ABSTRACT
The movement of mobile genetic elements (MGEs), including bacteriophages, insertion sequence (IS) elements, and integrative and conjugative elements (ICEs) can have profound effects on bacterial evolution by introducing novel genes, or disrupting the existing ones. Obligate endobacteria are a distinctive group of bacteria that reside within the intracellular compartments of their eukaryotic hosts. Many obligate endobacteria are reproductively dependent on their hosts. Vertical transmission, in addition to degenerative genome contraction and loss of MGEs, makes heritable endobacteria vulnerable to Muller's ratchet, a process that jeopardizes evolutionary longevity of small populations. Mycoplasma-related endobacteria (MRE) are ancient heritable endosymbionts of arbuscular mycorrhizal fungi. Their genomes harbour numerous MGEs. To explore the significance of MGEs in the evolution of MRE and other obligate endobacteria, we analyze the impact of transmission mode, recombination, and evolutionary age on the maintenance of MGEs. Furthermore, we discuss the ability of MGEs to act as sites of gene conversion and recombination in endobacterial genomes. We propose that MGEs are important instruments of genome shuffling, contributing to population heterogeneity and evolutionary longevity in heritable obligate endobacteria.
Introduction
Inter- and intra-cellular movement of genetic information is a widespread phenomenon among bacteria. These mobile genetic elements (MGEs) come in many forms, including bacteriophages, insertion sequence (IS) elements, and integrative and conjugative elements (ICEs). The movement of MGEs can have profound effects on bacterial evolution, by decreasing and/or increasing the bacteria's genomic potential. As MGEs are selfish entities, their replication imposes fitness costs on the host cell. Moreover, integration events into new sites of the host genome may disrupt important regulatory and metabolic functions. Conversely, MGE contribute to the acquisition of new genetic information through horizontal gene transfer, whereas MGE-mediated genomic rearrangements may generate novel phenotypes.
The abundance and activity of MGEs in bacteria can be affected by their ecological niche and lifestyle. Free-living bacteria, for instance, have potentially limitless exposures to different external genetic elements, with natural selection effectively shaping beneficial DNA transfers. In contrast, bacteria that are confined to their eukaryotic host's intracellular environment and have no extracellular state (obligate endobacteria) are unlikely to be exposed to novel genetic elements.Citation1 Finally, bacteria that are capable of living both in eukaryotic cells and in extracellular environments (facultative endobacteria) can encounter novel MGEs during their extracellular state. A recent analysis of MGEs in 384 bacterial genomes revealed that facultative endobacteria, such as Legionella, Salmonella, or Bartonella, carry the highest percentage of MGEs in their genomes, followed by free-living bacteria, with obligate endobacteria harbouring the least.Citation2 Remarkably, there were no correlations between the number of MGEs present and genome size, except for within obligate endobacteria.
The strong correlation between MGE density and genome size, which differentiates obligate endobacteria from bacteria of other lifestyles, is intriguing, as genome evolution in obligate endobacteria is driven by several distinct forces. The intracellular niche defining the overall lifestyle of obligate endobacteria is a source of selective pressures that contribute to the elimination of bacterial genes encoding costly metabolites available from the host. Transmission mode is another important factor that affects the evolution of obligate endobacteria. Most obligate endobacteria are heritable, i.e. their transmission from one host generation to the next is either exclusively vertical or mixed, combining vertical transmission with occasional horizontal transfers among host individuals of the same generation. These transmission patterns are influenced by the degree of co-evolution between the partners, the age of the association, and the effects of endobacteria on host fitness, with endobacteria ranging from mutualists, either essential or non-essential to the host survival, to antagonists. Vertical transmission of endobacteria reduces their effective population size, which exposes them to genome-wide accumulation of slightly deleterious mutations and degenerative genome contraction.Citation3-5 Without recombination, such small populations are also vulnerable to random and irreversible losses of the most fit genotypes, eventually leading to population extinction, a phenomenon referred to as Muller's ratchet.Citation6 Genomic comparisons among heritable obligate endobacteria differing in the degree of genome contraction and transmission mode indicate that these factors may interact with the MGE load.Citation2,4
We recently reported on a metagenomic study of the mycoplasma-related endobacteria (MRE) of arbuscular mycorrhizal fungi.Citation7 MRE are obligate endobacteria and possess numerous MGEs in their genomes. In this commentary, we expand our analysis of MGEs in MRE and compare them to other heritable obligate endobacteria. The ability of certain endobacteria to maintain high levels of MGEs, and the role of these elements in evolutionary longevity of endobacterial lineages is discussed.
