Abstract
Diacylglycerol kinases (DGKs) belong to a family of cytosolic kinases that regulate the phosphorylation of diacylglycerol (DAG), converting it into phosphatidic acid (PA). There are 10 known mammalian DGK isoforms, each with a different tissue distribution and substrate specificity. These differences allow regulation of cellular responses by fine-tuning the delicate balance of cellular DAG and PA. DGK isoforms are best characterized as mediators of signal transduction and immune function. However, since recent studies reveal that DAG and PA are also involved in the regulation of endocytic trafficking, it is therefore anticipated that DGKs also plays an important role in membrane trafficking. In this review, we summarize the literature discussing the role of DGK isoforms at different stages of endocytic trafficking, including endocytosis, exocytosis, endocytic recycling, and transport from/to the Golgi apparatus. Overall, these studies contribute to our understanding of the involvement of PA and DAG in endocytic trafficking, an area of research that is drawing increasing attention in recent years.
Abbreviations
| DAG | = | diacylglycerol |
| DGK | = | diacylglycerol kinase |
| IP3 | = | inositol 1,4,5-trisphosphate |
| MICAL-L1 | = | Molecules Interacting with CAs-Like1 |
| PA | = | phosphatidic acid |
| PKC | = | protein kinase C |
| PKD | = | protein kinase D |
| PI | = | phosphatidylinositol |
| PIP2 | = | phosphatidylinositol 4,5-bisphosphate |
| RCP | = | rab coupling protein |
| SNX27 | = | sorting nexin 27 |
| TRE | = | tubular recyling endosome |
Introduction
The cytosolic diacylglycerol kinases (DGKs) are a family of kinases that regulate the phosphorylation of diacylglycerol (DAG), thus generating phosphatidic acid (PA). DGKs are widely conserved evolutionarily, and are found in organisms as diverse as bacteria,Citation1 fungi, Saccharomyces cerevisiae, plants,Citation2 multi-cellular organisms including Drosophila melanogaster and Caenorhabditis elegans,Citation3,4 and mammals.Citation5 DGK from Escherichia coli share little structural resemblance to the eukaryotic enzymes,Citation6 although eukaryote DGKs have a highly conserved catalytic domain. Drosophila melanogaster DGK shares homologous amino acid sequences at the carboxyl-terminal domain with porcine DGK.Citation7 In plants such as Arabidopsis thaliana, DGK has evolved into 3 phylogenetic clusters. Cluster I, encoded by AtDGK1 and AtDGK2, resembles the mammalian DGKε, while the other 2 clusters, contain only the conserved kinase domain but lack other accessory motifs, and accordingly are much smaller proteins.Citation5,8 Intriguingly, the yeast Saccharomyces cerevisiae DGK does not possess the signature catalytic domain with an ATP-binding domain, but utilizes CTP. It also has a much simpler and less varied amino-terminal regulatory domain than its ATP-dependent homologs.Citation9 To date, in mammals, 10 DGK isoforms have been identified.Citation10-19 All DGK isoforms have a conserved catalytic domain with an ATP-binding site that is required for kinase activity, and cysteine-rich regions that are homologous to the C1A and C1B motifs of protein kinase C (PKC) Citation20.
Beyond the conserved catalytic domains, the regulatory domains of mammalian DGK isoforms differ greatly, leading to differential localization and regulation of phospholipids in the cells. Based on these regulatory domains, DGK isoforms can be divided into 5 subtypes. summarizes the catalytic and diverse regulatory domains in each DGK isoform of each type, and summarizes current knowledge of their subcellular localization. In addition to the conserved domains, type I DGKs (DGKα, β, and γ) contain a calcium-binding EF hand motif Citation21. Upon activation, DGKα rapidly translocates from the cytosol to the plasma membrane.Citation22 Type II DGKs (DGKδ, η and κ) have a unique pleckstrin homology (PH) domain and a sterile α motif (SAM) domainCitation10 that can bind to the endoplasmic reticulum (ER) and affect anterograde transport.Citation23 Type III DGK (DGKε) has a unique substrate specificity for arachidonate-DAG.Citation24,25 This substrate specificity renders DGKε the most important isoform that catalyzes the first step of phosphatidylinositol (PI) re-synthesis. Although there is no evidence supporting a direct role for DGKε in membrane trafficking, it is possible that of the DGK isoforms, only DGKε exerts an additional function mediating membrane trafficking via PtdIns cycling. Type IV DGKs (DGKζ and ι) have a nuclear localization signal in a MARCKS homologous domain,Citation26 4 ankyrin repeats, and carboxyl terminal PDZ-binding domains.Citation27 Type V DGK (DGKθ) has 3 C1 domains, a putative PH domain, and a Ras association (RA) domain. In humans, each subtype of the DGK family isoforms displays a tissue-specific expression profile. For example, DGKα is mostly detected in brain and immunologic organs, such as spleen and thymus.Citation22 DGKβ is expressed in neurons in caudate-putamen, the nucleus accumbens, and the olfactory tubercle.Citation12,28 DGKδ is substantially expressed in spleen, ovary and skeletal muscle.Citation10,29,30 DGKζ is also highly expressed in spleen and thymus, as well as in heart and pancreas.Citation31
Figure 1. Schematic diagram illustrating the domain architecture of diacylglycerol kinase isoforms. The ten members of the mammalian DGK family are grouped into 5 types according to their regulatory domains. PH, pleckstrin homology domain; SAM, sterile α motif domain.
