ABSTRACT
Acytota is a kingdom of life covering satellites, plasmids, transposable elements, viroids and viruses, all outside the conventional tree of life but satisfying most life definitions. This review focuses on some aspects of Acytota, their “genomes” and life styles, the dominance of transposable elements and their evolutionary influence on other life forms in order to vindicate the Acytota as a life kingdom no more polyphyletic than other kingdoms and its members no more parasitic than other life forms.
Introduction
We recently revived the Acytota as a kingdom of life covering genetic entities such as satellites, plasmids, transposable elements, viroids and viruses, all outside the traditional tree of life but qualifying nonetheless as life according to very basic definitions.Citation50 The “genomes” of these entities range from simple sequence elements to sizes comparable to the genomes of prokaryotes. In our view, Acytota predate the origin of the cell and interconnect all branches of the tree of life, inseparable from other life forms and indispensable for them. The persisting debate about whether viruses are alive or notCitation15,38 still reverberates but recent discoveries of e.g. giant viruses infected by virophages and extensive horizontal gene transfer have established a suitable milieu for finally embracing both viruses and other replicating genetic entities into a newly built tree of life.
Here, we focus on the plethora of Acytota forms dominated by a large variety of transposable elements and on the great variety of life styles involving e.g., numerous modes of DNA and RNA migrations.
Acytota genomes – from RNA to DNA and greater complexity
Some members of Acytota have retained the ancient RNA genomes (certain viruses, viroids and the RNA phase of retrotransposons) but others (satellites, plasmids and DNA viruses) have dsDNA and enzymes for DNA replication.Citation14
The complexity of Acytota “genomes” has increased during evolution, reaching the stage of giant viruses which are comparable to bacteria not only in size but also in the length of their genomes. The close link of Acytota to other life forms is characterized by the gene flow both from Acytota members (e.g. phages) to cellular domainsCitation32 and, vice versa, for example from cellular genomes to mimiviruses that then encode the proteins involved in protein synthesis (genes acquired from host species – amoeba). This gene exchange blurs the distinction between viral and cellular worlds. Large Polintons, exemplified by Maverick transposons, encode, in addition to retroviral-like integrase, proteins with homology to replication and packaging proteins of some bacteriophages and diverse eukaryotic double-stranded DNA viruses.Citation39, 21
The inter-connections and some degree of hierarchy in Acytota are exemplified by the infection of giant virus by virophages (satellite viruses) common in plants and animals.Citation47 Virophages infect and hijack the viral factory of giant viruses in order to replicate, encapsidate and increase their own yield at the expense of the declining yield of host giant virus.Citation28 For example, Mimivirus (>1 Mb), infecting protist Acanthamoeba, is itself infected by the virophage “Sputnik” (after the first man-made satellite) - 18kb genome with just 21 genes, that is, virophages can perform horizontal gene transfer between viruses like bacteriophages do between bacteria.Citation38 This phenomenon is probably widespread because mimiviruses constitute a diverse, quantitatively important and ubiquitous component of the population of large eukaryotic DNA marine viruses.Citation34
The dependence of virophages on giant viruses resembles non-autonomous TEs that utilize the replication machinery of autonomous TEs (e.g., non-autonomous Stowaway MITE and autonomous mariner-like among DNA transposons, or SINEs and LINEs among non-LTR retrotransposons and many others). These were naturally labeled “parasites of parasites”.Citation41 The intracellular environment represents an ecosystem where transposon-encoded proteins (both structural gag and enzymes) are shared by autonomous and non-autonomous elements. Some of these have similar and others, different mechanisms of amplification. Another example of inter-connections between members of Acytota is primitive TEs - insertion sequences, e.g. IS26, that can be inserted into plasmidsCitation18 or amplification of satellites within TEs.Citation31,23 Structural and functional liaisons between TEs and satellite DNAs were described recently.Citation33
Life styles of Acytota
In contrast to higher cellular organisms, Cytota, all of which appear as cells or multicellular organisms, representatives of the acellular kingdom often do not have a distinct “image” (with the exception of viruses) and can be viewed more like molecules. Their life cycles, however, are sometimes intricate, as exemplified by the above inter-connection episodes.