Trajectories of the mobilome evolution in heritable obligate endobacteria
The trajectory of MGE loss during the process of degenerative genome contraction associated with exclusively vertical transmission can be inferred from comparisons between the minimal genomes of ancient endobacteria and the intermediately sized genomes of bacteria that have recently switched to the host-restricted environment.Citation4 MGEs are nearly entirely absent from the genomes of ancient maternally transmitted nutritional mutualists of insects, such as Buchnera aphidicola endosymbionts of aphids. With 422 to 641 kb genomes, B. aphidicola is one of the oldest arthropod-associated lineages of obligate endobacteria, dating back to 160-280 MYA.Citation3,8-11 In these highly reduced genomes, the absence of MGEs emerges as a factor responsible for their evolutionary stasis, illustrated by the lack of chromosomal rearrangements or novel gene acquisitions between B. aphidicola strains from 2 different aphid host species that diverged over 50 MYA.Citation8 In contrast, Wigglesworthia glossinidia, which originated as recently as ~40 MYA, supports a phage load of over 3% in the genome of 698 kb.Citation2,3,12 MGEs are also found in obligate endobacteria of cereal weevils Sitophilus oryzae, and S. zeamais, known as SOPE and SZPE, respectively, from their role as essential nutritional mutualists, or primary endosymbionts (PEs). Given their relatively recent origin at less than 25 MYA as well as genome sizes of 4.5 Mb in SOPE and 3.5 Mb in SZPE, these endobacteria are believed to represent early stages of genome contraction.Citation13-15 SZPE displays an unusually high IS element proliferation, with 10 times more IS copies per genome than any other known bacterium.Citation15 Analysis of multiple SZPE genomes revealed that the number of IS copies varies between populations, with a range of 1,000 to 10,000 IS256 copies and 10 to 100 IS903 copies per chromosome.Citation16 IS elements are also numerous in SOPE, occupying around 20% of the genome.Citation14,17 Collectively, these examples of MGE content across obligate endobacteria with exclusively vertical transmission but different genome sizes illustrate that progressive genome degeneration over evolutionary time is associated with elimination of MGEs.
Genomic comparisons between closely related obligate endobacteria that differ in the level of preservation of their recombination machinery and the mode of transmission can offer insights into the role of these 2 factors in genome evolution, including the mobilome size. For example, different strains of Serratia symbiotica that form distinct types of mutualisms with their aphid hosts, vary in genome composition and recombination ability. S. symbiotica SAp is a nonessential defensive mutualist, whereas S. symbiotica SCc and S. symbiotica SCt are essential nutritional mutualists. While the age of S. symbiotica SAp is estimated at ~90 MY,Citation18 the antiquity of S. symbiotica SCc and S. symbiotica SCt is unknown. The 2.8 Mb genome of S. symbiotica SAp and the 2.5 Mb genome of S. symbiotica SCt are capable of recombination and comprise 4 and 13.5% MGEs, respectively.Citation18,19 These elements are believed to be responsible for massive rearrangements setting the 2 genomes apart, in addition to the role in differential gene inactivation.Citation19 In contrast, the 1.8 Mb genome of S. symbiotica SCc does not show evidence of being able to support recombination and lacks MGEs. In turn, the impact of the transmission mode on MGE load can be illustrated by Wolbachia pipientis, an obligate intracellular endobacterium of nematodes and arthropods. In nematodes, Wolbachia behaves as an essential nutritional mutualist and its transmission appears to be exclusively vertical.Citation20 In arthropods, Wolbachia manipulates host reproduction in favor of its own transmission and thus acts as a parasite,Citation21 though it can also play roles of both a defensive and nutritional mutualist.Citation22 In addition to transovarian transmission, arthropod-associated Wolbachia transmits horizontally, and multiple infections of a single host are not uncommon.Citation23 The time of divergence between the nematode- and arthropod-associated Wolbachia lineages is estimated at 50-55 MYA.Citation24 The 1.1 Mb genome of nematode-associated Wolbachia wBm contains 2.4% of IS elements that appear to be completely non-functional due to genome erosion.Citation25,26 Interestingly, recombinatorial gene conversions occur at the homologous IS elements, continually restoring the degrading IS copies in the genome.Citation26 This pattern represents a stark contrast with the arthropod-associated Wolbachia in which MGEs take up a greater proportion of the genome and are functionally active.Citation27,28 For example, 10% of the 1.3 Mb genome of Wolbachia wMel are MGEs.Citation2,27 Even more strikingly, MGEs constitute 21% of the 1.5 Mb Wolbachia wPip genome.Citation2,29 Analysis of the IS element ISWpi1 across 40 arthropod-associated Wolbachia strains, showed that this element is widespread, active and transfers among these endobacteria not only within strains but also between them.Citation30 Another study of the arthropod-associated Wolbachia IS element, ISRpe1, found that this element was also transferring to other obligate endobacteria, Cardinium, and Rickettsia, found in the same insect host as Wolbachia.Citation31 These examples suggest that recombination and horizontal transmission contribute to preservation of MGE activity in the contracting genomes of heritable obligate endobacteria.
