ABSTRACT
Although Helitrons were discovered 15 y ago, they still represent an elusive group of transposable elements (TEs). They are thought to transpose via a rolling-circle mechanism, but no transposition assay has yet been conducted. We have recently characterized a group of Helitrons in Drosophila, named DINE-TR1, that display interesting features, including pronounced enrichment at β-heterochromatin, multiple tandem insertions (TIs) of the entire TE, and that experienced at least 2 independent expansion events of its internal tandem repeats (TRs) in distant Drosophila lineages. Here we discuss 2 aspects of TE dynamics displayed by the DINE-TR1 Helitrons: (i) the general evolutionary impact of piRNA-guided heterochromatin formation via TE-derived TR expansion and (ii) the possible mechanisms that could account for the recurrent TIs of Helitrons.
Introduction
Eukaryotic genomes are notably rich in several types of repetitive sequences which, although mostly parasitic in nature, have been fundamental in shaping these genomes and have generated many beneficial side effects by means of exaptation.Citation1-3 The most abundant type of repetitive DNA are the transposable elements (TEs), which are stretches of DNA that are able to move between loci in the host genome and make copies of themselves in the process.Citation4 In spite of the large number of studies conducted on these elements over the last decades, TE abundance and diversity is so massive that we still do not fully appreciate their impact on genome evolution.
One particularly elusive group of TEs are the Helitrons, DNA transposons thought to mobilize using a mechanism similar to the rolling circle replication of some plasmids, single stranded DNA viruses and bacterial transposons.Citation5 These TEs were discovered 15 y ago in Caenorhabditis elegans, Arabidopsis thaliana and Oryza sativa, and they are now known to be present in a wide phylogenetic range, from fungi to mammals.Citation5-7
Despite many advances in Helitron research over the past years, data are still lacking for their mode of transposition, genome organization, and chromosome distribution, for most of the studied species. In the genus Drosophila, for example, the metaphase chromosome distribution of the prolific DINE-1 HelitronsCitation8 is unknown for most species, including Drosophila melanogaster.
In a recent work, we improved characterization of Helitrons from the DINE-1 group in 2 Drosophila species from the virilis group, D. virilis and D. americana, and defined a subgroup of DINEs present in Acalyptratae (Diptera) that we called DINE-TR1.Citation9 This group is characterized by the presence of internal tandem repeats (TRs) of ∼150 bp which share a conserved region of 30–40 bp in their 5′ end, suggesting that this area may exhibit functionality. In D. virilis and D. americana, DINE-TR1 is located in many euchromatic loci but is particularly enriched in chromatin/heterochromatin boundaries (β-heterochromatin) and in the Y chromosome. In D. virilis, DINE-TR1 further colonized the centromeric region of chromosome 5 (Muller element C). Small RNAs matching DINE-TR1 are ∼25 nt in length, abundant in 0–2 h embryos and adult gonads, but are almost absent in adult carcasses, suggesting that DINE-TR1 copies are targeted for silencing by the piRNA pathway in D. virilis.Citation9
Surprisingly, we detected 2 independent TR amplification events from within DINE-TR1 that occurred in Drosophila lineages which diverged over 60 My ago (virilis subgroup and D. biarmipes). A few other Drosophila species and the Queensland fruit fly Bactrocera tryoni also showed signs of incipient expansion of DINE-TR1 internal TRs. These results point to DINE-TR1 as a recurrent source of satellite DNAs in Acalyptratae.
In this commentary, we focus on 2 aspects of TE dynamics displayed by the DINE-TR1 Helitrons. First, we discuss the evolutionary impact of TR expansion within TEs that are targeted by the piRNA pathway, such as DINE-TR1, to gene and genome regulation and evolution. Second, we draw attention to the numerous DINE-TR1 tandem insertions (TIs) we detected in the D. virilis genome assembly, with up to 11 sequential elements. We discuss the literature concerning Helitron TIs and highlight some unresolved questions regarding Helitron transposition.