Acytota possess a large range of amplification modes. LTR retrotransposons migrate between the nucleus where they are transcribed and cytoplasm where a new DNA copy is formed by reverse transcription. In non-LTR retrotransposons, transcripts migrate into cytoplasm where they serve as templates for translation but new DNA copies of the elements are synthetized in the nucleus by target-primed reverse transcription (TPRT). For propagation, DNA transposons use either a “cut and paste” mechanism or duplications within the genome. Helitrons are amplified extrachromosomally by a rolling-circle mechanism though the details of this process are unknown.Citation22
The transposition of Polintons, self-synthesising DNA transposons, uses a completely different mechanism, hitherto unseen in transposons.Citation21 Some features of self-synthesising eukaryotic DNA transposons are shared by the newly identified DNA transposons, denoted Casposons that have been found in bacteria and archea.Citation26 Casposons encode Cas1 endonuclease, a key enzyme of the CRISPR-Cas adaptive immunity system of archea and eubacteria. This enzyme catalyzes integration and excision via mechanisms similar to the integration of new spacers into CRISPR loci.
The spectrum of mechanisms used by replicating and propagating Acytota is broadened by non-equal crossover or DNA slippage characteristic for satellite DNA as well as by the large variability in modes utilized by DNA and RNA viruses. Satellites also probably include formation of extra-chromosomal DNA (eccDNA) as has been demonstrated in several species.Citation6,7,5 The formation of extrachromosomal DNA circles appears to be an efficient way of amplifying repeats into high copy number and reinserting them back into homologous regions of the genome (). Another important recombination-based process is a gene conversion that homogenizes repeats (TEs, satellites, rDNA) and leads to their concerted evolution.Citation29,24
Figure 1. DNA (red) and RNA (blue) traffic inside cell and between cells during processes of endosymbiotic gene transfer (green background), retrotransposition of LTR retrotransposons and retroviruses (violet background), extrachromosomal amplification of satellite DNA and rDNA (blue background), silencing of TE by RNA interference (yellow background), transfer of plasmid DNA, infection of cell by viroid RNA and transformation of cell by Agrobacterium tumefaciens. All processes can take place in one cell, 2 cells are shown here to better visualize migration of nucleic acids between cells.
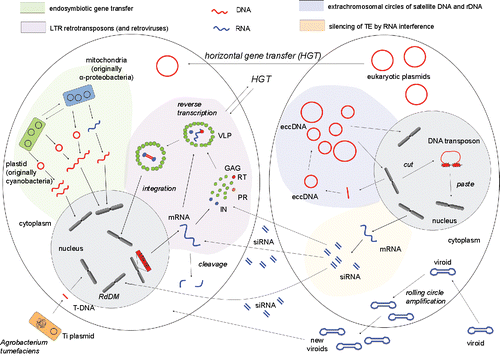
Looking at the modes of amplification, we can see that some members of Acytota change nuclear and cytoplasmic phases (genomic copies of TEs and satellites versus their cytoplasmatic intermediates) while others are confined to extra-nuclear stages (DNA plasmids). Viruses and viroids alternate between nuclear, cytoplasmatic and extra-cellular phases. “Template switching” between 2 co-packaged genomic RNAs of retroviruses during reverse transcription, represents the most primitive form of genetic recombination.Citation37
The migration of various DNA or RNA Acytota genomes (retrotransposons, viruses, viroids, plasmids) between cellular compartments and between cells () together with migration of small RNA also between cells,Citation45 and migration of released organellar DNA fragments from cytoplasm to nucleus are processes that all elevate genome dynamics. The cytoplasm is a cellular compartment with more space and fewer constraints where such evolutionary experiments can take place, irrespective of DNA- or RNA-based amplification. Nucleic acid traffic can trigger other events that shape the genomic architecture, e.g., subsequent reshuffling, deletions or gene conversions and this might contribute to the biological diversity of cells and species.
