Abstract
Cinchona officinalis, commonly called cascarilla or quina, has medicinal value; and is on Peru’s national coat of arms representing its plant wealth (flora), however, it is threatened by anthropogenic activities. This study aimed to determine the effect of the commercial product Myco Grow® on the growth of C. officinalis in nursery. A randomized design was used with two treatments, one with Myco Grow® application (WM) and the other without incorporating this commercial product (NM). Each treatment had three replicates consisting of 30 plants each. Monthly evaluations were performed, during which the number of dead plants, plant height, and plant diameter were recorded. Additionally, at the end of the study, the anhydrous weight of leaves, stems, and roots; leaf area; mycorrhizal frequency; mycorrhizal colonization index; and the length of extra-radicular mycelia were determined. The WM treatment achieved 36.6% lower mortality, 38.01% greater height, and 48.52% greater diameter than the NM treatment. Additionally, inoculation with arbuscular mycorrhizae (AM) improved the anhydrous weights of the leaves, stems, roots, and leaf area by 84.31%, 84.28%, 70.85%, and 76.91%, respectively. Regarding the three fungal variables analyzed for the WM treatment; mycorrhizal frequency was 87%, AM application led to a mycorrhizal intensity of 7.7% and an extra-radicular mycelium length of 90.3 cm. This study confirmed that AM positively influences the growth of C. officinalis in the nursery and can be used to sustainably produce this species on a large scale.
Introduction
Cinchona officinalis L. is a forest species whose bark contains alkaloids that have medicinal importance. This has been used for centuries as one of the main treatments for malaria until the 1940s (Cóndor et al. Citation2009; Bharadwaj et al. Citation2018). Cinchona alkaloids are considered to be the most influential tree bark-derived medicines in human history (Prendergast and Dolley Citation2001); moreover, this species is found on Peru’s national coat of arms representing its plant wealth (Flora) (García et al. Citation2022).
Cinchona officinalis requires special conditions to grow and its distribution is limited (Armijos-González and Pérez-Ruiz Citation2016). As of 2022, expanding agricultural and livestock land have degraded the C. officinalis habitat (Huamán et al. Citation2019; Fernandez et al. Citation2022). Additionally, this species has slow growth at the nursery level which is affected by edaphoclimatic (Fernandez et al. Citation2021) and microbiological (mycorrhizae) characteristics (Fernandez-Zarate et al. Citation2022).
Associations between plant roots and mycorrhizal fungi that can influence plant growth have been previously reported (Martínez and Pugnaire Citation2009). Arbuscular mycorrhizas (AM) adhere to the root system, forming extra-root hyphae that allow greater root elongation and, consequently, plants to absorb additional nutrients and water, thus improving their growth and development (Mehmood et al. Citation2022), in addition, fungal structures are directly related to the absorption of phosphate, ammonium, nitrate and amino acids by plants (Parniske Citation2008).
Mycorrhizal associations in forest nurseries have been studied in economically important forest species, such as pines, oaks, and spruces (Iwański et al. Citation2006; Menkis and Vasaitis Citation2011; Pietras et al. Citation2013; Rudawska et al. Citation2017). However, studies on this type of symbiotic association in native tree species used in various local restoration and recovery strategies are scarce (Timonen and Kauppinen Citation2008; Stanturf et al. Citation2014; Rudawska et al. Citation2017). Therefore, generating information on C. officinalis behavior at the nursery stage to initiate plans for its management and preservation is necessary (Fernandez et al. Citation2022). Thus, this study aimed to determine whether applying AM to the substrate influences the growth of C. officinalis in the nursery stage. Additionally, AM could be used as a bio-fertilizer and biostimulant to accelerate C. officinalis growth in the future.
Materials and methods
Study area
The research was carried out in the La Cascarilla locality (5° 40′ 21.12″ S and 78° 53′ 55.65″ W), province of Jaén, Peru, which is located at 1810 m altitude, with an annual rainfall of 1730 mm, and an average temperature of 16.5 °C (Fernandez et al. Citation2021).