While the absence of recombination and horizontal transmission is associated with the loss of MGEs, heritable obligate endobacteria with reduced genomes that retain these 2 processes display a wide range of mobilome sizes. One of the largest mobilomes is found in Orientia tsutsugamushi. O. tsutsugamushi diverged from the Rickettsia lineage ~200 MYA;Citation32 Rickettsia are also obligate endobacteria with mixed transmission and sizeable mobilomes.Citation33 O. tsutsugamushi is the causative agent of scrub typhus. It is transmitted maternally by trombiculid mites and, to a lesser degree, horizontally through vertebrates. Nearly 47% of the O. tsutsugamushi 2.0 Mb genome is occupied by repetitive sequences derived from 10 types of transposable elements and the ICE OtAGE (O. tsutsugamushi amplified genetic element).Citation34 OtAGE is extensively amplified throughout the genome, with copies showing evidence of erosion through gene loss and genome reduction expected of heritable endobacteria. OtAGE copies are thus likely no longer mobile on their own, but transmission via a shuttle is still highly possible. The O. tsutsugamushi genome exhibits poor colinearity with other Rickettsia genomes, which is related to the amplification and movement of the MGEs.Citation34 Other notable examples of substantial mobilomes come from 2 sister species Ca. Hamiltonella defensa and Ca. Regiella insecticola, nonessential defensive mutualists of aphids. While the exact age of these 2 lineages is unknown, they may be as old as the aphid-associated lineage of Buchnera aphidicola.Citation11 The genomes of Ca. Hamiltonella defensa and Ca. Regiella insecticola are 2.1 and 2.0 Mb in size, with MGEs making up 34 and 20% of all coding sequences, respectively.Citation35,36
Collectively, the pattern of MGE distribution across arthropod-associated obligate endobacteria with mixed transmission and reduced genomes suggests that recombination machinery and horizontal transmission are key determinants of an active mobilome. Together, these 3 factors could be expected to promote evolutionary longevity in bacteria that are vulnerable to extinction because of their reproductive dependence the host. However, the lineages with and without mobilomes appear to have existed equally long within the time frame of arthropod evolution. Consequently, while obligate endobacteria with active mobilomes may be more adaptable, the overall evolutionary longevity of endobacterial lineages associated with arthropods does not seem to be mobilome-dependent.
Mobile genetic elements of mycoplasma-related endobacteria
MRE are heritable obligate endobacteria that reside in the cytoplasm of fungi representing all major lineages of Glomeromycota (arbuscular mycorrhizal fungi, AMF) and the Endogone lineage of Mucoromycotina.Citation37,38 MRE association with fungi is ancient, hypothesized to have occurred during the early Palaeozoic, over 400 MYA.Citation39 While laboratory observations indicate that MRE are heritable, phylogenetic analyses suggest that they may also transmit horizontally.Citation39 The role of MRE in the biology of their fungal hosts is unknown. MRE are members of Mollicutes, and phylogenetic reconstructions place them in the family Mycoplasmataceae.Citation7,38,40 Interestingly, mycoplasmas (and all other Mollicutes) are characterized by minimal genomes, with highly reduced functional capabilities, despite the majority of their members existing as horizontally transmitted extracellular bacteria. Because of rapid accumulation of mutations, the small genomes of Mycoplasma species were believed to be products of degenerative evolution, not unlike those of essential mutualists of insects with exclusively vertical transmission.Citation41 However, recent comparative analyses have indicated that the Mycoplasma genomes are highly plastic, with numerous functional MGEs, such as IS elements, phages, and ICEs.Citation41-43 For instance, IS elements were not only found to be abundant in many Mycoplasma genomes, but also transferable between species.Citation44 The most important elements for Mycoplasma genetic mobility, however, seem to be ICEs, which encode the machinery for their excision, conjugative transfer, and eventual integration into the recipient chromosome.Citation43 ICEs have been identified in various mycoplasmas, and are also believed to transfer between species.Citation45-47 Horizontal transfer of MGEs seems to occur in Mycoplasma species when they infect and share the same host.Citation48
A metagenomic study of 3 populations of MRE associated with the AMF species Claroideoglomus etunicatum (MRE-CE), Racocetra verrucosa (MRE-RV), and Rhizophagus clarus (MRE-RC) revealed that MRE reduced genomes are highly plastic and undergo frequent recombination.Citation7 Genomes from all 3 populations harbour IS elements, which are often located at sites marking alterations in gene synteny. In addition, MRE-RV and MRE-CE contain remnants of a past plectroviral invasion, a phage known to infect Spiroplasma species.Citation49
To determine the role of MGEs in MRE biology, we performed an extensive search of the 3 MRE metagenomes for additional MGEs, and included the MRE associated with a fourth AMF species, Dentiscutata heterogama (MRE-DH) in our analysis.Citation40 We found that, in addition to the previously identified IS elements and phage,Citation7 all MRE harbour key components of ICEs in high copy numbers, with MGEs taking up slightly over 5% of the MRE genomes ().