Transposable elements can migrate outside the cells in body fluids. “Naked” RNA and DNA molecules can circulate in blood serum, saliva, lymph and milk.Citation2 Although the mechanism of RNA/DNA release into circulation is unknown, the greater proportion of DNA homologous to TEs in blood serum, compared to its genome proportion, indicates greater resistance of repetitive DNA (e.g. human Alu elements) to degradation.Citation46
Influence of Acytota on other life forms and the dominance of transposable elements
Since Acytota, presumably predated the origin of the cell, they are inextricably embedded in the tree of life with widespread effect on other life forms, although their contribution may alter over time. Acytota “hegemons” probably also changed during the course of evolution: (local) expansions of satellites may be important in the early stages of genome evolution when genomes lacked complexity, while amplifications of TE could contribute rather later when genes appeared in genomes, had longer-distance genomic effects and could provide substrates for recombination. Retrotransposons, that retained the ancient RNA phase in the life-cycle, probably constituted the first genomes, along with satellites. The first genes were formed later.
Like in Cytota, interrelationships between species include (i) autonomous existence (though in the environment of the rest of the Biota), (ii) semi-autonomous (food chain dependence), (iii) pathological coexistence, (iv) symbiosis and (v) obligatory symbiosis. The autonomous existence of Acytota perhaps characterized the earliest stages of life. Most of the Acytota, in particular, viruses and phages and, especially, plasmids and TEs, are involved in the vibrant horizontal gene transfer (HGT) between Acytota and Cytota and within them. This traffic of genome parts can be considered a form of symbiosis (plasmids with bacterial genomes) and obligatory symbiosis (domesticated TEs with cellular genomes).
Of all Acytota, invasive by nature, the TEs are, perhaps, least damaging to their hosts following invasion. Many TEs reside in the host genomes temporarily or permanently, often without any visible phenotypic effects - seemingly neutral passengers of the genomes. However, numerous cases have been documented of transposition events resulting in advantageous changes in host genome function which are apparently picked up by selection pressure. This evolutionarily refreshing role makes TEs a major vehicle of evolution, introducing changes much faster than point mutations. Moreover, their promiscuous life style allows them to by-pass the limitations of strictly vertical genetic transmission, and permits all kingdoms to a certain degree to share their accumulated genomic memory and evolutionary achievements - by HGT of new functionalities by TEs. Rapidly accumulating knowledge of the HGT already justifies the claim that TEs and other mobile elements are major contributors to the evolution of all Biota, including Acytota themselves.
A large number of domesticated TEs have been published over the last decadeCitation44,13 and there is no doubt that TEs play a role as the regulators of gene expression, often building whole regulatory networks.Citation12 Another example of the relationship between Acytota and “host” genome are Helitrons that can capture genic sequences, spread them across the genome or even reshuffle and put them together.Citation27
Although it is thought that epigenetic mechanisms arose as a genome defense against TEs, an alternative view that has emerged recently is that epigenetic mechanisms were formed first and only secondarily enabled massive expansion of TEs.Citation11 In this scenario, TEs dominated rather later in evolution - during diversification of eukaryotes. At this stage, repeats (mostly TEs) could serve as linkers of gene modules (as in phage lambda where modules correlate with morphogenesis) or as recombination hotspots that could allow reshuffling of genic modules. A similar scenario could later lead to RNA splicing that could also combine 2 different molecules as we observe in bimolecular RNA splicing in trypanosomes.Citation42
While transposable elements operate more at the genomic level, viruses arrived with their inter-organism influence, and having a more physiological or ecological effect. For example, marine viruses regulate the populations of many sea organisms.Citation17 Moreover, the interplay between giant viruses and satellite viruses regulates the growth and death of plankton. This could have a major effect on ocean nutrient cycles and climate and these viruses could then be major players in global ecosystems.Citation38