Plant material
For producing C. officinalis plants, seeds collected were used from a single population of C. officinalis from the community of San Luis (6°22′ 6.68″ S and 79°3′ 29.50″ E) at 2489 m altitude. Mature capsules (1 kg, brown to dark brown) were collected and transported in cloth bags to the La Cascarilla community, where they were stored in the shade. Seeds without visible cracks and/or contaminated by fungi were selected 20 d later for use in this study (Fernandez-Zarate et al. Citation2022). Seed storage was avoided because they rapidly lose their germination capacity owing to their recalcitrant nature (Caraguay et al. Citation2016). A subirrigation chamber was used for geminating C. officinalis seeds as described by Fernandez et al. (Citation2021). Seeds were sown in a forest substrate, whose textural class was sandy loam, pH = 3.87, electrical conductivity = 0.15 dS m−1, phosphorus = 9.95 ppm, potassium = 187.42 ppm, nitrogen = 9.53%, organic matter = 10.55% (Fernandez et al. Citation2022), autoclaved at 105 °C for 1 h, and this process was repeated for three consecutive days. C. officinalis seedlings were transplanted into 280 WM3 cylindrical polyethylene bags (5.8 diameter and 10.6 height).
Microbiological inoculation
An inoculum of Rhizophagus irregularis, Funneliformis mosseae and Glomus aggregatum contained in the commercial product MycoGrow® – Complex was used. Six kilograms of this product were applied to one cubic meter of substrate, then uniformly mixed and placed in polyethylene bags.
Substrate
For the transplanting of C. officinalis, a substrate was prepared based on forest soil and sand (2:1), which was subjected to a sterilization process in an oven at 105 °C for 1 h. This process was replicated for three consecutive days. The physical and chemical properties of the substrate were as follows: sandy loam texture, pH = 4.18, electrical conductivity = 0.51 dS m−1, organic matter = 6.03%, total nitrogen = 0.3%, and phosphorus = 8.5 ppm.
Experimental design and set-up
Two treatments were used, one with arbuscular mycorrhizae (WM) and one without mycorrhizae (NM), and three replicates per treatment. Thirty C. officinalis plants were used per replicate and 180 plants were used for the entire investigation. The C. officinalis seedlings were placed in polyethylene bags and monitored every month for 10 months.
The effect of AM on C. officinalis growth in the nursery was estimated by recording the number of dead plants, plant height, and plant diameter (measured using a digital Vernier at the substrate level). Additionally, the weight of leaves, stems, roots, leaf area, and fungal variables (mycorrhizal frequency, mycorrhizal intensity, and length of extraradicular mycelia) were calculated at the end of the trial.
The dry matter of the leaves, stems, and roots was determined by drying in an oven at 60 °C for 72 h, and the weight of each plant part was subsequently obtained.
The leaf area (cm2) was calculated by taking photographs of leaves on a blank background (20 × 12 cm cardboard) that had a 2 cm long line drawn next to the leaf location site necessary to maintain the scale in image processing. The leaves were prevented from wrinkling by covering them with transparent glass (20 × 12 cm and 2 mm thick) and were photographed using a Huawei cell phone with a camera model MAR-LX3A of 24 megapixels. Images were processed using ImageJ (Baker et al. Citation1996).
Finally, mycorrhizal frequency (MF) (Sieverding et al. Citation1991), mycorrhizal intensity (MI) (Trouvelot et al. Citation1986) and length of extraradical mycelium (LEM) (Newman Citation1966; Carballar Citation2010) were determined.
Data analysis
The means of the replicates of each treatment were compared using a t-test (α = 0.05) after testing for compliance with the normality assumption (Shapiro–Wilk) and homogeneity of variance (Levene’s test). The results were analyzed using StatGraphics Centurion XVI (StatPoint Technologies Inc., Warrenton, VA, USA. JU).
