Abstract
Ulmus davidiana has been used as food or medicine in oriental due to its safety, including its roots, bark, and fruits. The roots and bark of Ulmus were used as a diuretic and for treatment due to their anti-cancer, antioxidant, and anti-inflammatory effects. Ulmus species are known to contain flavan-3-ols as their main component. In previous research the presence of (+)-catechin 5-O-β-D-apiofuranoside was isolated from the roots of Ulmus davidiana, and (+)-catechin 7-O-β-D-xylopyranoside was confirmed in the bark Ulmus Americana, and the presence of catechin 7-O-β-D apiofuranoside in the stems and bark of Ulmus davidiana var. japonica. The catechin-glycosides of flavan-3-ols have various effects, including antiulcer properties, and have also been used as analgesics. Additionally, chemotaxonomy studies of the Ulmus species have been reported. However, the chemotaxonomy of Ulmus species native to Asia has not been reported. In this study, we report the screening of catechin 7-O-beta-D apiofuranoside from various Ulmus species native to Asia. We confirmed the presence of catechin 7-O-β-D-apiofuranoside in a total of 22 Ulmus samples, including 18 Ulmus species native to China, and 1 sample each from Mongolian, Nicaragua, Vietnam, and Korea through HPLC and LC-MS/MS analysis. We also confirmed the content of the indicator materials. Therefore, the potential of using native Ulmus species from Korea as a natural material has been confirmed.
1. Introduction
Ulmus species grow in the temperate regions of the northern hemisphere. These deciduous broad-leaf trees are commonly found in the Orient, regions such as Korea, China, and Japan. There are 30 species including Ulmus davidiana var. japonica for. Suberosa and Ulmus pumila Linne. (Ulmus mandshurica Nakai) (Choi et al. Citation2010; Kim et al. Citation2020). Ulmus has been used as food or medicine in Korean traditional medicine (Hong et al. Citation1990), such as root, bark, and fruit, because of its safety. The sticky mucus from which bark of the Ulmus and root of the Ulmus were contained in water was used as a diuretic, and was used for treatment with effects on antioxidant, anti-inflammatory, and anti-cancer. It was also used as a medicine to treat diseases, such as inflammation, burns, and frostbite (Lee Citation1996; Lee et al. Citation2004; Kim et al. Citation2010; Pan et al. Citation2017). Ulmus species are known to contain flavan-3-ols as their main component (Son et al. Citation1989; Mun and Lim Citation1995; Lee et al. Citation2008). In previous research the presence of (+)-catechin 7-O-β-D-xylopyranoside was confirmed in the bark Ulmus Americana (Doskotch et al. Citation1973), and (+)-catechin 5-O-β-D-apiofuranoside was isolated from the roots of Ulmus davidiana (Son et al. Citation1989), and the presence of catechin 7-O-β-D apiofuranoside in the stems and bark of Ulmus davidiana var. japonica. (Kim et al. Citation2020). The catechin-glycosides of flavan-3-ols have various effects, such as including and antiulcer, and were also used as analgesics (Wu et al. Citation2012). Additionally, chemotaxonomy studies of the Ulmus species have been reported (Rowe et al. Citation1972; Sherman and Giannasi Citation1988; Cheng et al. Citation2020; Kim et al. Citation2020; Shen et al. Citation2023). However, the chemotaxonomy of Ulmus species native to Asia has not been reported.
In this study, we report the screening of catechin 7-O-beta-D apiofuranoside from various Ulmus species native to Asia. We confirmed the presence of catechin 7-O-beta-D-apiofuranoside in a total of 22 Ulmus samples, including 18 Ulmus species native to China, and 1 sample each from Mongolian, Nicaragua, Vietnam, and Korea. We also confirmed the content of the indicator materials. Therefore, the potential of utilizing native Ulmus species from Korea as a natural material has been confirmed.
2. Materials and methods
2.1. Plant extracts of Ulmus species
Standard products are stored in the Department of Forest Biomaterials Engineering, Kangwon National University (Kwon et al. Citation2022). Standard products are a total of 21 samples native to Asia were purchased from the Korea Research Institute of Bioscience International Biological Material Research Center. The bark of Ulmus, native to Korea, was collected in an experimental forest by the Gangwon State Forest Science Institute (Jinaeri, Dong-myeon, Chuncheon-si, Gangwon-do, Republic of Korea). The bark extracts of Ulmus macrocarpa from Korea were obtained by extracting with 60% edible ethanol and used for the study. Shown the sample list in .