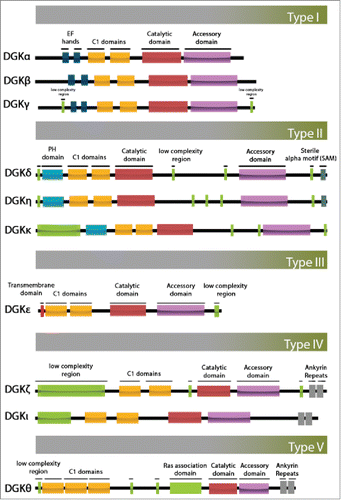
Table 1. Known subcellular localizations of different DGK isoforms
Studies using DGK knock-out mouse models have revealed the involvement of various DGK isoforms in different diseases. DGKα or DGKζ-null T cells display defects in immune function.Citation32,33 DGKδ haploinsufficiency causes the development of insulin resistance in skeletal muscle and adipose tissue.Citation30 In addition, DGKβ/DGKε knockout mice exhibit brain disorders and behavioral abnormalities.Citation34-36
Since DGKα was identified in the 1990s, studies on DGK family proteins have primarily focused on their function in regulating signaling pathways. DGK terminates DAG-based signals and accentuates PA signaling, and both DAG and PA serve as important second messengers in the cells. The activation of DGK usually involves the translocation of DGK to a membrane compartment.Citation37 Upon recruitment to the membrane, DGK can be activated by calcium binding to the EF hand motif. DGK activity is initiated or enhanced by some lipid components including the products of phosphatidylinositol-3-kinase (PI3K) such as phosphatidylinositol-3,4,5-trisphosphate (PIP3),Citation38 phosphatidylserine, and sphingosine.Citation6 It is also activated via Src tyrosine kinase phosphorylationCitation39 and serine phosphorylation by protein kinase C (PKC).Citation40 Some upstream players participating in DGK activation include interleukin2 (IL-2) receptor signaling,Citation41 epidermal growth factor receptor (EGFR),Citation42 T cell antigen receptor signal transduction,Citation22 and hepatocyte growth factor receptor (HGFR).Citation43
The substrate and product of DGK, DAG and PA respectively, play different roles in endocytic events. These include vesicular trafficking, the secretory pathway regulation, and Golgi apparatus function. The mechanisms by which DAG regulates membrane trafficking are diverse: 1) serving as a second messenger to activate PKC, PKD, and downstream signaling cascades;Citation44-46 2) involvement in PI cycling regulation of phosphatidylinositol 4,5-bisphosphate (PIP2) abundance, inositol 1,4,5-trisphosphate (IP3)Citation47 and subsequent Ca2+ influx.Citation48 On the other hand, the local concentration of PA is an important regulator of trafficking, possibly because: 1) PA-enriched membranes with higher curvature tend to undergo fission;Citation49 2) it serves as a docking site for recruiting specific proteins such as Rab coupling proteins (RCP),Citation50 Molecules Interacting with CAsL-Like1 (MICAL-L1),Citation51 and Sorting nexin 27 (SNX27) Citation52 to the membrane; 3) it is an intermediate for PtdIns re-synthesis.Citation24 Given the increasing awareness of the relevance of lipid metabolites such as DAG and PA in membrane trafficking, understanding the significance of DGK function in regulating the fine balance of DAG-to-PA levels is becoming an increasingly important research goal.