Results
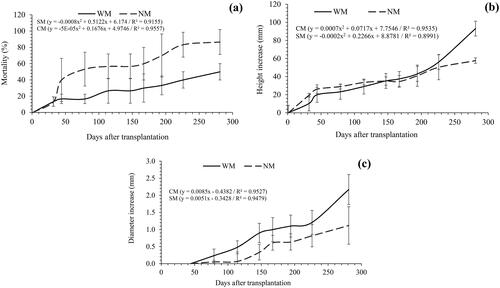
After 44 d of the C. officinalis transplant, the percentage of accumulated mortality in both treatments differed, reaching 86.6% dead plants at the end of the trial for the NM treatment, which was 36.6% higher than that in the AM treatment (). The cumulative increase in height at the end of the WM treatment was 38.01% higher than that of the NM treatment (). A similar result trend was obtained for the increase in diameter, which was 48.52% higher in the WM treatment than that in the NM treatment (). This indicated that AM had a significant and positive influence on the growth of C. officinalis at the nursery stage. Generally, C. officinalis plants inoculated with AM showed a lower mortality rate and reached greater heights and diameters than those without AM treatment.
Figure 1. Cumulative percent mortality of C. officinalis during the entire trial (a), height increment (B), and diameter (C) of C. officinalis.
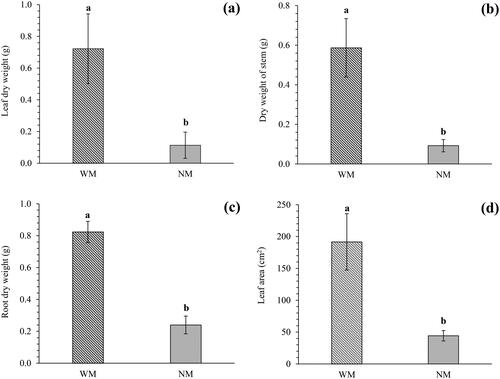
AM inoculation resulted in a significantly higher dry biomass yield of leaves (), stems (), and roots () than when no AM was inoculated. Furthermore, the leaf area of C. officinalis plants in the WM treatment was significantly higher than that in the NM treatment (), representing an increase of 79.91%.
Figure 2. The evaluated growth parameters. Anhydrous leaf weight (a), anhydrous stem weight (B), anhydrous root weight (C), and leaf area (D). Different lowercase letters indicate significant statistical differences (p = .05).
Regarding the three fungal variables analyzed for the WM treatment, the results are shown in .
Table 1. Fungal parameters analyzed for the WM treatment.
Discussion
AM application led to a 36.6% reduction in C. officinalis plant mortality at the nursery stage compared with NM treatment, this result could be associated with the positive effects of AM in protection against pathogens, leading to early plant death (Berruti et al. Citation2015; Devi et al. Citation2022; Dey and Ghosh Citation2022). Additionally, a significant increase in the height and diameter of C. officinalis was observed in the WM treatment, these results could be directly related to the production of more efficient root systems, since plants inoculated with AM had significantly higher root biomass than plants that were not inoculated with AM (Quoreshi and Khasa Citation2008). AM colonizing roots provides better nutrition (Pankaj et al. Citation2021) because mycorrhizae can increase root size by up to 200% (Falcón et al. Citation2021), allowing greater access to nutrients in the soil (Weisany et al. Citation2016; Khalediyan et al. Citation2021). Several studies have suggested that AM generates growth hormones such as auxins and indole acetic acid, which influence plant development, physiology, morphogenesis, and root growth (Quoreshi and Khasa Citation2008; Smith and Read Citation2008), this proves the potential benefits to be obtained with AM inoculation on C. officinalis plants at the nursery stage.
The anhydrous weights of leaves, stems, and roots were higher in plants inoculated with AM than in those without AM, suggesting that the binding between AM and the roots of C. officinalis improves nutrient uptake by the plant, as demonstrated for a Theobroma cacao crop, and water uptake (Jiang et al. Citation2017; Aggangan et al. Citation2019).