Acytota are polyphyletic like other kingdoms and not more parasitic than other organisms
It follows from the above, that the cell-based tree of life is incomplete without adding the Acytota. A capsid encoding “virosphere” cannot be separated from a ribosome encoding “biosphere” because they have similar genesCitation4,40, 15 and there is gene flow between viruses and cells. Viruses appear to have evolved from capsidless Acytota, like plasmids or TEs, and vice versa, on multiple occasions during evolution.Citation25
Life is networked by Acytota members – not only cellular genesCitation19 but TEs, plasmids and whole viruses in particular are horizontally transferred.Citation8,48, 43,35. TEs are present in all genomes not only because of their ancient origin but also partially thanks to HGT. The HGT of transposable elements has been described in many species (for review seeCitation43) and has great impact on genome evolution. It is influenced by the transposition mechanism of a given TE group. For example, Maverick transposons exhibit a patchy distribution pointing to both their ancient origin and horizontal transmission.Citation39
The Darwinian and Haeckelian tree of life has been replaced by the network of life (new paradigm) where branches are interconnected.Citation10 The contribution of vertical vs. horizontal branches could change over time. Nevertheless, all kingdoms are polyphyletic, although the proportions of genes having the same origin and genes from diverse phyla can differ between taxons. The tree is still incomplete because many Biota species and Acytota members in particular are still unknown – for example we know only about 9% of the viral sequences from ocean metagenomic studies and the majority is “dark matter” that should be characterized and included in the tree of life.Citation1
Acytota are often parasitic entities, either genomic (TEs and satellites) or cellular (viruses, viroids), although they are also beneficial for the cell (plasmids, TEs). In TEs in particular but also among other Acytota, we can find a continuum of relationships, from parasitism via competition and symbiosis to cooperation between various TE families in the genome ecosystem.Citation3,41, 51 This can be compared to interactions between classical organisms in classical ecosystems and somehow correlates with member mobility.Citation20 Parasitism in Acytota is no greater than parasitism in the organisms of other kingdoms. Even the term “parasitism” is relative if we accept the view that all entities from microsatellites to ecosystems constitute one functional body similar to Earth viewed as a single living organism like in the Gaia hypothesis.Citation30
Acytota and definition of life
The polemical question “are viruses alive?” is largely philosophical based only on choice of decision.Citation15 That notwithstanding, a number of scientists consider viruses as living forms since new findings reveal they exhibit features typical of life as we know it. Here, we propose not only adding viruses but extending a newly built tree of life to other acellular genetic entities such as satellites, plasmids and TEs.
Encompassing the prolific variety of RNA and DNA-containing entities that multiply within and propagate between cells in Acytota kingdom, challenges existing definitions of life. The common tendency is to include in the definition, all major manifestations of life. This trend, however, makes any attempt to imitate or model the origin of life highly unrealistic. On the other hand, some simple forms of life have a better chance. This invites simpler definitions which would include only the most basic definientia, such as “self-reproduction with variations”.Citation36,49 The Acytota can be viewed naturally as fore-runners to life owing to their simplicity. In a search for a pragmatic definition of life, for the purpose of its experimental reconstruction, the cell-less life would be a natural root for the rest of the tree. The reconstruction of the earliest events in emerging life may involve replication of simple repeating sequences, not unlike aggressive triplet repeat expansions in eukaryotes, however - extracellularly, under originally abiotic conditions e.g.Citation9
Abbreviations
| TEs | = | transposable elements |
| LTR | = | long terminal repeat |
| TPRT | = | target-primed reverse transcription |
| HGT | = | horizontal gene transfer |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research has been supported by the Czech Science Foundation (grant 15-02891S to EK).