Exosome Secretion
DAG mediates acrosome fusion with plasma membrane
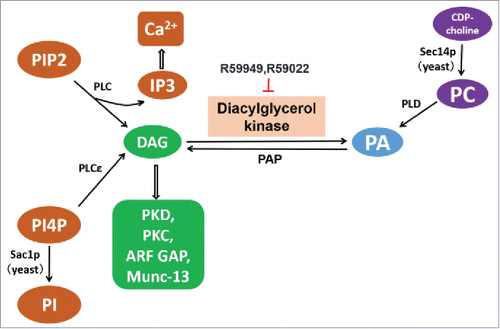
The acrosome is a secretory granule that is released from mammalian sperm and is essential for fertilization. Acrosomal secretion is a special type of regulated exocytosis, which uses conserved exocytic mechanisms also found in neuronal, endocrinal and other cells.Citation53 Upon inhibition of DGK in cells with the inhibitor R59022, impaired DGK activity leads to increased levels of DAG. Simultaneously, the level of PA correspondingly decreases in these cells. In this case, considerable stimulation of acrosomal exocytosis is observed.Citation53,54 Subsequent studies have identified DAG's role in stimulating acrosomal exocytosis through PKC and phospholipase D1 (PLD1) activation, promoting the continued production of PIP2 and subsequently, IP3 which is required for the intra-acrosomal calcium efflux during fusion with the plasma membrane of the spermatozoon (See for the metabolism of DAG and related phospholipids). However, PA alone could not trigger exocytosis.Citation55 Furthermore, it was demonstrated that DAG activates Rab3A leading to the assembly of SNARE complexes and membrane fusion via interaction with Munc-13.Citation55,56
Figure 2. Pathways involved in the metabolism of DAG and PA described in this review. DGK, diacylglycerol kinase. DAG, Diacylglycerol; PA, phosphatidic acid; PC, phosphatidylcholine; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3, inositol 1,4,5-trisphosphate; PLD, phospholipase D; PLC, phospholipase C; PI4P, Phosphatidylinositol 4-phosphate; PI, phosphatidylinositol; PKD, protein kinase D; PKC, protein kinase C; ARF GAP, ARF GTPase-activating protein. Solid arrows indicate the metabolic pathway, hollow arrows indicate the downstream effector activated by DAG or IP3.
PA regulation of the secretory pathway
Phospholipids may serve as an essential part of the machinery driving the fusion and/or fission of membranes, based on their shape and geometric features, and therefore may play a role in the budding and generation of secretory vesicles. Indeed, membrane domains enriched with acidic phospholipids, especially PA, are prone to membrane fusion, in conjunction with Ca2+.Citation57 However, the shape of PA varies greatly under different Ca2+ concentrations. Unsaturated PA has a cylindrical, bilayer-preferring structure under normal cytoplasmic conditions (37°C, pH 7.2, 0.5 mM free Mg2+); but at the mildly acidic intra-Golgi conditions (pH 5.9–6.6, 0.3 mM Ca2+), it displays a conical (type-II) shape,Citation49 which is prone to form a highly curved membrane facilitating the fission process.