Additionally, the present study revealed an increase in leaf area in the WM treatment, which could be attributed to the percentage of mycorrhizal colonization (Palacios et al. Citation2021; Fernandez-Zarate et al. Citation2022) and the length of the extraradicular mycelium, which was higher in plants inoculated with AM. AM colonization on C. officinalis roots can lead to increased uptake of water and nutrients, as it increases the surface area through the mycelium in the soil, providing the plant with greater access to the soil. This can lead to increased photosynthetic efficiency, improved plant growth, and, subsequently, increased leaf area (Huang et al. Citation2018; Khalediyan et al. Citation2021).
Conclusion
AM inoculation decreased in mortality and increased in height, biomass and diameter of C. officinalis plants, and improves the evaluated fungal parameters (MF, MI, and LEM). This suggests that AM can be used as a biofertilizer for C. officinalis at the nursery stage. This study did not analyze the effect of a specific mycorrhiza, but evaluated a mycorrhizal consortium formed by Rhizophagus intraradices, Funneliformis mosseae, and Glomus aggregatum, in this sense, if the effect of each species on the growth of C. officinalis is desired, each of the mycorrhizae should be analyzed independently. The results show promise for AM application in nurseries to produce C. officinalis plants and contribute to repopulation programs of the species.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aggangan NS, Cortes AD, Reaño CE. 2019. Growth response of cacao (Theobroma cacao L.) plant as affected by bamboo biochar and arbuscular mycorrhizal fungi in sterilized and unsterilized soil. Biocatal Agric Biotechnol. 22:101347. doi: 10.1016/j.bcab.2019.101347.
- Armijos-González R, Pérez-Ruiz C. 2016. In vitro germination and shoot proliferation of the threatened species Cinchona officinalis L (Rubiaceae). J For Res. 27(6):1229–1236. doi: 10.1007/s11676-016-0272-8.
- Baker B, Olszyk DM, Tingey D. 1996. Digital image analysis to estimate leaf area. J Plant Physiol. 148(5):530–535. doi: 10.1016/S0176-1617(96)80072-1.
- Berruti A, Lumini E, Balestrini R, Bianciotto V. 2015. Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front Microbiol. 6:1559. doi: 10.3389/fmicb.2015.01559.
- Bharadwaj KC, Gupta T, Singh RM. 2018. Chapter 9 – Alkaloid group of Cinchona officinalis: Structural, synthetic, and medicinal aspects. In: Tewari A, Tiwari S, editors. Synthesis of medicinal agents from plants. Elsevier; p. 205–227. doi: 10.1016/B978-0-08-102071-5.00009-X.
- Caraguay KA, Eras VH, Gonzalez D, Moreno J, Minchala J, Yaguana M, Valarezo C. 2016. Potencial reproductivo y análisis de calidad de semillas de Cinchona officinalis L., provenientes de relictos boscosos en la provincia de Loja – Ecuador. Revista Investigaciones Altoandinas. 18(3):271–280.
- Carballar S. 2010. Variación temporal de la diversidad de hongos de micorriza arbuscular y el potencial micorrizico en especies silvestres de agave en Oaxaca [Tesis de Magister]. Mexico: IPN. http://tesis.ipn.mx:8080/xmlui/handle/123456789/5973.
- Cóndor E, de Oliveira BH, Loayza Ochoa K, Reyna Pinedo V. 2009. Estudio químico de los tallos de Cinchona pubescens Vahl. Revista de la Sociedad Química Del Perú. 75(1):54–63.
- Devi NO, Tombisana Devi RK, Debbarma M, Hajong M, Thokchom S. 2022. Effect of endophytic Bacillus and arbuscular mycorrhiza fungi (AMF) against Fusarium wilt of tomato caused by Fusarium oxysporum f. Sp. Lycopersici. Egypt J Biol Pest Control. 32(1):1. doi: 10.1186/s41938-021-00499-y.