References
- Angly FE, Felts B, Breitbart M, Salamon P, Edwards RA, Carlson C, Chan AM, Haynes M, Kelley S, Liu H, et al. The marine viromes of four oceanic regions. PLoS Biol 2006; 4:2121–31; PMID:17090214; http://dx.doi.org/10.1371/journal.pbio.0040368
- Bremnes RM, Sirera R, Camps C. Circulating tumour-derived DNA and RNA markers in blood: a tool for early detection, diagnostics, and follow-up? Lung Cancer 2005; 49:1–12; PMID:15949585; doi:10.1016/j.lungcan.2004.12.008
- Brookfield JFY. The ecology of the genome – mobile DNA elements and their hosts. Nature Rev Genet 2005; 6:128–36; PMID:15640810; doi:10.1038/nrg1524
- Brussow H. The not so universal tree of life or the place of viruses in the living world. Phil Trans R Soc B 2009; 364:2263–74; PMID:19571246; http://dx.doi.org/10.1098/rstb.2009.0036
- Cohen S, Segal D. Extrachromosomal circular DNA in eukaryotes: possible involvement in the plasticity of tandem repeats. Cytogenet Genome Res 2009; 124:327–38; PMID:19556784; http://dx.doi.org/10.1159/000218136
- Cohen S, Houben A, Segal D. Extrachromosomal circular DNA derived from tandemly repeated genomic sequences in plants. Plant J 2008; 53:1027–34; PMID:18088310; http://dx.doi.org/10.1111/j.1365-313X.2007.03394.x
- Cohen S, Agmon N, Sobol O, Segal D. Extrachromosomal circles of satellite repeats and 5S ribosomal DNA in human cells. Mobile DNA 2010; 1:11; PMID:20226008; http://dx.doi.org/10.1186/1759-8753-1-11
- Diao X, Freeling M, Lisch D. Horizontal transfer of plant transposon. PLoS Biol 2006; 4:e5; PMID:16336045; http://dx.doi.org/10.1371/journal.pbio.0040005
- Di Mauro E, Saladino R, Trifonov EN. The path to Life's origins. Remaining hurdles. J Biomol Str Dyn 2014; 32:512–22; PMID:23582097; http://dx.doi.org/10.1080/07391102.2013.783509
- Doolittle WF. Fun with genealogy. Proc Natl Acad Sci USA 1997; 94:12751–3; PMID:9398070
- Fedorova NV. Transposable elements, epigenetics, and genome evolution. Science 2012; 338:758–67; PMID:23145453; http://dx.doi.org/10.1126/science.338.6108.758
- Feschotte C. Transposable elements and the evolution of regulatory networks. Nature Rev Genet 2008; 9:397–405; PMID:18368054; http://dx.doi.org/10.1038/nrg2337
- Feschotte C, Pritham EJ. DNA transposons and the evolution of eukaryotic genomes. Ann Rev Genet 2007; 41:331–68; PMID:18076328; http://dx.doi.org/10.1146/annurev.genet.40.110405.090448
- Forterre P. Displacement of cellular proteins by functional analogues from plasmids or viruses could explain puzzling phylogenies of many DNA informational proteins. Mol Microbiol 1999; 33:457–65; PMID:10417637; http://dx.doi.org/10.1046/j.1365-2958.1999.01497.x
- Forterre P. Defining life: The virus viewpoint. Orig Life Evol Biosph 2010; 40:151–60; PMID:20198436; http://dx.doi.org/10.1007/s11084-010-9194-1
- Fuhrman JA. Marine viruses and their biogeochemical and ecological effects. Nature 1999; 399:541–8; PMID:10376593; doi:10.1038/21119
- He S, Burgess Hickman A, Varani AM, Siguier P, Chandler M, Dekker JP, Dyda F. Insertion sequence IS26 reorganized plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio 2015; 6:e00762–15; PMID:26060276; http://dx.doi.org/10.1128/mBio.00762-15