Table 1. Sample list.
2.2. Catechin 7-O-beta-D apiofuranoside (1)
Negative LC-MS/MS: m/z 422.38 [M-H]-, 1H-NMR (400 MHz, MeOH-d4): 6.82 (H-2′, 1H, d, J = 1.2 Hz), 6.76 (H-5′, 1H, d, J = 7.6 Hz), 6.72 (H-6′, 1H, dd J = 2, 8 Hz), 6.12 (H-8, 1H, d, J = 2.4 Hz), 6.07 (H-6, 1H, d, J = 2.4 Hz), 5.47 (H-1′′, 1H, d, J = 3.2 Hz), 4.58 (H-2, 1H, d, J = 7.6 Hz), 4.13 (H-2′′, 1H, d, J = 3.2 Hz), 4.06 (H-4a′′, 1H, d, J = 10 Hz), 3.99 (H-3, 1H, m), 3.84 (H-4b′′, 1H, d, J = 10 Hz), 3.60 (H-5′′, 2H, m), 2.82 (H-4a, 1H, dd, J = 5.6, 16.8 Hz), 2.56 (H-4b, 1H, dd, J = 8, 16.4 Hz), 13 C-NMR (100 MHz, MeOH-d4): 158.3 (C-7), 157.7 (C-5), 157.0 (C-9), 146.4 (C-4′), 146.4 (C-3′), 132.3 (C-1′), 120.1 (C-6′), 116.2 (C-5′), 115.3 (C-2′), 108.8 (C-1′′), 103.4 (C-10), 97.0 (C-8), 97.4 (C-6), 83.1 (C-2), 80.4 (C-3′′), 78.4 (C-2′′), 75.6 (C-4′′), 68.8 (C-3), 65.1 (C-5′′), 28.6 (C-4) (Kim et al. Citation2020).
2.3. Quantitative analysis of catechin 7-O-beta-D apiofuranoside of ulmus species using high pressure liquid chromatography (HPLC)
HPLC analysis used Waters 515 pump (Milford, MA) equipped with a Waters 717 autosampler, a 2487 dual λ absorbance detector and column compartment, was used to Phenomenex KJ0-4282 guard column and skypak c18 column (4.6 × 250 mm, 5 µm). The mobile phase consisted of 0.9% acetic acid in water and acetonitrile, and the specific are detailed in . The mobile phase was filtered under vacuum through a 0.45-µm, membrane filter and degassed prior to use. The column temperature was set to room temperature. The injection volume was 20 µL, and the flow rate was 1.0 mL/min. Total analysis time was 35 min. The system was monitored at 280 nm (λ max of catechin 7-O-beta-D apiofuranoside). Eluting at 13.32 ± 0.12 min, and catechin 7-O-beta-D apiofuranoside was detected in the extracts.
Table 2. HPLC method of solvent system.
Table 3. Results of standard compound peak area by HPLC chromatography.
2.4. LC-MS/MS analysis
The molecular weight of Ulmus species was analyzed to confirm the indicator material. AB SCIEX (QTRAP 4500) model (Foster City, CA) and LCMS-8050 (Shimadzu Ltd, Tokyo, Japan) were used, and a combination of skypak c18 column (5 μ) and Phenomenex KJ0-4282 guard column was used, and the temperature of the column oven was maintained at 25 °C. The mobile phase, 0.9% acetic acid in water, and acetonitrile were used, the flow rate was 1 mL/min, the wavelength was 280 nm, and the analysis time was 35 min.
3. Results and discussion
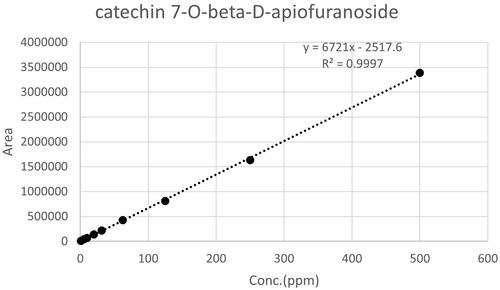
The catechin-7-O-beta-D-apiofuranoside (), which are the effective substance and indicator materials of Ulmus species, were identified through previous research (Kim et al. Citation2020). A standard calibration curve () and content values are shown in for the catechin −7-O-beta-D-apiofuranoside was created for the analysis of 22 Ulmus species extracts using HPLC, with the equation y = 6721x − 2517.6 and R2 = 0.9997 (). The limit of detection of the calibration curve is 1.38 ppm, and the limit of quantization is 4.19 ppm. The HPLC chromatogram of the 22 extracts of Ulmus species is shown in .