- Dey M, Ghosh S. 2022. Arbuscular mycorrhizae in plant immunity and crop pathogen control. Rhizosphere. 22:100524. doi: 10.1016/j.rhisph.2022.100524.
- Falcón E, Cobas M, Bonilla M, Rodríguez O. 2021. Efecto del sustrato y la micorriza arbuscular en el sistema radical y estado nutricional de Swietenia mahagoni L. Jacq. Revista Cubana de Ciencias Forestales. 9(3):395–411.
- Fernandez FH, Huaccha AE, Barturén LM, Quiñones L, Sanchez T. 2022. Efecto del sustrato en la propagación sexual de Cinchona officinalis L. (Rubiaceae). ECOS. 31(1):2314. doi: 10.7818/ECOS.2314.
- Fernandez FH, Huaccha AE, Quiñones LQ, Sánchez T. 2021. Influencia del tamaño de plántula de Cinchona officinalis (Rubiaceae) en la supervivencia y deformación del tallo posterior al repique. Revista Cubana de Ciencias Forestales. 9(3):3.
- Fernandez-Zarate FH, Huaccha-Castillo AE, Quiñones-Huatangari L, Vaca-Marquina SP, Sanchez-Santillan T, Morales-Rojas E, Seminario-Cunya A, Guelac-Santillan M, Barturén-Vega LM, Coronel-Bustamante D. 2022. Effect of arbuscular mycorrhiza on germination and initial growth of Cinchona officinalis L. (Rubiaceae). Forest Sci Technol. 18(4):182–189. doi: 10.1080/21580103.2022.2124318.
- García L, Veneros J, Chavez SG, Oliva M, Rojas-Briceño NB. 2022. World historical mapping and potential distribution of Cinchona spp. In Peru as a contribution for its restoration and conservation. J Nat Conserv. 70:126290. doi: 10.1016/j.jnc.2022.126290.
- Huamán L, Albán J, Chilquillo E. 2019. Aspectos taxonómicos y avances en el conocimiento del estado actual del árbol de la Quina (Cinchona officinalis L.) en el Norte de Perú. Ecol Apl. 18(2):145–153. doi: 10.21704/rea.v18i2.1333.
- Huang H, Zi X-M, Lin H, Gao J-Y. 2018. Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid, Dendrobium devonianum. J Microbiol. 56(1):42–48. doi: 10.1007/s12275-018-7225-1.
- Iwański M, Rudawska M, Leski T. 2006. Mycorrhizal associations of nursery grown Scots pine (Pinus sylvestris L.) seedlings in Poland. Ann For Sci. 63(7):715–723. doi: 10.1051/forest:2006052.
- Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D, Zhang X, Yang C, Chen X, Tang D, et al. 2017. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 356(6343):1172–1175. doi: 10.1126/science.aam9970.
- Khalediyan N, Weisany W, Schenk PM. 2021. Arbuscular mycorrhizae and rhizobacteria improve growth, nutritional status and essential oil production in Ocimum basilicum and Satureja hortensis. Ind Crops Prod. 160:113163. doi: 10.1016/j.indcrop.2020.113163.
- Martínez LB, Pugnaire FI. 2009. Interacciones entre las comunidades de hongos formadores de micorrizas arbusculares y de plantas. Algunos Ejemplos En Los Ecosistemas Semiáridos. Ecosistemas. 18(2):2. https://www.revistaecosistemas.net/index.php/ecosistemas/article/view/65.
- Mehmood H, Arif Ali M, Hussain S, Shehzad Baig K, Farooq U, Ajmal M, Atif Hasan Naqvi S, Sultan H, Datta R, Shabbir Dar J, et al. 2022. Synchronization of arbuscular mycorrhizae fungi inoculation with different zinc application methods for improvement in Basmati rice growth and yield in alkaline calcareous soil. J King Saud Univ Sci. 34(5):102053. doi: 10.1016/j.jksus.2022.102053.