Table 1. Mobile genetic elements in 4 populations of MRE. Numbers in parenthesis indicate percentage of total proteins of the individual MRE population metagenomes. Homologous proteins were identified based on BLASTp searches of the National Center for Biotechnology Information databases, with ICE proteins requiring their genetic loci to be in close proximity to each other.
The self-transmissible ICE follows a standard “life cycle,” including integration, excision, conjugation, and regulation.Citation50 For integration, the ICE encodes an integrase, with the majority of studied ICE integrases classified in the tyrosine recombinase family.Citation50 MRE genomes are full of integrases, specifically those of the XerCD integrase/recombinase family, which are members of the tyrosine recombinase family (). In addition to mediating integration events, the integrase is responsible for the excision of the ICE, and is aided in this process by small DNA-binding proteins, known as recombination directionality factors (RDFs).Citation50 MRE commonly encode homologues of the histone-like DNA-binding HU protein, which are located near the Xer integrase proteins and may act as RDFs (). In addition, to prevent the loss of excised ICE DNA, especially during cell division, the ICE encodes plasmid partitioning or maintenance proteins.Citation50 MRE carry multiple plasmid maintenance protein-encoding genes distributed throughout the genome and near the Xer integrases (). The presence of these proteins would allow for the proper maintenance of the ICE during the excision process. The genes involved in the conjugative transfer and regulation of ICE include DNA processing and secretion systems similar to type IV secretion systems as well as repressors, such as SetR.Citation50 However, most Gram-positive ICE secretion systems have no similarity to type IV secretion systems. Moreover, the genes in many ICEs of mycoplasmas are hypothetical and not well understood. In MRE, the genes that may be involved in ICE conjugative transfer or regulation are yet unknown. As more than half of the genes annotated in MRE are orphan, it is possible that some of these orphan genes are involved in these processes.Citation7 It is also possible that MRE ICEs are no longer capable of conjugation to other cells, but simply transfer within the same chromosome, not unlike the IS elements.
Of note, a number of mycoplasma species were reported to encode Xer recombinases that govern the high-frequency shuffling of surface protein gene families, resulting in phase variation.Citation51 In these same species, ICE-encoded DDE transposases are responsible for ICE-horizontal dissemination.Citation45,46 Interestingly, MRE genomes do not contain any homologues of these DDE transposases. Furthermore, the Xer recombinases, which are likely responsible for MRE ICE excision and integration, are more closely related to Xer recombinases of distant relatives, such as Acholeplasma, or highly divergent representatives of Firmicutes, Spirochaetes, and Bacteroidetes, rather than to those of other mycoplasmas. Thus, the ICE elements found in MRE may have been acquired after the divergence of MRE from their Mycoplasma relatives.
Mobile genetic elements as barriers against progression of Muller's ratchet and extinction
The presence of MGEs in MRE raises the question of their significance for evolutionary longevity of these ancient organisms. It is possible that, like in other obligate endobacteria with mixed transmission, MGEs may contribute to the acquisition of novel genetic information by MRE. In some AMF, MRE co-exist with the nonessential mutualist Ca. Glomeribacter gigasporarum,Citation39,52 which, together with MRE, is one of the oldest obligate endobacteria with mixed transmission.Citation53 The AMF-Ca. Glomeribacter gigasporarum association dates back to over 400 MYA.Citation53 Like MRE, Ca. Glomeribacter gigasporarum harbours MGEs, which constitute about 15% of its 1.7 Mb genome.Citation54 Clearly, further work is needed to address whether these 2 groups of endobacteria engage in gene exchanges.