Figure 1. Chemical structures of catechin 7-O-beta-D-apiofuranoside from ulmus species. This figure was made with ChemDraw (https://revvitysignals.com/products/research/chemdraw).
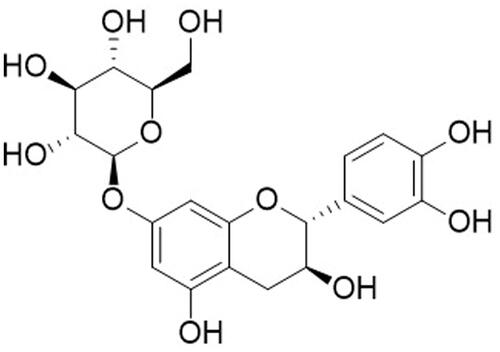
Figure 4. HPLC chromatogram of the extracts of ulmus species from Beijing in China. (A) sample 1 = extracts of Ulmus pumila L. 1000 ppm. (B) Sample 2 = extract of Ulmus davidiana Planch. 1000 ppm. (C) Sample 3 = leave and stem extracts of Ulmus macrocarpa Hance 1000 ppm. (D) Sample 4 = leave and stem extracts of Ulmus macrocarpa Hance 1000 ppm. (E) Sample 5 = the leave and stem extracts of Ulmus parvifolia Jacq. 1000 ppm. (F) Sample 7 = leave and stem extracts of Ulmus pumila L. 1000 ppm. (G) Sample 8 = leave and stem extracts of Ulmus lamellosa C. Wang & S.L. Chang 1000 ppm. (H) Sample 9 = leave and stem extracts of Ulmus laevis Pall. 1000 ppm. (I) Sample 10 = leave extracts of Ulmus parvifolia Jacq. 1000 ppm.
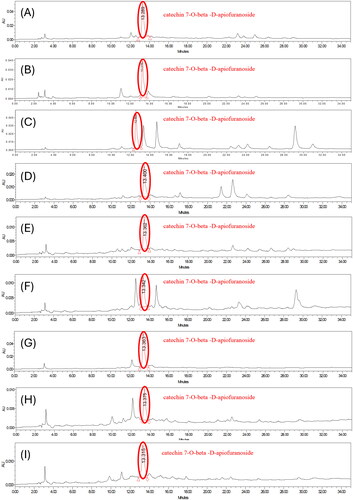
Figure 5. HPLC chromatogram of the leave and stem extracts of ulmus species from Yanbian in China. (A) Sample 11 = leave and stem extracts of ulmus laciniata (trautv.) mayr. 1000 ppm. (B) Sample 13 = leave and stem extracts of ulmus pumila L. 1000 ppm. (C) Sample 14 = leave and stem extracts of ulmus macrocarpa hance 1000 ppm. (D) Sample 15 = the leave and stem extracts of ulmus japonica (rehd.) sarg. 1000 ppm. (E) Sample 16 = stem extracts of ulmus macrocarpa hance 1000 ppm. (F) Sample 17 = stem extracts of ulmus davidiana planch. 1000 ppm.
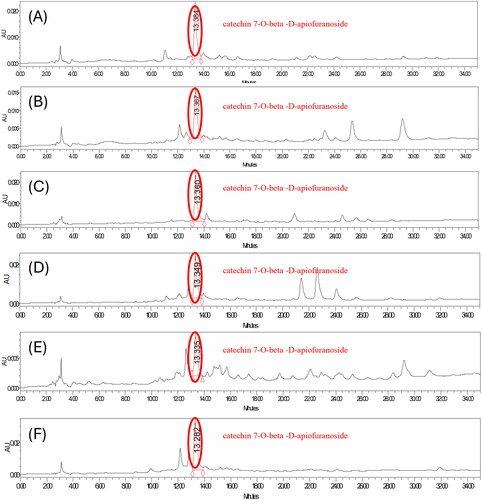
Figure 6. HPLC chromatogram of the bark extracts of Ulmus macrocarpa from Korea. Sample 22 = bark extracts of Ulmus macrocarpa 1000 ppm.