Another documented role for PA in secretion relates to the induction of neutrophil exocytosis from azurophilic granules by anti-neutrophil cytoplasmic antibodies (ANCAs). This likely results from the pathogenesis of endothelial cell damage in small vessel vasculitis, with serine proteases and myeloperoxidase (MPO) released from the activated neutrophils. DGK-generated PA is required for such exocytic activity, since treatment with DGK inhibitors reduces granule release by inhibiting granule fusion at the plasma membrane.Citation58 In this study, the addition of PA restored the release of MPO in DGK-inhibited cells, whereas supplementing cells with DAG failed to restore exocytosis. These findings collectively lead to the suggestion that ANCA-driven granule exocytosis is mediated by DGK-generated PA. Importantly, PA generated by PLD is not involved in this process.Citation59,60 PA production is also correlated with the ANCA-induced neutrophil adhesion in vitro.Citation61
DGK regulates MVB formation and secretion
MVBs are formed by the inward invagination of the limiting membrane of endosomes, giving rise to intraluminal vesicles (ILVs). Although previously considered a mechanism of cargo sorting for lysosomal degradation, MVBs also fuse with the plasma membrane, secreting their ILVs into the extracellular area.Citation62 Studies show that DGK is involved in multiple processes related to exosome secretion, including the formation and maturation of Multi-Vesicular Bodies (MVBs) (i.e., the number of MVBs per cell and inward vesiculation of MVBs), the budding and release of exosomes from MVBs, and their fusion with the plasma membrane.Citation63-66 In cytotoxic T lymphocytes, MVBs are responsible for releasing the pro-apoptotic Fas ligand (FasL) at the immunological synapseCitation67 with DGKα playing an important regulatory role in this process.Citation63,66 Upon receptor stimulation, FasL and DGKα relocate to FasL-containing MVB structures. DGKα is recruited to MVBs and to exosomes, where it plays a dual role; DGKα kinase activity exerts a negative role in the formation of mature MVBs, as experiments show that treatment with a type I DGK inhibitor, R59949, induces the maturation of CD63-positive/lysobisphosphatidic acid-positive MVBs, and increases the secretion of exosomes.Citation63,68,69 In contrast, down regulation of DGKα inhibits polarized exosome secretion, and affects degranulation of MVBs at the immune synapse, while the kinase inhibitor increases polarized secretion of exosomes. These studies imply that DGKα kinase activity negatively regulates the formation of mature MVBs, while regions outside the kinase domain are required for polarization of MVBs and exosome secretion.Citation69
The role of DGK on secretion from the Golgi apparatus is mediated via the cellular levels of DAG
The Golgi apparatus is an organelle found in most eukaryotic cells, and it is responsible for the processing, sorting and transporting of proteins and lipids. The formation of Golgi-to-plasma membrane transport carriers is accomplished via budding, elongation, constriction, and finally fission of the Golgi membrane. These events are facilitated by lipid bilayer deformation as well as the concerted efforts of many proteins that act at the various stages of secretion.Citation70 Previous studies have revealed that DAG plays a dual role in the generation of transport carriers, and thus mediates secretion from the Golgi. It serves in lipid signaling on the trans-Golgi network (TGN) for the recruitment and activation of essential proteins onto the TGN membrane. Such proteins include protein kinase D (PKD),Citation44 protein kinase Cη (PKCη),Citation45,46 and the ARF GTPase-activating proteins (ARF GAPs) Gcs1p, Age1p and Age2pCitation71-73 (see for the downstream effectors of DAG). Reducing cellular DAG levels inhibited recruitment and blocked TGN-to-plasma membrane trafficking.Citation74 On the other hand, the conical shape of DAG in the outer leaflet provides negative membrane curvature, which is thought to facilitate membrane fission.Citation75 Given the importance of DAG levels on TGN-to-plasma membrane transport, the metabolic pathways for the production or consumption of DAG intricately regulate the secretory pathway.Citation76-78 However, the molecular mechanisms mediating DAG cellular levels during vesicular trafficking under physiological conditions are not well understood.
DGK rapidly reduces the level of DAG, resulting in the inhibition of TGN-to-plasma membrane transport, implicating the negative regulation of DGK on Golgi secretory pathways. Earlier studies established that DGKα translocates to the TGN upon receptor stimulation.Citation79 Furthermore, work in yeast has revealed that the conversion of DAG to PA by increased DGK expression significantly impairs Golgi function. Sec14p is a phosphatidylinositol (PI)-transfer protein that generates the DAG precursor, phosphatidylcholine (PC). PLD then converts PC into PA, which is dephosphorylated by phosphatidic acid phosphatase (PAP) into DAG (). Studies have demonstrated that Sec14p is important in maintaining a favorable lipid environment for TGN-to-plasma membrane trafficking,Citation80-82 and thus is essential for yeast viability and secretory competence. To date, a large class of loss-of-function mutations in nonessential genes have been identified, the “bypass Sec14p”mutations, that restore cell viability and Golgi secretion with defective Sec14p, indicating that such mutations occur in regulators of TGN transportation downstream of Sec14p. Among the “bypass Sec14p” mutations, Sac1p deficiency functions by inducing accumulation of phosphatidylinositol 4-phosphate (PI4P), a pro-secretory phospholipid in the Golgi. Indeed, yeast DGK expression compromises the ability of Sac1p deficiency to effect “bypass Sec14p,” suggesting DGK's negative role in TGN secretory pathway, possibly through reducing the level of PI4P.Citation83
It is worth noting that the regulation of the DAG pool is more tightly controlled by Sec14p-dependent PC-PA-DAG conversion than through phosphorylation in the DAG-to-PA conversion pathway.Citation84 Currently, there is incomplete agreement over the involvement of DGK-generated PA in regulating secretion from the Golgi. On one hand, since PA is a direct product of DGK's activity on DAG and the up-regulation of DAG at the Golgi does not lead to a concomitant PA level increase, this implies that DGK might not be involved in this mechanism,Citation85 or that PA is rapidly transformed into other phospholipids. On the other hand, a study shows that DGK activity on PA production, rather than the consumption of DAG, regulates nascent vesicle secretion from the TGN.Citation86
Studies also show that the level of DAG at the Golgi mediates retrograde transport (Golgi-to-ER), while anterograde transport (ER-to-Golgi) is insensitive to DAG. PA phosphatase mediated DAG production is required for the formation of COPI vesicles and Golgi-to-ER transport.Citation74,87-89 Interestingly, the level of PA generated via DGK-mediated phosphorylation of DAG affects anterograde transport, which will be described below.