Another potential contribution of MGEs to evolutionary longevity of MRE is related to their role in genome plasticity and population diversity. The presence of MGEs and their movement and amplification across a single chromosome is expected to create multiple regions of repeated loci, which can be used as sites of recombination (). Furthermore, a multitude of MGEs in the MRE genome would allow for homologous recombination between regions of DNA that have no identity except in the elements themselves, leading to changes in gene synteny. In other bacteria, incidences of recombination involving ICEs were observed both in nature and in the laboratory.Citation55,56 Similarly, Wolbachia wBm uses its non-functional IS elements as sites for gene conversion.Citation26 In MRE, the recombination sites generated by amplified MGEs provide a potentially favorable environment for intra-population horizontal gene transfer and subsequent recombination. The passive release of DNA from dead MRE cells in a single host is likely the source of most DNA for horizontal transfer; not only is there a large amount of DNA present in the host cytoplasmic compartment, the DNA encountered by live MRE cells will always be that of the same population, thus regions of homology provided by MGEs will be present for recombination. Other barriers such as lethality of DNA, or functional compatibility will not exist. The reduced genomes of MRE may also lack the genes that limit homologous recombination. For instance, naturally occurring mutants of DNA repair genes have been shown to have a relaxed recombination potential, allowing for uptake of highly divergent DNA.Citation57 Similarly, the absence of DNA repair genes from the MRE genomes may allow for more permissive recombination. The sites of homology provided by MGEs may also pave way for homology-facilitated illegitimate recombination (HFIR), where recombination is initiated in a single region of homology, and the strand exchange extends into regions with no homology, which can be greater than 1 kb.Citation57 Whether the mechanism is simple homologous recombination or HFIR, MGEs provide regions of DNA that can be exploited to maintain genome plasticity and population diversity, allowing the eroding MRE genomes to escape Muller's ratchet.
Figure 1. Endobacterial MGEs as sources of genetic heterogeneity and population diversity. (A) In endobacterial populations lacking recombination machinery and MGEs, e.g. Buchnera aphidicola, genomic homogeneity is maintained, while Muller's ratchet continually progresses. (B) In endobacterial populations that have retained recombination machinery and MGEs, e.g., MREs and Wolbachia wBm, MGE sequences can be used as sites for gene conversion and homologous recombination, in addition to their traditional role in transposition. These processes create, through genome shuffling, a genetically heterogeneous population (without the introduction of novel genes) and ensure population longevity by decelerating Muller's ratchet.
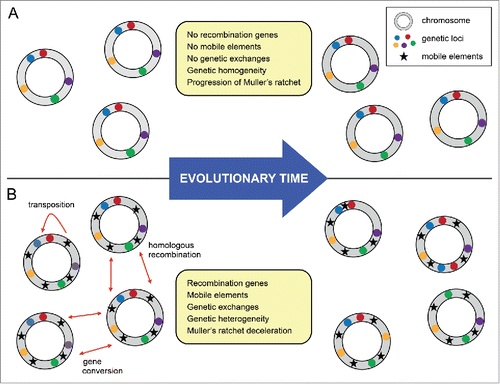
Like other obligate endobacteria with mixed transmission, MRE appear to harbour an active mobilome. The evolutionary antiquity of MRE, coupled with their present day worldwide distribution, suggests that this is a highly successful lineage. Moreover, MRE predate arthropod-associated obligate endobacteria that lost their mobilomes to genomic degeneration. Based on these patterns, we propose that MGEs may be the key to the evolutionary and ecological success of MRE and other heritable obligate endobacteria.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Science Foundation grant IOS-1261004.
References
- Bordenstein SR, Reznikoff WS. Mobile DNA in obligate intracellular bacteria. Nat Rev Microbiol 2005; 3:688-99; PMID:16138097; http://dx.doi.org/10.1038/nrmicro1233
- Newton IL, Bordenstein SR. Correlations between bacterial ecology and mobile DNA. Curr Microbiol 2011; 62:198-208; PMID:20577742; http://dx.doi.org/10.1007/s00284-010-9693-3
- Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 2008; 42:165-90; PMID:18983256; http://dx.doi.org/10.1146/annurev.genet.41.110306.130119
- McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol 2012; 10:13-26; PMID:22064560; http://dx.doi.org/10.1038/nrmicro2670
- Moran NA, Bennett GM. The tiniest tiny genomes. Annu Rev Microbiol 2014; 68:195-215; PMID:24995872; http://dx.doi.org/10.1146/annurev-micro-091213-112901
- Muller HJ. The relation of recombination to mutational advance. Mutat Res 1964; 1:2-9; http://dx.doi.org/10.1016/0027-5107(64)90047-8
- Naito M, Morton JB, Pawlowska TE. Minimal genomes of mycoplasma-related endobacteria are plastic and contain host-derived genes for sustained life within Glomeromycota. P Natl Acad Sci USA 2015; 112:7791-6; http://dx.doi.org/10.1073/pnas.1501676112
- Tamas I, Klasson L, Canback B, Naslund AK, Eriksson AS, Wernegreen JJ, Sandstrom JP, Moran NA, Andersson SGE. 50 million years of genomic stasis in endosymbiotic bacteria. Science 2002; 296:2376-9; PMID:12089438; http://dx.doi.org/10.1126/science.1071278
- Perez-Brocal V, Gil R, Ramos S, Lamelas A, Postigo M, Michelena JM, Silva FJ, Moya A, Latorre A. A small microbial genome: The end of a long symbiotic relationship? Science 2006; 314:312-3; PMID:17038625; http://dx.doi.org/10.1126/science.1130441
- Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 2000; 407:81-6; PMID:10993077; http://dx.doi.org/10.1038/35024074
- Moran NA, Munson MA, Baumann P, Ishikawa H. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. P Roy Soc Lond B Bio 1993; 253:167-71; http://dx.doi.org/10.1098/rspb.1993.0098
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet 2002; 32:402-7; PMID:12219091; http://dx.doi.org/10.1038/ng986
- Lefevre C, Charles H, Vallier A, Delobel B, Farrell B, Heddi A. Endosymbiont phylogenesis in the Dryophthoridae weevils: Evidence for bacterial replacement. Mol Biol Evol 2004; 21:965-73; PMID:14739242; http://dx.doi.org/10.1093/molbev/msh063
- Oakeson KF, Gil R, Clayton AL, Dunn DM, von Niederhausern AC, Hamil C, Aoyagi A, Duval B, Baca A, Silva FJ, et al. Genome degeneration and adaptation in a nascent stage of symbiosis. Genome Biol Evol 2014; 6:76-93; PMID:24407854; http://dx.doi.org/10.1093/gbe/evt210
- Plague GR, Dunbar HE, Tran PL, Moran NA. Extensive proliferation of transposable elements in heritable bacterial symbionts. J Bacteriol 2008; 190:777-9; PMID:17981967; http://dx.doi.org/10.1128/JB.01082-07
- Dougherty KM, Plague GR. Transposable element loads in a bacterial symbiont of weevils are extremely variable. Appl Environ Microbiol 2008; 74:7832-4; PMID:18952872; http://dx.doi.org/10.1128/AEM.01049-08
- Gil R, Belda E, Gosalbes MJ, Delaye L, Vallier A, Vincent-Monegat C, Heddi A, Silva FJ, Moya A, Latorre A. Massive presence of insertion sequences in the genome of SOPE, the primary endosymbiont of the rice weevil Sitophilus oryzae. Int Microbiol 2008; 11:41-8; PMID:18683631
- Burke GR, Moran NA. Massive genomic decay in Serratia symbiotica, a recently evolved symbiont of aphids. Genome Biol Evol 2011; 3:195-208; PMID:21266540; http://dx.doi.org/10.1093/gbe/evr002
- Manzano-Marín A, Latorre A. Settling down: The genome of Serratia symbiotica from the aphid Cinara tujafilina zooms in on the process of accommodation to a cooperative intracellular life. Genome Biol Evol 2014; 6:1683-98; http://dx.doi.org/10.1093/gbe/evu133
- Fenn K, Blaxter M. Are filarial nematode Wolbachia obligate mutualist symbionts? Trends Ecol Evol 2004; 19:163-6; PMID:16701248; http://dx.doi.org/10.1016/j.tree.2004.01.002
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 2008; 6:741-51; PMID:18794912; http://dx.doi.org/10.1038/nrmicro1969
- Zug R, Hammerstein P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Bio Rev 2015; 90:89-111; http://dx.doi.org/10.1111/brv.12098
- Vavre F, Fleury F, Lepetit D, Fouillet P, Boulétreau M. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol Biol Evol 1999; 16:1711-23; PMID:10605113; http://dx.doi.org/10.1093/oxfordjournals.molbev.a026084
- Clark MA, Moran NA, Baumann P. Sequence evolution in bacterial endosymbionts having extreme base compositions. Mol Biol Evol 1999; 16:1586-98; PMID:10555290; http://dx.doi.org/10.1093/oxfordjournals.molbev.a026071