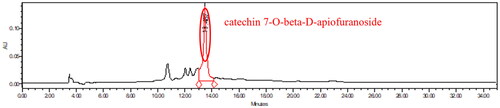
We quantified catechin −7-O-beta-D-apiofuranoside from Ulmus species native to Asia. The 22 sample were not detected so they were treated as Not Detected (N.D.. The full extracts of Ulmus pumila L. [sample 1, 101.51 ± 6.43 ppm], the full extract of Ulmus davidiana Planch. [sample 2, 76.93 ± 3.88 ppm], the leave and stem extracts of Ulmus macrocarpa Hance [sample 3, 73.02 ± 0.27 ppm], the leave and stem extracts of Ulmus macrocarpa Hance [sample 4, 55.61 ± 5.08 ppm], the leave and stem extracts of Ulmus parvifolia Jacq. [sample 5, 25.45 ± 2.26 ppm], the leave and stem extracts of Ulmus castaneifolia Hemsl. [sample 6, N.D], the leave and stem extracts of Ulmus pumila L. [sample 7, 23.11 ± 2.32 ppm], the leave and stem extracts of Ulmus lamellosa C. Wang & S.L. Chang [sample 8, 121.00 ± 9.91 ppm], the leave and stem extracts of Ulmus laevis Pall. [sample 9, 16.41 ± 2.34 ppm], the leave extracts of Ulmus parvifolia Jacq. [sample 10, 40.28 ± 3.30 ppm] from Beijing in China. The leave and stem extracts of Ulmus laciniata (Trautv.) Mayr. [sample 11, 26.74 ± 1.55 ppm], the full extracts of Ulmus suberosa (Turcz.) S. D. Zhao [sample 12, N.D], the leave and stem extracts of Ulmus pumila L [sample 13, 26.89 ± 3.14 ppm], the leave and stem extracts of Ulmus macrocarpa Hance [ample 14, 40.58 ± 0.69 ppm], the leave and stem extracts of Ulmus japonica (Rehd.) Sarg. [Sample 15, 40.31 ± 1.11 ppm], the stem extracts of Ulmus macrocarpa Hance [Sample 16, 18.59 ± 3.74 ppm], the stem extracts of Ulmus davidiana Planch. [Sample 17, 73.61 ± 7.93 ppm] from Yanbian in China. The leave and stem extracts of Ulmus androssowii var. subhirsuta (C.K. Schneid.) P.H. Huang [Sample 18, N.D] from Yunnan in China. The full extracts of Ulmus pumila L. [Sample 19, N.D] from Mongolia. The leave, stem, and flower extracts of Ulmus mexicana (Liebm.) Planch. [Sample 20, N.D] from Nicaragua. The leave and branch extracts of Ulmus lanceifolia Roxb. ex Wall. [Sample 21, N.D] from Vietnam. The bark extracts of Ulmus macrocarpa [Sample 22, 263.70 ± 22.55 ppm] from Korea ().
Table 4. Data of HPLC analysis of ulmus species native to Asia.
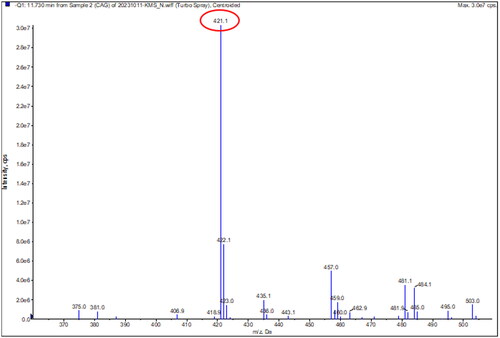
The samples were analyzed using LC-MS/MS. The spectrum was measured in negative mode, it was confirmed that it had a molecular weight of 422.38 g/mol (), which is the same as that of the catechin 7-O-beta-D-apiofuranoside, which is known as an indicator material for Ulmus species ( and ).
Figure 8. Molecular weight of the extracts of ulmus species from Beijing in China. (A) Sample 1 = extracts of Ulmus pumila L. 1000 ppm. (B) Sample 2 = extract of Ulmus davidiana Planch. 1000 ppm. (C) Sample 3 = leave and stem extracts of Ulmus macrocarpa Hance 1000 ppm. (D) Sample 4 = leave and stem extracts of Ulmus macrocarpa Hance 1000 ppm. (E) Sample 5 = the leave and stem extracts of Ulmus parvifolia Jacq. 1000 ppm. (F) sf ample 7 = leave and stem extracts of Ulmus pumila L. 1000 ppm. (G) Sample 8= leave and stem extracts of Ulmus lamellosa C. Wang & S.L. Chang 1000 ppm. (H) Sample 9 = leave and stem extracts of Ulmus laevis Pall. 1000 ppm. (I) Sample 10 = leave extracts of Ulmus parvifolia Jacq. 1000 ppm.