piRNA-targeted TEs as sources of TR expansion and heterochromatin formation
There are now several reports available on the expansion of TRs from within different classes of TEs.Citation10 The occurrence of internal TRs seems to be a relatively common feature for many TEs, although the functional role of these repeats (if any) is mostly unknown.Citation11 For TEs belonging to the terminal inverted repeat (TIR) order, it has been proposed that the increased number of internal TRs containing transposase binding sites could improve the specificity of recognition by the transposase leading to a more efficient mobilization.Citation12 In fact, the presence of multiple transposase binding sites improves in vitro transposition of the TE Sleeping Beauty in HeLa cells.Citation13 For retroelements from the large Ty3/Gypsy family, it has been proposed that TRs at the 3′ end could act as transposition termination signals by forming stable stem loops.Citation14 Similar examples of TRs residing within Helitrons have been reported,Citation15-17 although no explanation regarding their functional significance has yet been rendered.
Regardless of their involvement in TE movement itself, the expansion/contraction of internal TRs can impact genome structure in several ways. It has been shown recently that TEs induce local heterochromatin formation via deposition of H3K9me and that such epigenetic change may affect the expression of nearby genes.Citation18 In addition to the local effects of repeats on gene regulation, the overall dosage of heterochromatin was shown to affect position effect variegation (PEV) and viability in Drosophila.Citation19,20 This is known as the “chromatin sink” hypothesis, and accounts for the ability of chromatin to recruit modulator proteins in such a way that increases or decreases in heterochromatin dosage would lead to depletion or overabundance, respectively, of these modulators in other regions of the genome.Citation19
In light of this knowledge regarding the epigenetic effects of repetitive sequences, it is possible to advance 2 consequences of TR expansion from piRNA targeted TEs. They might act like “tuning knobs” for gene expression in euchromatin and/or as factories of satellite DNAs that influence both heterochromatin turnover and genome modulation ().
Figure 1. General layout for transposable element (TE)-derived tandem repeat (TR) expansion and heterochromatin formation. (A) At some point in the TE life cycle one of its copies inserts into an active piRNA cluster and then serves as a template for the generation of complementary piRNAs that silence homologous sequences in the genome via heterochromatin formation. (B) The heterochromatinized TE copies harboring internal TRs are prone to suffering unequal recombination. This TR concertina generates variation in the size of heterochromatin blocks what may in turn affect gene expression. TIR: Terminal Inverted Repeats; LTR: Long Terminal Repeats.
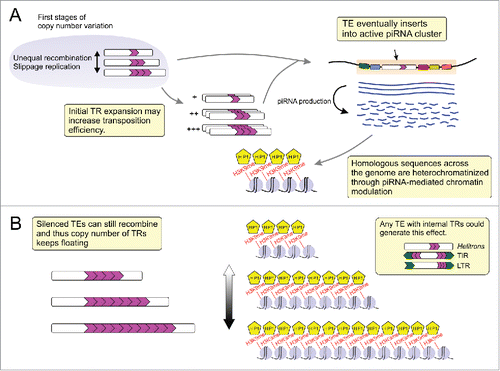
The TRs within TEs may expand or contract, and this variation is likely the result of either unequal recombination or slippage replication.Citation21,22 The initial variation in size may fluctuate by drift, or proceed toward expansion in the case that additional TRs increase transposition efficiency.Citation12,10 Constant transpositions will eventually result in the insertion of a TE copy within an active piRNA cluster. Such events ensue production of piRNAs that will recognize and silence the copies of this TE, reducing or stopping further transpositions (). The silencing is thought to occur at both transcriptional (via H3K9me and HP1)Citation23 and post-transcriptional levels (processing of TE transcripts by PIWI-clade proteins).Citation24 Although unable to transpose, the dispersed silenced copies of the TE can still be subject to unequal exchange mechanisms such as unequal recombination. In this context, expansion and contractions of internal TRs coupled with piRNA targeting now result in variations in the size of local heterochromatin blocks, acting like a heterochromatin concertina ().
