering is gaining popularity worldwide, especially in research and development in different species due to its specification in medium and hormones. The global plant tissue culture market is expected to witness significant growth during the forecast period, growing at a CAGR (Compound Annual Growth Rate) of 6.4% over the estimated period from 2022 to 2031. Regionally, Asia Pacific is projected to hold the largest market share (Global Plant Tissue Culture Market Predicted to Generate a Revenue of $2,202.9 Million and Rise at a CAGR of 6.4% throughout the Analysis Timeframe from 2022–2031 Citation2023).
The propagation of plant species has been mainly from seed. However, seeds from most trees are highly variable. Therefore, plant tissue culture technology has the potential to overcome this problem by enabling efficient and rapid clonal propagation of many economically important species, particularly in the pulp and paper industry. The pulp and paper industry is a sector that continues to grow in terms of utilization and is becoming a leading export industry in Indonesia. The rapid increase in the production capacity of the pulp and paper industry will be followed by an increasing demand for pulpwood in many industrial plantation forests as a source of wood fiber raw materials (Faqir Citation2022). The use of exotic wood species as raw materials for the pulp and paper industry currently has environmental limitations in terms of native plant biodiversity and pest and disease infestation. The invasion of exotic plant species into natural ecosystems is recognized as a major threat to biodiversity. The introduction and establishment of non-native invasive organisms are increasing globally with no signs of slowing down, facilitated by increased global trade and exacerbated by climate change (Prior et al. Citation2018). The spread of invasive species reduces the biodiversity of habitats, disrupts ecosystem function, and affects economic development in newly colonized areas and sustainability (Negi et al. Citation2022).
According to the results of research (Rizqiani et al. Citation2021), the quality of gerunggang wood fiber is grade III. The weight of gerunggang wood is greater than that of Acasia crasicarpa. Although the growth rate of local peat wood is generally low, it has the advantage of a high survival rate compared to exotic species. Breeding of this local species must be carried out to optimize its growth if it is to be developed in a plantation forest. Taxonomically, it belongs to the phylum Tracheophyta, family Hypericaceae and genus Cratoxylum, with the scientific name Cratoxylum arborescens Blume, gerunggang has a synonym Ancistrolobus glaucescens Turcz., Caopia arborescens (Vahl) Kuntze, C. arborescens var. miquelii King, C. cuneatum Miq. and Vismia arborescens (Vahl) Choisy. Compared to other local peatland types, gerunggang growth is relatively higher and faster, and the hollow is able to maintain good microclimate conditions due to the moisture of the litter being in a relatively non-flammable state. Gerunggang has the opportunity as an alternative raw material for pulp because the fiber of the wood is in class II. The wood is classified as strong class III-IV and durable class IV (Suwito et al. Citation2021).
Gerunggang is an alternative pulp raw material choice because the wood waste can be used as medicinal biomass. Gerunggang is a well-known ethnomedicine used in Asia for the treatment and prevention of peptic ulcers. As a natural product, a-mangostin (AM) is a prenylated xanthone isolated from C. arborescens by bio-guided fractionation. These natural compounds have been previously reported to have a variety of biological properties, including anti-inflammatory, antioxidant damage, and antioxidant activity. According to, the potent antioxidant and anti-Helicobacter pylori activity of a-mangostin, as well as the activation of Hsp70 protein, may contribute to its gastroprotective activity. The logs of this multi-purpose species are also used for siding and light construction of roofing, furniture, crates, plywood, and concrete moldings (Pattanaprateeb et al. Citation2005).