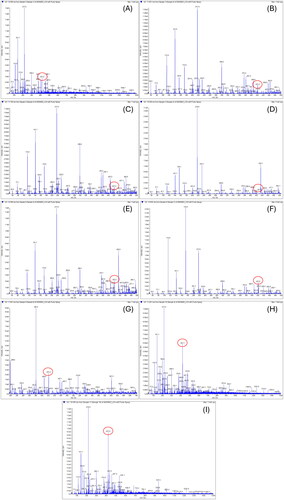
Figure 9. Molecular weight of the leave and stem extracts of ulmus species from Yanbian in China. (A) Sample 11 = leave and stem extracts of ulmus laciniata (trautv.) mayr. 1000 ppm. (B) Sample 13 = leave and stem extracts of ulmus pumila L. 1000 ppm. (C) Sample 14 = leave and stem extracts of ulmus macrocarpa hance 1000 ppm. (D) Sample 15 = the leave and stem extracts of ulmus japonica (rehd.) sarg. 1000 ppm. (E) Sample 16 = stem extracts of ulmus macrocarpa hance 1000 ppm. (F) Sample 17 = stem extracts of ulmus davidiana planch. 1000 ppm.
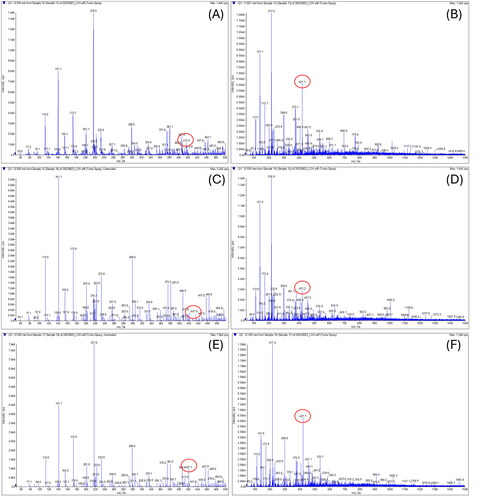
In total, 21 samples excluding sample 20 of Ulmus species showed peaks at the same retention time as catechin 7-O-beta-D-apiofuranoside, but the molecular weight of catechin 7-O-beta-D-apiofuranoside was not detected in 5 samples during LC-MS/MS analysis. The analysis results showed that the molecular weight was detection in the extracts of Ulmus species from China, except for samples 6, 12, and 18. However, the extracts from Ulmus species in Mongolia, Vietnam and Nicaragua did not show detection. Therefore, the content of catechin 7-O-beta-D-apiofuranoside in the extract of Ulmus from Korea was compared with the content of catechin 7-O-beta-D-apiofuranoside detected in extracts of Ulmus from China. The sample with the highest content of indicator materials Chinese samples is the leave and stem extracts of Ulmus lamellosa C. Wang & S.L. Chang [sample 8, 121.00 ± 9.91 ppm], and when compared to the bark extracts of Ulmus macrocarpa [sample 22, 263.70 ± 22.55 ppm] from Korea, it was confirmed that the extract of Ulmus native to Korea showed more than twice the amount of catechin 7-O-beta-D-apiofuranoside.
4. Conclusions
In this study, we confirmed the presence of catechin 7-O-beta-D-apiofuranoside in the Ulmus species native to Asia. In the HPLC analysis, it was confirmed that the presence of indicator substances in a total of 22 samples of native to Asia. Subsequent LC-MS/MS negative analysis revealed the presence of a molecule weight with the same molecular weight of 422.38 g/mol, identified as catechin 7-O-beta-D-apiofuranoside. Therefore, it was confirmed that 16 out of the 22 samples contained the indicator substance of Ulmus, catechin 7-O-beta-D-apiofuranoside. And particularly compared the amounts of indicator materials in the Ulmus species native to China and Korea. Therefore, the chemotaxonomic significance of native to Asia Ulmus species was conducted. The significantly higher levels of indicator materials Ulmus of native to Korea, showing more than a twofold difference, indicate highly meaningful results. Therefore, we have confirmed the value of natural materials native to Korea. The results of this research are expected to be valuable for the exploration of natural materials in the development of new functional materials.