The heterochromatin formation via piRNA-targeted TEs could have a great impact on driving the turnover of centromeric sequences, since new residents of the centromere must maintain the heterochromatic state of this region.Citation25,26 In plants, a boom-bust process was proposed to explain the radical shifts in sequence composition of homologous centromere regions before a favorable repeat becomes fixed.Citation27 This model could account for the quick colonization of the centromere of chromosome 5 in D. virilis by DINE-TR1, which is absent in the same region in the closely related species D. americana.Citation9 This turnover was likely achieved by both DINE-TR1 replicative transposition and the heterochromatin formation via TR expansion. Such rapid changes in heterochromatin content may influence genome stability and contribute to species divergence.Citation28,29 Thus, TRs derived from TEs targeted by piRNAs could be a recurrent source for heterochromatin turnover.
In cases where TEs with internal TRs are close to or within genes, the concertina-like range size variation of silenced TRs could modulate gene expression and be, in turn, subject to selection.Citation30 In D. virilis, for example, there are over 1,000 intronic insertions of DINE-1Citation15 and many of them represent DINE-TR1 elements. The tuning of DINE-TR1 internal repeat array length by natural selection is arguably more dynamic than the mere presence/absence of TEs close to genes. In fact, the epigenetic effect of TEs on gene expression was associated with the number and length of TEs, distance from the gene, and also the likelihood that these TEs were targeted by the piRNA pathway.Citation18 The same mechanisms of TE silencing are assumed to act upon TE-derived TRs as long as they are also present in the piRNA generating clusters. In this context, it would be interesting to investigate the extent of TR array size variation for DINE-TR1 associated with genes and in “gene desert” regions. The size variation of TE-derived TRs is also expected to contribute to the bulk amount of heterochromatin, which itself has regulatory properties.Citation31,20
In conclusion, the expansion/contraction of TRs within piRNA targeted TEs could impact genome regulation at several levels, according to both the chromatin sink hypothesis and the local regulatory effect exerted by repeats. This could be an important process in tuning global genome regulation and also a step toward population divergence.
Tandem insertions of Helitrons: a still unexplained phenomenon
Helitrons are thought to transpose through a semi-replicative rolling-circle (RC) mechanismCitation5-7 as they encode a protein similar to the replication initiator proteins (Rep) found in RC replicons.Citation5,32 The fact that several contigs in D. virilis show 2 or more DINE-TR1 insertions in tandem arraysCitation9 could be related to this mode of transposition.
Helitron TIs were also identified in organisms such as Myotis lucifugus,Citation33 Daphnia pulex,Citation34 Bombyx moriCitation35 and Zea mays.Citation36,37 These head-to-tail connections were explained as a feature of the rolling-circle (RC) transposition mechanism, which is thought to form tandem arrays if the termination signal is bypassed.Citation38 This hypothesis stems from the work conducted by Mendiola et al.Citation32 describing the formation of TIs (one-ended transposition) composed of pSU2572 plasmids containing IS91 elements with inactive termination signals. In this case, insertions of whole plasmid units were found fused in a head-to-tail fashion (). This is an expected outcome for the RC transposition mechanism in cases of termination signal bypassing, but only if the donor element is a circular dsDNA. Therefore, an inactive termination signal would only generate TIs of RC transposons (e.g. IS91, Helitrons) if the donor element is converted into a ‘head-to-tail’ circular unit (). However, if during transposition the donor TE is located in a chromosome or plasmid, one-ended transposition is expected to capture the 3′ flanking sequence, until an alternative termination signal is encountered downstream ().Citation39
Figure 2. Schematic representation of the tandem insertions observed by Mendiola et al.Citation32 When the termination signal is missing at IS91 terminus, the RC transposition loops-out the entire plasmid and generate TIs of the whole construct (IS91 + pSU2572).
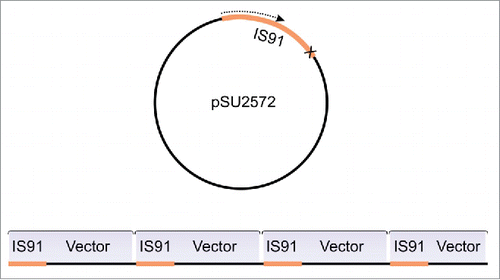
Figure 3. Two RC transposition models proposed for Helitrons. (A) The “Concerted” model of RC transposition was proposed for the IS91 prokaryotic elements and used to explain Helitron transposition.Citation5,39 Redrawn from Garcillán-Barcia et al.Citation41 (B) The “Sequential” model of RC transposition was proposed to explain the formation of episomal circular intermediates of IS91. Schematic representation based upon the model described in Garcillán-Barcia et al.Citation41 Single-stranded binding Proteins (SSBs) were not represented in the B section to improve clarity. RepHel: Replication Initiator Protein and Helicase; DNA Pol: host DNA polymerase.