Gerunggang can be propagated both generatively and vegetatively. Seeds are readily available, but silvicultural research of this type is still limited, so generative propagation relies on gerunggang seedlings growing naturally. Genetically superior gerunggang seeds have not been obtained to date. The gerunggang breeding program is still in its early stages or at the base population stage. To prepare for the high demand for high-yielding seeds in the pulp and paper industry, it is necessary to develop effective and efficient large-scale propagation techniques. Tissue culture micropropagation is an alternative to large-scale vegetative propagation of high-yielding varieties that are considered effective and efficient. Tissue culture refers to the in vitro cultivation of plant parts, such as stems, leaves, roots, flowers, nodes, shoots, and embryos. Tissue culture starts with pieces from all parts of the plant. Explants are small organs or pieces of tissue that are isolated in vitro and cultured on a sterile artificial (George et al. Citation2008a): (1) the required explants are small in its multiplication, (2) little space is required to maintain it, (3) multiple and extensive propagation in a short time, (4) generate clones of some types of plants that are slow and difficult to propagate, (5) characteristics obtained the same as the parent, obtain plants with different characteristics new, propagation can be done at any time and is not affected by seasonal changes. BAP (6-Benzyl Amino Purine) is a cytokinin hormone commonly used in tissue culture techniques that functions to induce cell division, apical dominance, and differentiation of adventitious shoots from callus and organs. Whereas the synthetic auxin NAA (Naphthalene Acetic Acid) functions in tissue culture to induce cell division, organogenic and embryogenic differentiation. At low concentrations, auxin can induce root initiation, whereas at high concentrations it can induce callus formation (George et al. Citation2008b). However, there has been little study of tissue culture of gerunggang, a versatile native species of tropical peatland. Micropropagation of peatland plants has many obstacles, especially to obtain axenic cultures, the conditions where contamination does not inhibit in vitro tissue regeneration. Collecting explants from nature in wet peatland habitats increases the potential for high contamination. The current study aims to develop an efficient protocol for the micropropagation technique for large-scale seedling establishment by tissue culture using a combination of BAP and NAA in MS (Murashige & Skoog) basal media.
2. Materials and methods
This research was conducted at the Tissue Culture Laboratory of the Center for Forest Biotechnology and Tree Improvement (CFBTI), Yogyakarta (7.6165° S, 110.4113° E). The study was conducted for 15 months (from November 2019 to February 2021). The 6 months old seedlings of gerunggang were obtained from Perawang, Riau, Indonesia (00 40.2091″ N, 1010 36.7445″ E). Approximately 300 seedlings were prepared as a source of explant material from the natural habitat in Riau () (Pattanaprateeb et al. Citation2005) and seedlings in the nursery as a source of explants (). Explant preparation was carried out by spraying pesticides and 3 axillary nodules from the new shoots were selected for explants.
Figure 1. Gerunggang in its natural habitat in Riau (a) and the 6-month old seedlings in the nursery.

2.1. Explants sterilization
The seedling nodules were used as explant material. In this study, several modifications of the sterilization method developed by George et al. (Citation2008a) were used. Sterilization modifications were made to the outside and inside of the Laminar Air Flow (LAF). To sterilize the outside of the LAF, the explants are soaked in distilled water with detergent for 1 min and a fungicide for 3 min, rinsed with a fine sponge and tap water for 20 min. The explants are sterilized internally by immersion in a 6% (v/v) solution of the antimicrobial compound BI (biocide isothiazolone) containing 5.25% sodium hypochlorite, hydrogen peroxide, sodium perborate, and sodium percarbonate. A few drops of Tween 80 were added to the solution for 0 min, followed by 70% ethanol for 1 min. After each sterilization, the explants were rinsed with sterile distilled water. The percentage of axenic culture was observed for each treatment (one explant per tube).