- Foster J, Ganatra M, Kamal I, Ware J, Makarova K, Ivanova N, Bhattacharyya A, Kapatral V, Kumar S, Posfai J, et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biology 2005; 3:599-614; http://dx.doi.org/10.1371/journal.pbio.0030121
- Cordaux R. Gene conversion maintains nonfunctional transposable elements in an obligate mutualistic endosymbiont. Mol Biol Evol 2009; 26:1679-82; PMID:19414524; http://dx.doi.org/10.1093/molbev/msp093
- Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N, et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Biology 2004; 2:327-41; http://dx.doi.org/10.1371/journal.pbio.0020327
- Leclercq S, Giraud I, Cordaux R. Remarkable abundance and evolution of mobile group II introns in Wolbachia bacterial endosymbionts. Mol Biol Evol 2011; 28:685-97; PMID:20819906; http://dx.doi.org/10.1093/molbev/msq238
- Klasson L, Walker T, Sebaihia M, Sanders MJ, Quail MA, Lord A, Sanders S, Earl J, O'Neill SL, Thomson N, et al. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol Biol Evol 2008; 25:1877-87; PMID:18550617; http://dx.doi.org/10.1093/molbev/msn133
- Cordaux R, Pichon S, Ling A, Perez P, Delaunay C, Vavre F, Bouchon D, Greve P. Intense transpositional activity of insertion sequences in an ancient obligate endosymbiont. Mol Biol Evol 2008; 25:1889-96; PMID:18562339; http://dx.doi.org/10.1093/molbev/msn134
- Duron O. Lateral transfers of insertion sequences between Wolbachia, Cardinium and Rickettsia bacterial endosymbionts. Heredity 2013; 111:330-7; PMID:23759724; http://dx.doi.org/10.1038/hdy.2013.56
- Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM. Evolution and diversity of Rickettsia bacteria. BMC Biol 2009; 7:6; PMID:19187530; http://dx.doi.org/10.1186/1741-7007-7-6
- Merhej V, Raoult D. Rickettsial evolution in the light of comparative genomics. Biol Rev Camb Philos Soc 2011; 86:379-405; PMID:20716256; http://dx.doi.org/10.1111/j.1469-185X.2010.00151.x
- Nakayama K, Yamashita A, Kurokawa K, Morimoto T, Ogawa M, Fukuhara M, Urakami H, Ohnishi M, Uchiyama I, Ogura Y, et al. The whole-genome sequencing of the obligate intracellular bacterium Orientia tsutsugamushi revealed massive gene amplification during reductive genome evolution. DNA Res 2008; 15:185-99; PMID:18508905; http://dx.doi.org/10.1093/dnares/dsn011
- Degnan PH, Yu Y, Sisneros N, Wing RA, Moran NA. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. P Natl Acad Sci USA 2009; 106:9063-8; http://dx.doi.org/10.1073/pnas.0900194106
- Degnan PH, Leonardo TE, Cass BN, Hurwitz B, Stern D, Gibbs RA, Richards S, Moran NA. Dynamics of genome evolution in facultative symbionts of aphids. Environ Microbiol 2010; 12:2060-9; PMID:21966902
- Desir∫ A, Faccio A, Kaech A, Bidartondo MI, Bonfante P. Endogone, one of the oldest plant-associated fungi, host unique Mollicutes-related endobacteria. New Phytol 2014; 205:1464-72; http://dx.doi.org/10.1111/nph.13136
- Naumann M, Schüßler A, Bonfante P. The obligate endobacteria of arbuscular mycorrhizal fungi are ancient heritable components related to the Mollicutes. ISME J 2010; 4:862-71; PMID:20237515; http://dx.doi.org/10.1038/ismej.2010.21
- Toomer KH, Chen X, Naito M, Mondo SJ, den Bakker HC, VanKuren NW, Lekberg Y, Morton JB, Pawlowska TE. Molecular evolution patterns reveal life history features of mycoplasma-related endobacteria associated with arbuscular mycorrhizal fungi. Mol Ecol 2015; 24:3485-500; PMID:26011293; http://dx.doi.org/10.1111/mec.13250
- Torres-Cortés G, Ghignone S, Bonfante P, Schüßler A. Mosaic genome of endobacteria in arbuscular mycorrhizal fungi: Transkingdom gene transfer in an ancient mycoplasma-fungus association. P Natl Acad Sci USA 2015; 112:7785-90; http://dx.doi.org/10.1073/pnas.1501540112
- Sirand-Pugnet P, Citti C, Barre A, Blanchard A. Evolution of mollicutes: down a bumpy road with twists and turns. Res Microbiol 2007; 158:754-66; PMID:18023150; http://dx.doi.org/10.1016/j.resmic.2007.09.007