Anterograde transport is mediated by local PA levels
A lipid micro-domain containing interconverting LPA, PA and diacylglycerol has the potential to drive membrane fission through changes in membrane deformation.Citation49 Several proteins have been identified that may induce fission at the Golgi apparatus: CtBP3/BARS and endophilin facilitate the conversion of lysophosphatidic acid (LPA) to PA,Citation90,91 PLD mediates generation of PA,Citation92,93 and protein kinase D binds to DAG.Citation94 Although no direct evidence shows that DGK is involved in the regulation of membrane fission, since PA is required for the fission of Golgi structures, it is possible that membrane fission and fusion events may require the DGK-generated PA, potentially implicating DGK as a major player in mediating vesicle trafficking.
Although there are 10 isoforms in the DGK family, only DGKα has been localized to the TGN,Citation63 and a portion of cytosolic DGKδ localizes to the ER via its SAM domain.Citation23 DGKδ expression blocks the formation of COPII-coated structures in the ER and slows ER-to-Golgi transport of Vesicular Stomatitis Virus G Glycoprotein (VSV-G), indicating that the anterograde transport is inhibited by DGKδ. On the other hand, COPI structures are unaffected,Citation23 suggesting that retrograde transport is independent of DGKδ. Apparently, lipid conversion is not required for anterograde transport, since the overexpression of a DGKδ kinase-dead mutant led to similar inhibition of ER-to-Golgi transport. As described above, the level of DAG affects the formation of COPI endosomes and retrograde transport (Golgi-to-ER), but not anterograde transport (ER-to-Golgi).Citation74,89 It is possible that the effect of DGK on anterograde transport from the ER to the Golgi is mediated through the production of PA, rather than DAG.
Others
There are some indications that DGK mediates the release of neurotransmitters, possibly through the recruitment of munc-13.Citation95 Studies on DGKι knock-out mice show that it is involved in presynaptic glutamate release during 3,5-dihydroxyphenylglycine (DHPG)-induced long-term potentiation.Citation96 DGK1 knockout (the homolog of DGKθ) in Caenorhabditis elegans, led to an increase in acetylcholine release,Citation97 suggesting that DGK negatively regulates synaptic transmission. However, the molecular mechanism underlying this role of DGK has yet to be established.
Endocytic Recycling
Role of DAG in recycling
DGK affects the DAG/Ras/ERK signaling pathway, either through regulating the DAG levels or directly by mediating the activity of downstream proteins such as PKC, and/or Munc-13.Citation33,98-101 In either case, PKC serves as an important downstream signaling protein involved in multiple trafficking processes. Classical and novel PKC bind to DAG.Citation102,103 PKCα localizes to transferrin-positive recycling endosomes in the peri-nuclear area, and PKCα stimulation by DAG/ phorbol myristate acetate (PMA) accelerates the recycling of transferrin to the plasma membrane,Citation104,105 while the sorting of LDLR to the lysosome remains unaffected.Citation105 Furthermore, perturbation of this DAG gradient through the inhibition of DGK impaired both dynein recruitment and microtubule organizing center (MTOC) polarization.Citation64
Role of PA in recycling
Endocytic recycling is mediated by local PA levels
DGKα-derived PA binds and recruits the Rab-coupling protein (RCP) to the tips of invasive pseudopods. Since RCP is required for the recycling of α5β1 integrin during cell migration,Citation106 DGKα is essential for RCP to drive the recycling of α5β1 integrin.Citation50
Another effect of DGK-derived PA in endocytic recycling lies in the regulation of tubular recycling endosomes (TRE) that are decorated by Molecules Interacting with CAsL-Like1 (MICAL-L1). MICAL-L1 and Syndapin2 promote the biogenesis of TRE (thus regulating TRE function), and they are both recruited to the TRE membrane through direct interactions with PA.Citation51 DGKα-derived PA mediates the recycling of Major Histocompatibility Complex Class I (MHC I) without affecting its internalization. In addition, the MICAL-L1-decorated TRE are disrupted upon DGKα-depletion, leading to general defects in endocytic recycling.Citation107When the compromised synthesis of PA by DGKα-knock-down is preserved by preventing it catabolism, with the PA phosphatase inhibitor propranolol, the loss of TRE is reversed. This indicates that DGKα regulates endocytic recycling through the level of PA.