2.2. Initiation, multiplication, and rooting medium
Full strength MS (Murashige and Skoog) basal medium was used for initiation with the addition of hormones: N6-benzyl amino purine (BAP) 0.5, 1, and 1.5 mg/l; and α-naphthalene acetic acid (NAA) 0.1 and 0.5 mg/l. All treatments were supplemented with 6 ml/l PPM biocide. The best response of initiation of germination in axenic cultures was used for the multiplication phase without the addition of PPM. Half strength MS basal medium was used for rooting medium with the best concentration of initiation hormones added. All media were adjusted to pH 5.7 at 27 °C with KOH and HCl and compacted with pure agar 8%, and autoclaved (1.1 kg cm−2 at 1210 C for 20 min) after the addition of hormones as required. They were cultured in a light photoperiod of 16 h (50–70 μmol/m2/s). They were then sub-cultured every month until 12 months of incubation for initiation, multiplication, and rooting. The effect of the hormone combination was tested on 50 replications of the plantlet for each treatment. The potential initiation, multiplication, and rooting of gerunggang axillary shoot in vitro was measured based on bud break response, shoot length, nodule multiplication coefficient (NMC), and root length. NMC is a modification of the coefficient equation of shoot multiplication coefficients by Ibáñez et al. (Citation2009). NMC was defined as:
The best response of axenic plantlets in combining BAP (6-Benzyl Amino Purine) and NAA (Naphthalene Acetic Acid) were continued to acclimatization
2.3. Acclimatization
Health plantlets with well-developed roots from the best BAP and NAA treatments were removed from the culture tube, washed under running tap water, soaked in fungicide for a few minutes, and transplanted into a polybag containing a 1:1 mixture of soil and compost. The seedlings were grown in a greenhouse with 30–40% natural light and sprayed with water once a day. In this experiment, 25 plantlets were transplanted individually into plastic-covered polybags in 4 weeks of incubation. The percentage of plantlet survival was measured 24 weeks after the uncovered plastic wrapper and the percentage of seedling survival was calculated using the following formula was calculated using the following formula:
One year of seedling development was observed in a nursery. The entire study lasted 15 months, with time spent in the laboratory, greenhouse, nursery, data processing, and writing. The experimental units were arranged in a completely random design (CRD). ANOVA was used to analyze the data, and the means were separated using Duncan’s multiple range test (DMRT) with a significance level of 0.05. The statistical package SPSS (version 24) was used for analysis.
3. Results and discussion
3.1. Bud break and shoots initiation
Initiation of gerunggang explants begins with bud break at each nodule (). Gerunggang explants did not show bud break formation in the first nodule or nodules close to shoots. Bud break reaction occurs in the middle nodule (second nodule) and the lower nodule (third nodule) far from the shoot bud. This is in line with research conducted by Putri and Jayusman (Citation2012). The occurrence of bud break in certain nodules may be due to apical dominance. Apical dominance is lateral meristem activity that is inhibited by auxin when the lateral meristem is located adjacent to the apical meristem, causing limited shoot formation. As stated by Setyaningsih et al. (Citation2020) that most plants, if the stem growth is sufficient, will naturally grow lateral branches on the lower nodes which are quite far from the end of the stem, this is because as it gets further from the end of the stem the influence of apical dominance decreases. This is in agreement with George et al. (Citation2008a) that lateral shoots are initially in a dormant state due to the influence of apical dominance, lateral shoots can grow along with the influence of apical dominance decreases with increasing distance from the shoot apical. Thus, the nodes farther from the tip of the stem respond more rapidly. The fastest bud burst of gerunggang occurred 2 weeks (before the first subculture) after the transfer of explants to in vitro initiation media supplemented with 1 mg/l BAP and 0.5 mg/l NAA (). The response of gerunggang bud burst is more rapid compared to other peat plants, such as ramin (G. bancanus), which takes 6 months (after the sixth subculture) (Putri et al. Citation2017) ().
Figure 2. Response of gerunggang explant in vitro: bud break from gerunggang explant initiation (a), shoot initiation, 1 month after bud break (b), and elongation shoot initiation after 6 months (c).