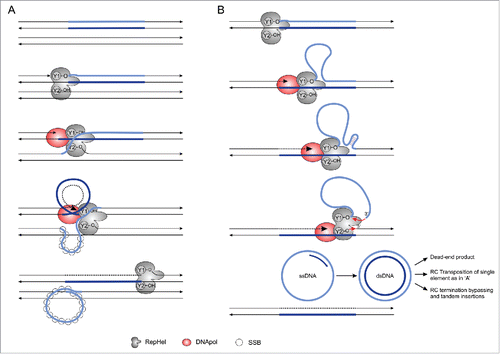
The current model used to explain the transposition of Helitrons ()Citation6,39 is adapted from the first proposed mechanism for RC transposition, based on the prokaryotic element IS91.Citation32 After the detection of circular ss- and dsDNA intermediates from IS91,Citation40 a second model was suggested by Garcillán-Barcia et al.Citation41 (). They called the first model “concerted” and the second “sequential” (). As noted by Thomas and Pritham,Citation7 because there is no description of Helitron circular DNA species to date, the concerted model has been chosen to explain the transposition mechanism of Helitrons, although other indirect lines of evidence point to the occurrence of a sequential mechanism in this TE, at least occasionally. For example, the presence of Helitron insertions in the form of tandem arrays of the same elementCitation9,33 agrees with the sequential model.
It is not clear how other typical Helitron features, like the tendency to form proximal (but not tandem) insertions or clusters in variable types of arrangementsCitation9,36,37 could be explained by either model alone. Surely, the development of transposition assays will be essential to clarify all the aforementioned issues. Additionally, some important aspects of Helitron insertions found in the genomic data have not yet been addressed by the existing models and should be properly outlined in the future.
Perspectives
Given their abundance and wide phylogenetic distribution, Helitrons are expected to interact in many ways with their host genomes, and the evolutionary routes and outcomes of such interactions have only recently begun to be unraveled in a few organisms (e.g., see refs. Citation35, 42–46).
Here we discuss 2 features displayed by Helitrons: the recurrent phenomenon of TIs composed of entire elements, which seems to be specific to this group of TEs and related to their mobilization mechanism; and the more general evolutionary impact of TR expansion from piRNA-targeted TEs. Advances in computational identification of Helitrons and the development of transposition assays will greatly improve our knowledge about these prolific elements. Also, the combined molecular, cytogenetic and computational characterization of Helitrons in unexplored genomes will surely continue to provide many interesting insights on Helitron biology, and to the overall impact of repetitive DNA for genome evolution.
Abbreviations
| DINE | = | Drosophila INterspersed Element |
| HP1 | = | Heterochromatin Protein 1 |
| My | = | million years |
| RC | = | rolling-circle |
| Rep | = | replication initiator protein |
| TE | = | transposable element |
| TI | = | tandem insertion |
| TR | = | tandem repeat |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by “Fundação de Amparo à Pesquisa do Estado de Minas Gerais” (FAPEMIG) (Proc: APQ-01563-14), “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq), a doctoral fellowship from “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) to GBD, and a student fellowship to PH from “Programa Institucional de Auxílio à Pesquisa de Doutores. Recém-Contratados da Universidade Federal de Minas Gerais.”