- Jain R, Rivera MC, Lake JA. Horizontal gene transfer among genomes: The complexity hypothesis. Proc Natl Acad Sci USA 1999; 96:3801–6; PMID:10097118; http://dx.doi.org/10.1073/pnas.96.7.3801
- Jalasvuori M, Koonin EV. Classification of prokaryotic genetic replicators: between selfishness and altruism. Ann N Y Acad Sci 2015; 1341:96–105; PMID:25703428; http://dx.doi.org/10.1111/nyas.12696
- Kapitonov VV, Jurka J. Self-synthesizing DNA transposons in eukaryotes. Proc Natl Acad Sci USA 2006; 103:4540–5; PMID:16537396; http://dx.doi.org/10.1073/pnas.0600833103
- Kapitonov VV, Jurka J. Helitrons on a roll: eukaryotic rolling-circle transposons. Trends Genet 2007; 23:521–9; PMID:17850916; doi:10.1016/j.tig.2007.08.004
- Kejnovsky E, Kubat Z, Macas J, Hobza R, Mracek J, Vyskot B. Retand: A novel family of gypsy-like retrotransposon harboring an amplified tandem repeat. Mol Genet Genomics 2006; 276:254–63; PMID:16826419; DOI 10.1007/s00438-006-0140-x
- Kejnovsky E, Hobza R, Kubat Z, Widmer A, Marais GAB, Vyskot B. High intrachromosomal similarity of retrotransposon long-terminal repeats: Evidence for homogenization by gene conversion on plant sex chromosomes? Gene 2007; 390:92–7; PMID:17134852; doi:10.1016/j.gene.2006.10.007
- Koonin EV, Dolja VV. Virus world as an evolutionary network of viruses and capsidless selfish elements. Microbiol Mol Biol Rev 2014; 78:278–303; PMID:24847023; http://dx.doi.org/10.1128/MMBR.00049-13
- Krupovic M, Makarova KS, Forterre P, Prangishvili D, Koonin EV. Casposons: a new superfamily of self-synthesizing DNA transposons at the origin of prakaryotic CRISPR-Cas immunity. BMC Biol 2014; 12:36; PMID:24884953; http://dx.doi.org/10.1186/1741-7007-12-36
- Lal SK, Hannah LC. Massive changes of the maize genome are caused by Helitrons. Heredity 2005; 95:421–2; PMID:16222326; doi:10.1038/sj.hdy.6800764
- LaScola B, Desnues C, Pagnier I, Robert C, Barrassi L, Fournous G, Merchat M, Suzan-Monti M, Forterre P, Koonin E, et al. The virophage as a unique parasite of the giant mimivirus. Nature 2008; 455:100–4; PMID:18690211; http://dx.doi.org/10.1038/nature07218
- Liao D. Concerted evolution: molecular mechanism and biological implications. Am J Hum Genet 1999; 64:24–30; PMID:9915939; http://dx.doi.org/10.1086/302221
- Lovelock JE. Gaia – A new look at life on Earth. Oxford University Press, 1979
- Martinez-Izquierdo JA, Garcia-Martinez J, Vicient CM. What makes Grande1 retrotransposon different? Genetica 1997; 100:15–28; PMID:9440255; http://dx.doi.org/10.1007/978-94-011-4898-6_2
- McGeoch A, Bell SD. Eukaryotic/archeal primase and MCM proteins encoded in a bacteriophage genome. Cell 2005; 120:167–8; PMID:15680323
- Mestrovic N, Mravinac B, Pavlek M, Vojvoda-Zeljko T, Satovic E, Plohl M. Structural and functional liaisons between transposable elements and satellite DNAs. Chromosome Res 2015; 23:583–596; PMID:26293606; http://dx.doi.org/10.1007/s10577-015-9483-7.