PA as a binding partner for SNX27
Sorting nexin 27 (SNX27), a member of the SNX family of proteins involved in intracellular sorting and traffickingCitation108,109 interacts with DGKζ. This interaction is required for the localization of SNX27 to the sorting endosomes in Jurkat T cells. DGKζ-siRNA accelerated the recycling of transferrin receptor from the endocytic recycling compartment to the plasma membrane in T lymphocytes.Citation52 Although the reason why DGKζ–knock-down in T lymphocytes has such a dramatically different impact than DGKα-knock-down in other cell types remains unknown, it is possible that compensation by other DGKs and/or other PA-generating pathways in T cells is more robust.
Endocytosis
Dynamin is a key regulator of membrane constriction and fission during endocytosis that binds to anionic lipids (including PA) through its Pleckstrin Homology (PH) domain. The presence of PA increases dynamin's enzymatic activity, and induces its deep penetration into the membrane.Citation110 Moreover, in experiments where liposomes of different lipid components were co-incubated with dynamin, PA-containing liposomes had the most efficient dynamin-coated tubule formation.Citation111
Genome-wide short interfering RNA screening analysis was performed to assess the involvement of different human kinases on endocytosis, measuring the rate of VSV-G entry via clathrin-mediated endocytosis (CME) and Simian virus 40 (SV40) internalization via clathrin-independent endocytosis (CIE).Citation112 In this study it was predicted that DGKβ negatively influences CIE while DGKγ positively mediates CIE, and DGKδ seems to have a dual effect promoting CIE and inhibiting CME.
By inhibiting type I DGK, total cellular PA levels decreased. Correspondingly, the internalization of epidermal growth factor (EGF) was significantly impaired. However, the uptake of transferrin remained unaffected, suggesting that EGF internalization depends on DGK activity, whereas the uptake of transferrin is independent of the kinase activity. Moreover, upon inhibition of type I DGK, fewer clathrin-coated pits (CCP) formed, indicating a role for DGK activity in CCP formation.Citation113
In addition to its kinase activity regulating DAG-PA conversion, DGKs also serve as scaffolding proteins that recruit regulatory proteins required for endocytosis. For example, during clathrin-dependent endocytosis, CCP are formed with the assistance of clathrin and the Adaptor Protein 2 (AP-2) complex. DGKδ co-localizes with these CCP through its interaction with AP-2 via F369DTFRIL and D746PF sequences in the catalytic domain. Furthermore, the uptake of both transferrin and EGF were significantly reduced in the absence of DGKδ. Importantly, the kinase activity is also required for the endocytic process, as the kinase-dead mutant could not reverse the impaired uptake observed upon DGKδ knock-down.Citation114 This is probably due to the regulatory effect of PA on CME. Although DAG stimulates the internalization of transferrin in some organisms such as Trypanosomatids brucei, in human cells DAG levels do not influence transferrin endocytosis.Citation115 These studies suggest that DGK mediates clathrin-dependent endocytosis either through kinase activity leading to PA production, or by serving as a scaffold protein that recruits AP-2 for CCP formation.