Table 1. Effect of hormone treatment on bud break time after transfer of explants and shoot initiation response of gerunggang after bud break (1 month in vitro), shoots elongation, percentage of explants mortality, and axenic culture (after 3 months in vitro).
also shows that the percentage of bud break under low contamination conditions (axenic culture) ranges from 80 to 87%. According to Rathore et al. (Citation2015) on tissue culture research of Acacia nilotica using mature tree nodule segment with MS media resulted in 90.8% bud break. While in the research of Chaturvedi et al. (Citation2004) tree nodule segments of Azadirachta indica A. Juss. (Neem) 40 years old with modified MS base media showed 81.2% bud break. Thus, gerunggang has a bud break percentage range that is not different from other peat plant species in general.
The results showed that the combination of 1 mg/l BAP and 0.5 mg/l NAA in full strength MS medium resulted in the highest shoot initiation (3.0 ± 0.2 cm) after 4 weeks of incubation of axillary shoot explants. The quicker the explants respond to shoot initiation, the quicker the plant propagation material will be produced. As stated by Li et al. (Citation2013), the formation of shoots indicates the successful regeneration of explants inoculated on tissue culture media. Explant death is due to bacterial and fungal contamination. A contamination level of <25% is considered reasonable in tissue culture techniques with explants derived from natural tree plants. Obtaining 80% axenic cultures for up to 6 months of incubation indicated an appropriate explant sterilization protocol for this study, although it is possible to reduce the number of axenic cultures obtained due to human factors and the laboratory environment. The initiation of gerunggang shoots is followed by the growth and elongation of the shoots and leaf formation. Each gerunggang node can grow 1 to 3 buds, each shoot elongation grows one leaf per node. Shoots are the differentiation of explants. Explants that produce buds can be considered one of the successes of tissue culture. Shoot formation can be influenced by the composition of the media and the concentration of the hormone used. shows that the balance of the combination of the hormones BAP (1 mg/l) and NAA (0.5 mg/l) can increase the shoot length of the germplasm. The composition of the media with hormone concentrations that are too high or too low can inhibit shoot initiation. According to Kumar and Keshamma (Citation2022), the factors to be considered in tissue culture include the genetic characteristics of the plant cells/tissues as the source of explants, the culture media (hormones, minerals, organic substances, and support agents), the environment (light, temperature, and humidity), time (subculture period) and the interaction between these four factors. The formation of buds and shoots can be used as a source for further propagation, namely the multiplication and acclimatization stages.
3.2. Multiplication and rooting
Gerunggang propagation using plantlets as the source of explants from axenic culture () has a low risk of contamination; all propagated cultures were axenic. Axenic culture is an in vitro state that is free or very low in contamination. The multiplication process starts by separating the axenic plantlets into several new culture media with the best treatment composition in the initial phase (full MS basal media with 1 mg/l BAP and 0.5 mg/l NAA added). After one month of incubation, 25 replicates of viable explants showing good growth elongation conditions were used as propagation material. Explants were cut along a nodule from each shoot (microcutting), observed until 3 months subcultures (1 month/subculture), and transferred to rooting media or we can use to another multiplication material. Gerunggang in vitro propagation was based on shoot elongation from axillary shoot as explants. Multiplication is an in vitro propagation method that uses tissue culture-based technology to propagate plants using axillary shoot as explants. Explants were multiplied by the increase in nodule number caused by shoot elongation. In a previous study (Cuenca et al. Citation2017), species that only have the ability to form multiple shoots were used to obtain the shoot multiplication coefficient; however, gerunggang has the ability to form multiple nodules from elongation in one shoot as well as multiple shoots.