References
- Kidwell MG, Lisch DR. Transposable elements and host genome evolution. Trends Ecol Evol 2000; 15(3):95-9; PMID:10675923; http://dx.doi.org/10.1016/S0169-5347(99)01817-0
- Shapiro JA, von Sternberg R. Why repetitive DNA is essential to genome function. Biol Rev 2005; 80(02):227-50; PMID:15921050; http://dx.doi.org/10.1017/S1464793104006657
- Elliott TA, Linquist S, Gregory TR. Conceptual and empirical challenges of ascribing functions to transposable elements. Am Nat 2014; 184(1):14-24; PMID:24921597; http://dx.doi.org/10.1086/676588
- Gregory TR. Synergy between sequence and size in large-scale genomics. Nat Rev Genet 2005; 6(9):699-708; PMID:16151375; http://dx.doi.org/10.1038/nrg1674
- Kapitonov VV, Jurka J. Rolling-circle transposons in eukaryotes. Proc Natl Acad Sci USA 2001; 98(15):8714-9; http://dx.doi.org/10.1073/pnas.151269298
- Kapitonov VV, Jurka J. Helitrons on a roll: eukaryotic rolling-circle transposons. Trends Genet 2007; 23(10):521-9; PMID:17850916; http://dx.doi.org/10.1016/j.tig.2007.08.004
- Thomas J, Pritham E. Helitrons, the Eukaryotic Rolling-circle Transposable Elements. Microbiol Spectr 2015; 3(4):MDNA3-0049-2014; PMID:26350323; http://dx.doi.org/10.1128/microbiolspec.MDNA3-0049-2014
- Locke J, Howard LT, Aippersbach N, Podemski L, Hodgetts RB. The characterization of DINE-1, a short, interspersed repetitive element present on chromosome and in the centric heterochromatin of Drosophila melanogaster. Chromosoma 1999; 108(6):356-66; PMID:10591995; http://dx.doi.org/10.1007/s004120050387
- Dias GB, Heringer P, Svartman M, Kuhn GC. Helitrons shaping the genomic architecture of Drosophila: enrichment of DINE-TR1 in α- and β-heterochromatin, satellite DNA emergence, and piRNA expression. Chromosome Res 2015; 23(3):597-613; PMID:26408292; http://dx.doi.org/10.1007/s10577-015-9480-x
- Meštrović N, Mravinac B, Pavlek M, Vojvoda-Zeljko T, Šatović E, Plohl M. Structural and functional liaisons between transposable elements and satellite DNAs. Chromosome Res 2015; 23(3):583-96; PMID:26293606; http://dx.doi.org/10.1007/s10577-015-9483-7
- Macas J, Koblížková A, Navrátilová A, Neumann P. Hypervariable 3′ UTR region of plant LTR-retrotransposons as a source of novel satellite repeats. Gene 2009; 448(2):198-206; PMID:19563868; http://dx.doi.org/10.1016/j.gene.2009.06.014
- Marzo M, Liu D, Ruiz A, Chalmers R. Identification of multiple binding sites for the THAP domain of the Galileo transposase in the long terminal inverted-repeats. Gene 2013; 525(1):84-91; PMID:23648487; http://dx.doi.org/10.1016/j.gene.2013.04.050
- Zayed H, Izsvák Z, Walisko O, Ivics Z. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol Ther 2004; 9(2):292-304; PMID:14759813; http://dx.doi.org/10.1016/j.ymthe.2003.11.024
- Martínez-Izquierdo JA, García-Martínez J, Vicient CM. What makes Grande1 retrotransposon different? Genetica 1997; 100(1):15-28; PMID:9440255; http://dx.doi.org/10.1023/A:1018332218319
- Yang HP, Barbash DA. Abundant and species-specific DINE-1 transposable elements in 12 Drosophila genomes. Genome Biol 2008; 9(2):R39; PMID:18291035; http://dx.doi.org/10.1186/gb-2008-9-2-r39
- Kuhn GC, Heslop-Harrison JS. Characterization and genomic organization of PERI, a repetitive DNA in the Drosophila buzzatii cluster related to DINE-1 transposable elements and highly abundant in the sex chromosomes. Cytogenet Genome Res 2011; 132:79-88; PMID:20938165; http://dx.doi.org/10.1159/000320921