Phagocytosis and macropinocytosis are processes essential for innate immunity and tissue homeostasis, during which cells such as macrophages ingest particulate (phagocytosis) or soluble (macropinocytosis) pathogens into membrane-bound vacuoles. The molecular mechanisms mediating phagocytosis include protein tyrosine kinases, GTP-binding proteins, PKC, actin polymerization and membrane movement.Citation116 Phosphoinositide metabolism serves as critical regulation of the initiation of both processes. A local phagosomal DAG accumulation is observed by biochemical means during particle ingestion.Citation117 The phosphorylation by DGK is a critical determinant of DAG at the phagocytic sites.Citation118 DGK inhibition by R59002 or R59949 increases the DAG-positive phagosomes, and enhances reactive oxygen species (ROS) generation by these phagosomes,Citation118,119 indicating that DGK terminates the DAG signaling mediating phagosomal ROS production. On the other hand, PA is detected at the plasma membrane in phagocytes. Both DGK and PLD are responsible for the PA production at phagocytic sites.Citation120,121 Among the 10 isoforms of DGK, DGKβ, DGKγ, and DGKζ are found in the plasma membrane of macrophages, suggesting that multiple DGK isoforms are involved in the regulation of macropinocytosis. The abundance of PA in the plasma membrane correlates with membrane ruffling; accordingly, DGK inhibitor R59002 treatment depresses both the rate and extent of ruffle formation. Upon DGK inhibition, macropinosome formation and dextran internalization are impaired.Citation120 In addition to regulating the DAG-PA equilibrium, DGKζ plays a crucial role in phagocytosis and macropinocytosis via the activation of the Rho-GTPase family protein, Rac1.Citation122,123
Summary and Conclusion
In addition to the important role DGKs play in cell signaling and immune activity, their effect on endocytic trafficking should not be underestimated. By catalyzing the conversion of DAG to PA, DGKs regulate different stages of endocytic trafficking, including the transport from/to the Golgi apparatus, the formation and secretion of MVBs, endocytosis, and the biosynthesis of recycling endosomes. There are 10 mammalian DGK isoforms, each with different regulatory domains, substrate specificities, and tissue/subcellular distribution, resulting in differential regulation of membrane trafficking. The existence of multiple isoforms suggests that there may be different DGKs at distinct subcellular structures, and that there is localized regulation of DAG/PA levels mediating endocytic trafficking. However, lipid metabolism is a dynamic, bi-directional processes, and DGKs may need to cooperate with other lipid modifiers to create and/or maintain an optimal membrane environment for trafficking. It is also possible that DGKs function in a kinase-independent manner. One example is that DGKδ facilitates CME through its binding to AP-2, but not through its kinase activity in regulating DAG or PA levels.
By mediating membrane trafficking, DGKs control cell morphology, migration, apoptosis, protein biosynthesis, and receptor activation. Although there is no established pathology caused by a malfunctioning DGK isoform, knock-out mouse models reveal significant pathological consequences, including insulin insensitivity, immune function abnormalities and brain disorders, suggesting a potential role for DGKs as therapeutic targets in disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Badola P, Sanders CR, 2nd, Escherichia coli diacylglycerol kinase is an evolutionarily optimized membrane enzyme and catalyzes direct phosphoryl transfer. J Biol Chem 1997; 272(39):24176-82; PMID:9305868; http://dx.doi.org/10.1074/jbc.272.39.24176
- Gomez-Merino FC, Brearley CA, Ornatowska M, Abdel-Haliem ME, Zanor MI, Mueller-Roeber B, et al. AtDGK2, a novel diacylglycerol kinase from Arabidopsis thaliana, phosphorylates 1-stearoyl-2-arachidonoyl-sn-glycerol and 1,2-dioleoyl-sn-glycerol and exhibits cold-inducible gene expression. J Biol Chem 2004; 279(9):8230-41; PMID:14665624; http://dx.doi.org/10.1074/jbc.M312187200
- Harden N, Yap SF, Chiam MA, Lim L, et al. A Drosophila gene encoding a protein with similarity to diacylglycerol kinase is expressed in specific neurons. Biochem J 1993; 289(Pt 2):439-44; PMID:8380995
- Jose AM, Koelle MR. Domains, amino acid residues, and new isoforms of Caenorhabditis elegans diacylglycerol kinase 1 (DGK-1) important for terminating diacylglycerol signaling in vivo. J Biol Chem 2005; 280(4):2730-6; PMID:15563467; http://dx.doi.org/10.1074/jbc.M409460200
- Arisz SA, Munnik T. Diacylglycerol kinase, in Lipid signaling in plants. 2010, Springer. 107-114.