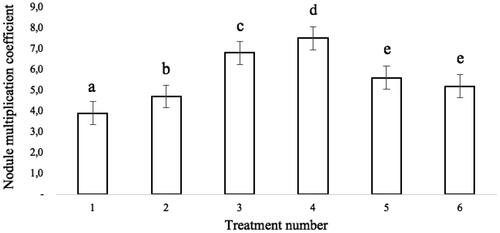
The ability of gerunggang to form single and multiple shoots from a single explant () can be attributed to an imbalance in the media components, such as the source of BAP and NAA hormones used. The ratio of auxin to cytokine in tissue culture varies depending on the plant species used; this plays a critical role in the success of in vitro plant regeneration. Single shoots can be propagated from the shoot elongation nodules, while multiple shoots can be separated. The ability of gerunggang to form two types of shoots requires further investigation. The effect of the combination of BAP and NAA hormones on the nodule multiplication coefficient (NMC) () showed a significant difference in the treatment of BAP and NAA, the lowest and highest hormones in this study gave a negative response. Contrary to the hormone treatment at a certain concentration balance, which gives the best results (7.46). Treatment with BAP and NAA in this study showed the correct concentration range to balance the addition of exogenous hormones with the endogenous hormone status of plants in vitro. In general, BAP is the most effective growth hormone for shoot development. It acts as an inhibitor of apical dominance in shoot induction and bud formation. Several studies have shown that media supplemented with BAP and NAA are beneficial for shoot propagation. According to one study, MS media supplemented with 2 mg/l BA + 2 mg/l NAA gave the best results (Yusnita et al. Citation2011; Sahoo and Rout Citation2014). Another study found that 2 mg/l BAP resulted in the greatest amount of shoot multiplication (Putri et al. Citation2022).
Figure 3. The effect of BAP and NAA hormones combination treatments (1, 2, 3, 4, 5, and 6) on the gerunggang nodule multiplication coefficient (NMC). Mean with different letters (a, b, c, d, and e) are significantly different from each other at 0.5 probability level by Duncan multiple range test.
The ability to root regenerating shoots in vitro () is required to facilitate their formation in acclimatization. The role of different hormonal processes in root induction was investigated in this study for three months of subculture. The best combination of BAP and NAA on initiation and multiplication media was used for enrichment in rooting media but with half strength MS basal media. Root response to multiplication explants showed good growth with an average root length of 4.8 ± 0.2 cm. After 9 months of in vitro multiplication and root regeneration, healthy plantlets with well-developed roots were removed from culture tubes for ex vitro acclimatization in a greenhouse. In this study, 100% of the micro-propagated gerunggang explants had healthy plantlets. The high mortality experienced by micropropagation plants during or after transfer from the laboratory to the greenhouse and transfer to the soil in the nursery is a major limitation in the large-scale application of this technology. Abiotic acclimatization will experience changes in temperature, light intensity, humidity conditions, and biotic stress conditions when transferred to ex vitro conditions. Thus, it requires a proper acclimatization process for the successful formation and survival of ex vitro plantlets, even though in vitro root growth is in a good response.
3.3. Acclimatization
The transfer of gerunggang plantlets to ex vitro conditions gave good growth at 2 months in the greenhouse and 3 months in the nursery (). This acclimatization can be successful, not resulting in mortality, it is possible because the stomata can function properly, the root system is strong to grow well, and the cuticle can still develop. Plantlets that received excess phytohormones showed morphological and anatomical abnormalities and were referred to as vitrified plants or hyperhydrate plants. Due to the physiological and anatomical characteristics of micro-propagated plantlets, they have to be gradually acclimatized to a greenhouse or field environment. The main cause of death of seedlings during acclimatization in all treatments was their sudden exposure (especially the root system) to the microbial community present in the soil, as they were less resistant to soil microflora (Sahoo and Rout Citation2014).
Acclimatization is very important to increase plantlet survival and the success of getting seeds ready for planting. Due to the high mortality rate of plants, direct planting of plant tissue cultured seedlings into the field is not possible. In vitro control of humidity, temperature, light, and nutrient availability requires ex vivo adjustments. Acclimatization to various biotic environments in soil media also requires special attention.