Additional information
Funding
References
- Cheng S, Li N, Yu Y, Elshafei A, Jin M, Li G, Zheng M. 2020. A new flavonoid from the bark of Ulmus pumila L. Biochem Syst Ecol. 88:103956. doi: 10.1016/j.bse.2019.103956.
- Choi SY, Lee S, Choi WH, Lee Y, Jo YO, Ha TY. 2010. Isolation and anti-inflammatory activity of Bakuchiol from Ulmus davidiana var. japonica. J Med Food. 13(4):1019–1023. doi: 10.1089/jmf.2009.1207.
- Doskotch RW, Mikhail AA, Chatterji SK. 1973. Structure of the water-soluble feeding stimulant for Scolytus multistriatus: a revision. Phytochemistry. 12(5):1153–1155. doi: 10.1016/0031-9422(73)85032-0.
- Hong ND, Rho YS, Kim NJ, Kim JS. 1990. A study on efficacy of Ulmi cortex. Korean J Pharmacogn. 21(3):217–222.
- Kim JK, Kwon DJ, Lim SS, Bae YS. 2010. Antioxidant and Anti-inflammatory Activity of Stem Bark Extracts from Ulmus davidiana var. japonica. J Korean Wood Sci Tech. 38(5):444–449. doi: 10.5658/WOOD.2010.38.5.444.
- Kim M, Lee YJ, Shin JC, Choi SE. 2020. Chemotaxonomic significance of catechin 7-O-beta-D-apiofuranoside in Ulmus species. J Korean Wood Sci Technol. 48(6):888–895. doi: 10.5658/WOOD.2020.48.6.888.
- Kwon YE, Choi SE, Park KH. 2022. Regulation of cytokines and dihydrotestosterone production in human hair follicle papilla cells by supercritical extraction-residues extract of Ulmus davidiana. Molecules. 27(4):1419. doi: 10.3390/molecules27041419.
- Lee GY, Jang DS, Kim J, Kim CS, Kim YS, Kim JH, Kim JS. 2008. Flavan-3-ols from Ulmus davidiana var. japonica with inhibitory activity on protein glycation. Planta Med. 74(15):1800–1802. doi: 10.1055/s-0028-1088324.
- Lee SE, Kim YS, Kim JE, Bang JG, Seong NS. 2004. Antioxidant of ulmus and hemiptelea. Korean J Med Crop Sci. 12(4):321–327.
- Lee SJ. 1996. Korean folk medicine, monographs series No. 3. Seoul, South Korea: Publishing Center of Seoul National University; p. 39.
- Mun YH, Lim GR. 1995. A study on the composition of the Ulmus parvifolia. Nat Prod Sci. 26(1):1–7.
- Pan JH, Lim Y, Kim JH, Heo W, Lee KY, Shin HJ, Kim JK, Lee JH, Kim YJ. 2017. Root bark of Ulmus davidiana var. japonica restrains acute alcohol-induced hepatic steatosis onset in mice by inhibiting ROS accumulation. PLoS One. 12(11):e0188381. doi: 10.1371/journal.pone.0188381.
- Rowe JW, Seikel MK, Roy DN, Jorgensen E. 1972. Chemotaxonomy of ulmus. Phytochemistry. 11(8):2513–2517. doi: 10.1016/S0031-9422(00)88527-1.
- Son BW, Park JH, Zee OP. 1989. Catechin glycoside from Ulmus davidiana. Arch Pharm Res. 12(3):219–222. doi: 10.1007/BF02855558.
- Shen L, An M, Liang R, Li Y, He X, Zhao G. 2023. Polyphase taxonomy and genome analysis reveal the adaptability of Luteolibacter rhizosphaerae sp. nov. to the rhizosphere soil of Ulmus pumila L. Antonie Van Leeuwenhoek. 116(8):763–772. doi: 10.1007/s10482-023-01845-w.
- Sherman SL, Giannasi DE. 1988. Foliar flavonoids of Ulmus in eastern North America. Biochem Syst Ecol. 16(1):51–56. doi: 10.1016/0305-1978(88)90117-2.
- Wu C, Xu H, Héritier J, Andlauer W. 2012. Determination of catechins and flavonol glycosides in Chinese tea varieties. Food Chem. 132(1):144–149. doi: 10.1016/j.foodchem.2011.10.045.