- Monier A, Claverie J-M, Ogata H. Taxonomic distribution of large DNA viruses in the sea. Genome Biol 2008; 9:R106; PMID:18598358; http://dx.doi.org/10.1186/gb-2008-9-7-r106
- Naik GA, Bhat LN, Chopade BA, Lynch JM. Transfer of broad-host-range antibiotic resistance plasmids in soil microcosms. Curr Microbiol 1994; 28:209–15; http://dx.doi.org/10.1007/BF01575963
- Oparin A, Fesenkov V. Life in the universe. Twayne Publishers, New York, 1961
- Pathak VK, Hu W-S. “Might as well jump!” Template switching by retroviral reverse transcriptase, defective genome formation, and recombination. Seminars Virol 1997; 8:141–50; http://dx.doi.org/10.1006/smvy.1997.0114
- Pearson H. “Virophage” suggests viruses are alive. Nature 2008; 454:677; http://dx.doi.org/10.1038/454677a
- Pritham EJ, Putliwala T, Feschotte C. Maverick, a novel class of giant transposable elements widespread in eukaryotes and related to DNA viruses. Gene 2007; 390:3–17; PMID:17034960; http://dx.doi.org/10.1016/j.gene.2006.08.008
- Raoult D, Forterre P. Redefining viruses: lesson from mimivirus. Nat Rev Microbiol 2008; 6:315–9; PMID:18311164; http://dx.doi.org/10.1038/nrmicro1858
- Sabot F, Schulman AH. Parasitism and the retrotransposon life cycle in plants: a hitchhiker's guide to the genome. Heredity 2006; 97:381–8; PMID:16985508; http://dx.doi.org/10.1038/sj.hdy.6800903
- Siegel TN, Tan KSW, Cross GAM. Systematic study of sequence motifs for RNA trans splicing in Trypanosoma brucei. Mol Cell Biol 2005; 25: 9586–94; PMID:16227607; http://dx.doi.org/10.1128/MCB.25.21.9586-9594.2005
- Schaack S, Gilbert C, Feschotte C. Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol Evol 2010; 25:537–46; PMID:20591532; http://dx.doi.org/10.1016/j.tree.2010.06.001
- Sinzelle L, Izsvak Z, Ivics Z. Molecular domestication of transposable elements: From detrimental parasites to useful host genes. Cell Mol Life Sci 2009; 66:1073–93. PMID: 19132291; http://dx.doi.org/10.1007/s00018-009-8376-3
- Slotkin RK, Vaughn M, Borges F, Tanurdžič M, Becker JC, Feijó JA, Martienssen RA. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 2009; 136:461–72; PMID:19203581; http://dx.doi.org/10.1016/j.cell.2008.12.038
- Stroun M, Lyautey J, Lederrey C, Mulcahy HE, Anker P. Alu repeat sequences are present in increased proportions compared to a unique gene in plasma/serum DNA: Evidence for a preferential release from viable cells? Ann N Y Acad Sci 2001; 945:258–64; PMID:11708488
- Talianski ME, Palukaitis PF. Satellite RNAs and satellite viruses. In Encyclopedia of Virology (eds A. Granoff & R.G. Webster), pp. 1607–1615. San Diego, CA, Academic Press 1999
- Thomas J, Schaack S, Pritham EJ. Pervasive horizontal transfer of rolling-circle transposons among animals. Genome Biol Evol 2010; 2:656–64; PMID:20693155; http://dx.doi.org/10.1093/gbe/evq050
- Trifonov EN. Vocabulary of definitions of life suggests a definition. J Biomol Struct Dyn 2011; 29:259–66; PMID:21875147; http://dx.doi.org/10.1080/073911011010524992
- Trifonov EN, Kejnovsky E. Acytota – associated kingdom of neglected life. J Biomol Struct Dyn 2016; Mar 1:1-8. [Epub ahead of print], PMID:26305806; http://dx.doi.org/10.1080/07391102.2015.1086959.
- Venner S, Feschotte C, Biemont C. Dynamic of transposable elements: towards a community ecology of the genome. Trends Genet 2009; 25:317–23; PMID:19540613; http://dx.doi.org/10.1016/j.tig.2009.05.003