Biotechnology of gerunggang propagation is expected to provide alternative techniques that are more efficient and effective for multipurpose utilization. Gerunggang not only having an important function in natural succession, restoration, and rehabilitation and has high value in tropical peat ecosystems, it also has great potential in the economic field as a producer of fiber including for pulp and paper as well as a producer of biopharmaceuticals. The development of this micro propagation protocol of gerunggang is very important, especially to support the needs of large-scale production in various industries that are based on gerunggang.
4. Conclusions
Gerunggang (Cratoxylum arborescens (Vahl) Blume), a multipurpose native species of Indonesian peatland, was micropropagated in order to develop a protocol for large-scale seedling by tissue culture using BAP (6-Benzyl Amino Purine) and NAA (Naphthalene Acetic Acid) in MS basal media. Gerunggang bud break was the quickest 2 weeks (before the first subculture) in full strength MS media with 1 mg/l BAP and 0.5 mg/l NAA added. This combining hormone also resulted in the highest shoot initiation (3.0 ± 0.2 cm) and shoot elongation (7.4 ± 0.1 cm) after 4 weeks of incubation from axillary buds explants. Hormone concentration has a significant influence on the nodule multiplication coefficient (NMC) of gerunggang (7.5). Root growth in response to multiplication explants was good, with an average root length of 4.8 ± 0.2 cm. Acclimatization of plantlets in ex vitro conditions was successful, with good growth after 2 months in the greenhouse and 3 months in the nursery. The gerunggang micropropagation method will be very useful for future research, such as application to real-world systems. As a result, trials on a laboratory scale have been conducted in this field, necessitating a more intensive and comprehensive study.
Author contributions
Asri Insiana Putri (A.I.P.), Noor Khomsah Kartikawati (N.K.K.), Arif Nirsatmanto (A.N.), Sri Sunarti (S.S.), Liliek Haryjanto (L.H.), Toni Herawan (T.H.), Purwanto Budi Susanto (P.B.S.), Reni Setyo Wahyuningtyas (R.S.W.), Fajar Lestari (F.L.), and Anto Rimbawanto (A.R.) contributed in conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing-review and editing, visualization, supervision, and project administration. All authors had an equal role as main contributors in discussing the conceptual ideas and the outline, providing critical feedback for each section, and writing the manuscript. All authors have read and agreed to the published version of the manuscript.
References
- Allied Market Research, Plant Tissue Culture Market, Global Opportunity Analysis and Industry Forecast, 2021–2030. 2022. Portland (OR).
- Chaturvedi R, Razdan MK, Bhojwani SS. 2004. In vitro clonal propagation of an adult tree of neem (Azadirachta indica A. Juss.) by forced axillary branching. Plant Sci. 166(2):501–506.
- Cuenca B, Sanches C, Aldrey A, Bogo B. 2017. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tissue Organ Cult. 131(3):1–14.
- Faqir A. 2022. Paper industry becomes the mainstay of exports in Indonesia. Merdeka; p. 1.
- George EF, Hall MA, Klerk G-JD. 2008a. Plant growth regulators I: introduction; auxins, their analogues and inhibitors. In: Plant propagation by tissue culture; Dordrecht Netherland: Springer, p. 175–204.
- George EF, Hall MA, Klerk G-JD. 2008b. Plant growth regulators II: cytokinins, their analogues and antagonists. In: Plant propagation by tissue culture; Dordrecht, Netherland: Springer, p. 205–226.
- Global Plant Tissue Culture Market Predicted to Generate a Revenue of $2,202.9 Million and Rise at a CAGR of 6.4% throughout the Analysis Timeframe from 2022–2031. 2023. GlobeNewswire. https://www.google.com/search?q=globenewswire.com%2C+2023+plant+tissue+culture&rlz.
- Haque MI, Singh PK, Ghuge S, Kumar A, Rai AC, Kumar A, Modi A. 2022. A general introduction to and background of plant tissue culture: past, current, and future aspects. In: Rai M, Kumar AC, Modi A, Singh A, editors. Advances in plant tissue culture. United Kingdom and United State of America: Academic Press, Science Direct, (in print) Elsevier; p. 1–30.