- Menkis A, Vasaitis R. 2011. Fungi in roots of nursery grown Pinus sylvestris: ectomycorrhizal colonisation, genetic diversity and spatial distribution. Microb Ecol. 61(1):52–63. doi: 10.1007/s00248-010-9676-8.
- Newman EI. 1966. A method of estimating the total length of root in a sample. J Appl Ecol. 3(1):139–145. doi: 10.2307/2401670.
- Palacios YM, Gleadow R, Davidson C, Gan W, Winfrey B. 2021. Do mycorrhizae increase plant growth and pollutant removal in stormwater biofilters? Water Res. 202:117381. doi: 10.1016/j.watres.2021.117381.
- Pankaj U, Kurmi A, Lothe NB, Verma RK. 2021. Influence of the seedlings emergence and initial growth of palmarosa (Cymbopogon martinii (Roxb.) Wats. Var. Motia Burk) by arbuscular mycorrhizal fungi in soil salinity conditions. J Appl Res Med Aromat Plants. 24:100317. doi: 10.1016/j.jarmap.2021.100317.
- Parniske M. 2008. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 6(10):763–775. doi: 10.1038/nrmicro1987.
- Pietras M, Rudawska M, Leski T, Karliński L. 2013. Diversity of ectomycorrhizal fungus assemblages on nursery grown European beech seedlings. Ann for Sci. 70(2):115–121. doi: 10.1007/s13595-012-0243-y.
- Prendergast HDV, Dolley D. 2001. Jesuits’ bark (Cinchona [Rubiaceae]) and other medicines. Econ Bot. 55(1):3–6. doi: 10.1007/BF02864540.
- Quoreshi AM, Khasa DP. 2008. Effectiveness of mycorrhizal inoculation in the nursery on root colonization, growth, and nutrient uptake of aspen and balsam poplar. Biomass Bioenergy. 32(5):381–391. doi: 10.1016/j.biombioe.2007.10.010.
- Rudawska M, Leski T, Aučina A, Karliński L, Skridaila A, Ryliškis D. 2017. Forest litter amendment during nursery stage influence field performance and ectomycorrhizal community of Scots pine (Pinus sylvestris L.) seedlings outplanted on four different sites. For Ecol Manage. 395:104–114. doi: 10.1016/j.foreco.2017.04.002.
- Sieverding E, Friedrichsen J, Suden W. 1991. Vesicular-arbuscular mycorrhiza management in tropical agrosystems. Schriftenreihe Der GTZ (Germany). https://scholar.google.com/scholar_lookup?title=Vesicular-arbuscular+mycorrhiza+management+in+tropical+agrosystems&author=Sieverding%2C+E.&publication_year=1991.
- Smith SE, Read D. 2008. Mycorrhizas in agriculture, horticulture and forestry. In: Smith SE, Read D, editors. Mycorrhizal symbiosis. 3rd ed. London: Academic Press; p. 11–18. doi: 10.1016/B978-012370526-6.50019-2.
- Stanturf JA, Palik BJ, Dumroese RK. 2014. Contemporary forest restoration: a review emphasizing function. For Ecol Manage. 331:292–323. doi: 10.1016/j.foreco.2014.07.029.
- Timonen S, Kauppinen P. 2008. Mycorrhizal colonisation patterns of Tilia trees in street, nursery and forest habitats in southern Finland. Urban For Urban Green. 7(4):265–276. doi: 10.1016/j.ufug.2008.08.001.
- Trouvelot A, Kough JL, Gianinazzi-Pearson V. 1986. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. https://typeset.io/papers/mesure-du-taux-de-mycorhization-va-d-un-systeme-radiculaire-1wu2mxnpdv.
- Weisany W, Zehtab-Salmasi S, Raei Y, Sohrabi Y, Ghassemi-Golezani K. 2016. Can arbuscular mycorrhizal fungi improve competitive ability of dill + common bean intercrops against weeds? Eur J Agron. 75:60–71. doi: 10.1016/j.eja.2016.01.006.