- Šatović E, Plohl M. Tandem repeat-containing MITEs in the clam Donax trunculus. Genome Biol Evol 2013; 5(12):2549-59; PMID:24317975; http://dx.doi.org/10.1093/gbe/evt202
- Lee YCG. The role of piRNA-mediated epigenetic silencing in the population dynamics of transposable elements in Drosophila melanogaster. PLoS Genet 2015; 11(6):e1005269; PMID:26042931; http://dx.doi.org/10.1371/journal.pgen.1005269
- Dimitri P, Pisano C. Position effect variegation in Drosophila melanogaster: relationship between suppression effect and the amount of Y chromosome. Genetics 1989; 122(4):793-800; PMID:2503420
- Berloco M, Palumbo G, Piacentini L, Pimpinelli S, Fanti L. Position effect variegation and viability are both sensitive to dosage of constitutive heterochromatin in Drosophila. G3 (Bethesda) 2014; 4(9):1709-16; PMID:25053704; http://dx.doi.org/10.1534/g3.114.013045
- Charlesworth B, Sniegowski P, Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 1994; 371:215-20; PMID:8078581; http://dx.doi.org/10.1038/371215a0
- Scalvenzi T, Pollet N. Insights on genome size evolution from a miniature inverted repeat transposon driving a satellite DNA. Mol Phylogenet Evol 2014; 81:1-9; PMID:25193611; http://dx.doi.org/10.1016/j.ympev.2014.08.014
- Le Thomas A, Rogers AK, Webster A, Marinov GK, Liao SE, Perkins EM, Hur JK, Aravin AA, Tóth KF. Piwi induces piRNA-guided transcriptional silencing and establishment of a repressive chromatin state. Genes Dev 2013; 27(4):390-9; PMID:23392610; http://dx.doi.org/10.1101/gad.209841.112
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon GJ. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 2007; 128:1089-103; PMID:17346786; http://dx.doi.org/10.1016/j.cell.2007.01.043
- Henikoff S, Ahmad K, Malik HS. The centromere paradox: stable inheritance with rapidly evolving DNA. Science 2001; 293(5532):1098-102; PMID:11498581; http://dx.doi.org/10.1126/science.1062939
- Plohl M, Meštrović N, Mravinac B. Centromere identity from the DNA point of view. Chromosoma 2014; 123(4):313-25; PMID:24763964; http://dx.doi.org/10.1007/s00412-014-0462-0
- Zhang H, Koblížková A, Wang K, Gong Z, Oliveira L, Torres GA, Wu Y, Zhang W, Novák P, Buell CR, Macas J, Jiang, J. Boom-bust turnovers of megabase-sized centromeric DNA in Solanum species: rapid evolution of DNA sequences associated with centromeres. Plant Cell 2014; 26(4):1436-47; PMID:24728646; http://dx.doi.org/10.1105/tpc.114.123877
- Kuhn GC, Sene FM, Moreira-Filho O, Schwarzacher T, Heslop-Harrison JS. Sequence analysis, chromosomal distribution and long-range organization show that rapid turnover of new and old pBuM satellite DNA repeats leads to different patterns of variation in seven species of the Drosophila buzzatii cluster. Chromosome Res 2008; 16(2):307-24; PMID:18266060; http://dx.doi.org/10.1007/s10577-007-1195-1
- Ferree PM, Barbash DA. Species-specific heterochromatin prevents mitotic chromosome segregation to cause hybrid lethality in Drosophila. PLoS Biol 2009; 7(10):2310; PMID:19859525; http://dx.doi.org/10.1371/journal.pbio.1000234
- King DG, Soller M, Kashi Y. Evolutionary tuning knobs. Endeavour 1997; 21(1):36-40; http://dx.doi.org/10.1016/S0160-9327(97)01005-3
- Zuckerkandl E. A possible role of “inert” heterochromatin in cell differentiation. Action of and competition for “locking” molecules. Biochimie 1974; 56:937-54; PMID:4614863
- Mendiola MV, Bernales I, De La Cruz F. Differential roles of the transposon termini in IS91 transposition. Proc Natl Acad Sci USA 1994; 91(5):1922-26; PMID:8127907; http://dx.doi.org/10.1073/pnas.91.5.1922