- Ibáñez CM, Camus PA, Rocha F. 2009. Diversity and distribution of cephalopod species off the coast of Chile. Mar Biol Res. 5(4):374–384.
- Kumar SC, Keshamma E. 2022. Perspectives on plant tissue culture technology: a review. Int J All Res Educ Sci Methods. 10(7):94–101.
- Li Q, Deng M, Zhang J, Zhao W, Song Y, Li Q, Huang Q. 2013. Shoot organogenesis and plant regeneration from leaf explants of Lysionotus serratus D. Don. Sci World J. 2013(8):1–7.
- Negi Z, Rawte S, Jha N. 2022. Plant tissue culture progress across the globe. Int J Adv Multidiscip Res Stud. 2(6):952–955.
- Pattanaprateeb P, Ruangrungsi N, Geoffrey A, Cordell GA. 2005. Cytotoxic constituents from Cratoxylum arborescens. Planta Med. 71(2):181–183.
- Prior KM, Adams DC, Klepzig KD, Hulcr I. 2018. When does invasive species removal lead to ecological recovery? Implications for management success. Biol Invasions. 20(2):267–283.
- Putri A, Herawan I, Prastyono T, Haryjanto L. 2017. Pengaruh Teknik Sterilisasi Explan Terhadap Tingkat Perolehan Kultur Jaringan Aksenik Ramin (Gonystylus bancanus). J Pemuliaan Tanam Hutan. 11(2):131–138.
- Putri A, Jayusman I. 2012. Axillary buds and callus initiation from stem cutting of Toona sinensis and Toona sureni. J Pemuliaan Tanam Hutan. 6(3):167–180.
- Putri AI, Kartikawati NK, Nirsatmanto A, Sunarti S, Haryjanto L, Herawan L, Santosa PB, Wahyuningtyas RS, Lestari F, Rimbawanto A. 2022. In vitro multiplication of Lophostemon suaveolens (Sol.ex Gaertn.) Peter G. Wilson & J. T. Waterh): peatland tree species for rehabilitation. Sustainability. 14(14720):1–14.
- Rathore JS, Phulwaria M, Rai MK, Shekhawat S, Shekhawat NS. 2015. A liquid culture system for improved micropropagation of mature Acacia nilotica (L.) Del. ssp. indica and ex vitro rooting. Ind J Plant Physiol. 20(2):172–176.
- Rizqiani KD, Aprianis Y, Junaedi A. 2021. Potensi Tiga Jenis Kayu Tanah Gambut Sumatera sebagai Bahan Baku Pulp dan Kertas. J Ilmu Teknol Kayu Trop. 17(2):112–121.
- Roosslee J. 2021. Tissue culture around the globe. Plant Cell Technology. https://www.plantcelltechnology.com/pct-blog/tissue-culture-around-the-globe/.
- Sahoo S, Rout G. 2014. Plant regeneration from leaf explants of Aloe barbadensis Mill. and genetic fidelity assessment through DNA markers. Physiol Mol Biol Plants. 20(2):235–240.
- Setyaningsih A, Indrioko S, Putri AI. 2020. Pengaruh Media Serta Posisi Nodul Pada Regenerasi Eksplan Kultur Jaringan Geronggang (Cratoxylum arborescens (Vahl) Blume).
- Suwito D, Poedjirahajoe, E, Suratman . 2021. The potency of Cratoxylum arborescens Blume (Geronggang) and Combrecarpus rotundatus Dans (Tumih) as natural regeneration in degraded tropical peat swamp forest. J Indones Sustain Dev Plan. 2(3):272–289.
- Yusnita , Pungkastiani W, Hapsoro D. 2011. In vitro organogenesis of two Sansevieria cultivars on different concentrations of benzyladenine (BA). AGRIVITA. 33(2):147–153.