- Nouvel LX, Sirand-Pugnet P, Marenda MS, Sagne E, Barbe V, Mangenot S, Schenowitz C, Jacob D, Barre A, Claverol S, et al. Comparative genomic and proteomic analyses of two Mycoplasma agalactiae strains: clues to the macro- and micro-events that are shaping mycoplasma diversity. BMC Genomics 2010; 11:86; PMID:20122262; http://dx.doi.org/10.1186/1471-2164-11-86
- Dordet-Frisoni E, Sagne E, Baranowski E, Breton M, Nouvel LX, Blanchard A, Marenda MS, Tardy F, Sirand-Pugnet P, Citti C. Chromosomal transfers in mycoplasmas: when minimal genomes go mobile. mBio 2014; 5:e01958; PMID:25425234; http://dx.doi.org/10.1128/mBio.01958-14
- Thomas A, Linden A, Mainil J, Bischof DF, Frey J, Vilei EM. Mycoplasma bovis shares insertion sequences with Mycoplasma agalactiae and Mycoplasma mycoides subsp. mycoides SC: Evolutionary and developmental aspects. FEMS Microbiol Lett 2005; 245:249-55; PMID:15837379; http://dx.doi.org/10.1016/j.femsle.2005.03.013
- Tardy F, Mick V, Dordet-Frisoni E, Marenda MS, Sirand-Pugnet P, Blanchard A, Citti C. Integrative conjugative elements are widespread in field isolates of Mycoplasma species pathogenic for ruminants. Appl Environ Microb 2015; 81:1634-43; http://dx.doi.org/10.1128/AEM.03723-14
- Dordet Frisoni E, Marenda MS, Sagne E, Nouvel LX, Guerillot R, Glaser P, Blanchard A, Tardy F, Sirand-Pugnet P, Baranowski E, et al. ICEA of Mycoplasma agalactiae: a new family of self-transmissible integrative elements that confers conjugative properties to the recipient strain. Mol Microbiol 2013; 89:1226-39; PMID:23888872; http://dx.doi.org/10.1111/mmi.12341
- Calcutt MJ, Lewis MS, Wise KS. Molecular genetic analysis of ICEF, an integrative conjugal element that is present as a repetitive sequence in the chromosome of Mycoplasma fermentans PG18. J Bacteriol 2002; 184:6929-41; PMID:12446643; http://dx.doi.org/10.1128/JB.184.24.6929-6941.2002
- Sirand-Pugnet P, Lartigue C, Marenda M, Jacob D, Barre A, Barbe V, Schenowitz C, Mangenot S, Couloux A, Segurens B, et al. Being pathogenic, plastic, and sexual while living with a nearly minimal bacterial genome. PLoS Genet 2007; 3:744-58; http://dx.doi.org/10.1371/journal.pgen.0030075
- Carle P, Saillard C, Carrere N, Carrere S, Duret S, Eveillard S, Gaurivaud P, Gourgues G, Gouzy J, Salar P, et al. Partial chromosome sequence of Spiroplasma citri reveals extensive viral invasion and important gene decay. Appl Environ Microbiol 2010; 76:3420-6; PMID:20363791; http://dx.doi.org/10.1128/AEM.02954-09
- Wozniak RAF, Waldor MK. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat Rev Microbiol 2010; 8:552-63; PMID:20601965; http://dx.doi.org/10.1038/nrmicro2382
- Citti C, Nouvel LX, Baranowski E. Phase and antigenic variation in mycoplasmas. Future Microbiol 2010; 5:1073-85; PMID:20632806; http://dx.doi.org/10.2217/fmb.10.71
- Desiró A, Salvioli A, Ngonkeu EL, Mondo SJ, Epis S, Faccio A, Kaech A, Pawlowska TE, Bonfante P. Detection of a novel intracellular microbiome hosted in arbuscular mycorrhizal fungi. ISME J 2014; 8:257-70; http://dx.doi.org/10.1038/ismej.2013.151
- Mondo SJ, Toomer KH, Morton JB, Lekberg Y, Pawlowska TE. Evolutionary stability in a 400-million-year-old heritable facultative mutualism. Evolution 2012; 66:2564-76; PMID:22834753; http://dx.doi.org/10.1111/j.1558-5646.2012.01611.x
- Ghignone S, Salvioli A, Anca I, Lumini E, Ortu G, Petiti L, Cruveiller S, Bianciotto V, Piffanelli P, Lanfranco L, et al. The genome of the obligate endobacterium of an AM fungus reveals an interphylum network of nutritional interactions. ISME J 2012; 6:136-45; PMID:21866182; http://dx.doi.org/10.1038/ismej.2011.110
- Garriss G, Waldor MK, Burrus V. Mobile antibiotic resistance encoding elements promote their own diversity. PLoS Genet 2009; 5:e1000775; PMID:20019796; http://dx.doi.org/10.1371/journal.pgen.1000775
- Marini E, Palmieri C, Magi G, Facinelli B. Recombination between Streptococcus suis ICESsu32457 and Streptococcus agalactiae ICESa2603 yields a hybrid ICE transferable to Streptococcus pyogenes. Veterinary microbiology 2015; 178:99-104; PMID:25935120; http://dx.doi.org/10.1016/j.vetmic.2015.04.013
- Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol 2005; 3:711-21; PMID:16138099; http://dx.doi.org/10.1038/nrmicro1234