- Pritham EJ, Feschotte C. Massive amplification of rolling-circle transposons in the lineage of the bat Myotis lucifugus. Proc Natl Acad Sci USA 2007; 104(6):1895-900; PMID:17261799; http://dx.doi.org/10.1073/pnas.0609601104
- Schaack S, Choi E, Lynch M, Pritham EJ. DNA transposons and the role of recombination in mutation accumulation in Daphnia pulex. Genome Biol 2010; 11(4):R46; PMID:20433697; http://dx.doi.org/10.1186/gb-2010-11-4-r46
- Thomas J, Schaack S, Pritham EJ. Pervasive horizontal transfer of rolling-circle transposons among animals. Genome Biol Evol 2010; 2:656-64; PMID:20693155; http://dx.doi.org/10.1093/gbe/evq050
- Du C, Caronna J, He L, Dooner HK. Computational prediction and molecular confirmation of Helitron transposons in the maize genome. BMC Genomics 2008; 9(1):51; PMID:18226261; http://dx.doi.org/10.1186/1471-2164-9-51
- Thomas J, Vadnagara K, Pritham EJ. DINE-1, the highest copy number repeats in Drosophila melanogaster are non-autonomous endonuclease-encoding rolling-circle transposable elements (Helentrons). Mob DNA 2014; 5(1):18; PMID:24959209; http://dx.doi.org/10.1186/1759-8753-5-18
- Yang L, Bennetzen JL. Distribution, diversity, evolution, and survival of Helitrons in the maize genome. Proc Natl Acad Sci USA 2009; 106:19922-27; PMID:19926865; http://dx.doi.org/10.1073/pnas.0908008106
- Feschotte C, Wessler SR. Treasures in the attic: rolling circle transposons discovered in eukaryotic genomes. Proc Natl Acad Sci USA 2001; 98:8923-4; PMID:11481459; http://dx.doi.org/10.1073/pnas.171326198
- Garcillán-Barcia MP, Bernales I, Mendiola M, De La Cruz F. Single-stranded DNA intermediates in IS91 rolling-circle transposition. Mol Microbiol 2001; 39(2):494-502; PMID:11136468; http://dx.doi.org/10.1046/j.1365-2958.2001.02261.x
- Garcillán-Barcia MP, Bernales I, Mendiola MV, de La Cruz F. 2002. IS91 Rolling-circle transposition, p 891-904. In Craig NL, Craigie R, Gellert M, Lambowitz A (ed), Mobile DNA II. ASM Press, Washington D. C. http://dx.doi.org/10.1128/9781555817954.ch37
- Ellison CE, Bachtrog D. Dosage compensation via transposable element mediated rewiring of a regulatory network. Science 2013; 342(6160):846-50; PMID:24233721; http://dx.doi.org/10.1126/science.1239552
- Barberán-Soler S, Fontrodona L, Ribó A, Lamm AT, Iannone C, Cerón J, Lehner B, Valcárcel J. Co-option of the piRNA Pathway for Germline-Specific Alternative Splicing of C. elegans TOR. Cell Rep 2014; 8(6):1609-16; http://dx.doi.org/10.1016/j.celrep.2014.08.016
- Carareto CM, Hernandez EH, Vieira C. Genomic regions harboring insecticide resistance-associated Cyp genes are enriched by transposable element fragments carrying putative transcription factor binding sites in two sibling Drosophila species. Gene 2014; 537(1):93-9; PMID:24361809; http://dx.doi.org/10.1016/j.gene.2013.11.080
- Castanera R, Pérez G, López L, Sancho R, Santoyo F, Alfaro M, Galbadón T, Pisabarro G, Oquiza JA, Ramírez L. Highly expressed captured genes and cross-kingdom domains present in Helitrons create novel diversity in Pleurotus ostreatus and other fungi. BMC Genomics 2014; 15(1):1071; PMID:25480150; http://dx.doi.org/10.1186/1471-2164-15-1071
- Hoffmann FG, McGuire LP, Counterman BA, Ray DA. Transposable elements and small RNAs: Genomic fuel for species diversity. Mob Genet Elements 2015; 5(5):1-4; http://dx.doi.org/10.1080/2159256X.2015.1066919