- Topham MK, Prescott SM. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem 1999; 274(17):11447-50; PMID:10206945; http://dx.doi.org/10.1074/jbc.274.17.11447
- Masai I, Hosoya T, Kojima S, Hotta Y, et al. Molecular cloning of a Drosophila diacylglycerol kinase gene that is expressed in the nervous system and muscle. Proc Natl Acad Sci U S A 1992; 89(13):6030-4; PMID:1321433; http://dx.doi.org/10.1073/pnas.89.13.6030
- Arisz SA, Testerink C, Munnik T. Plant PA signaling via diacylglycerol kinase. Biochim Biophys Acta 2009; 1791(9):869-75; PMID:19394438; http://dx.doi.org/10.1016/j.bbalip.2009.04.006
- Han GS, O'Hara L, Siniossoglou S, Carman GM, et al. Characterization of the yeast DGK1-encoded CTP-dependent diacylglycerol kinase. J Biol Chem 2008; 283(29):20443-53; PMID:18458076; http://dx.doi.org/10.1074/jbc.M802866200
- Sakane F, Imai S, Kai M, Wada I, Kanoh H, et al. Molecular cloning of a novel diacylglycerol kinase isozyme with a pleckstrin homology domain and a C-terminal tail similar to those of the EPH family of protein-tyrosine kinases. J Biol Chem 1996; 271(14):8394-401; PMID:8626538; http://dx.doi.org/10.1074/jbc.271.14.8394
- Klauck TM, Xu X, Mousseau B, Jaken S, et al. Cloning and characterization of a glucocorticoid-induced diacylglycerol kinase. J Biol Chem 1996; 271(33):19781-8; PMID:8702685; http://dx.doi.org/10.1074/jbc.271.33.19781
- Goto K, Kondo H. Molecular cloning and expression of a 90-kDa diacylglycerol kinase that predominantly localizes in neurons. Proc Natl Acad Sci U S A 1993; 90(16):7598-602; PMID:7689223; http://dx.doi.org/10.1073/pnas.90.16.7598
- Kai M, Sakane F, Imai S, Wada I, Kanoh H, et al. Molecular cloning of a diacylglycerol kinase isozyme predominantly expressed in human retina with a truncated and inactive enzyme expression in most other human cells. J Biol Chem 1994; 269(28):18492-8; PMID:8034597
- Tang W, Bunting M, Zimmerman GA, McIntyre TM, Prescott SM et al. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J Biol Chem 1996; 271(17):10237-41; PMID:8626589; http://dx.doi.org/10.1074/jbc.271.17.10230
- Bunting M, Tang W, Zimmerman GA, McIntyre TM, Prescott SM et al. Molecular cloning and characterization of a novel human diacylglycerol kinase zeta. J Biol Chem 1996; 271(17):10230-6; PMID:8626588; http://dx.doi.org/10.1074/jbc.271.17.10237
- Ding L, Traer E, McIntyre TM, Zimmerman GA, Prescott SM et al. The cloning and characterization of a novel human diacylglycerol kinase, DGKiota. J Biol Chem 1998; 273(49):32746-52; PMID:9830018; http://dx.doi.org/10.1074/jbc.273.49.32746
- Houssa B, Schaap D, van der Wal J, Goto K, Kondo H, Yamakawa A, Shibata M, Takenawa T, van Blitterswijk WJ et al. Cloning of a novel human diacylglycerol kinase (DGKtheta) containing three cysteine-rich domains, a proline-rich region, and a pleckstrin homology domain with an overlapping Ras-associating domain. J Biol Chem 1997; 272(16):10422-8; PMID:9099683; http://dx.doi.org/10.1074/jbc.272.16.10422
- Sakane F, Yamada K, Kanoh H, Yokoyama C, Tanabe T et al. Porcine diacylglycerol kinase sequence has zinc finger and E-F hand motifs. Nature 1990; 344(6264):345-8; PMID:2156169; http://dx.doi.org/10.1038/344345a0
- Imai S, Kai M, Yasuda S, Kanoh H, Sakane F et al. Identification and characterization of a novel human type II diacylglycerol kinase, DGK kappa. J Biol Chem 2005; 280(48):39870-81; PMID:16210324; http://dx.doi.org/10.1074/jbc.M500669200
- Hurley JH, Newton AC, Parker PJ, Blumberg PM, Nishizuka Y et al. Taxonomy and function of C1 protein kinase C homology domains. Protein Sci 1997; 6(2):477-80; PMID:9041654; http://dx.doi.org/10.1002/pro.5560060228
- Yamada K, Sakane F, Matsushima N, Kanoh H et al. EF-hand motifs of α, β and gamma isoforms of diacylglycerol kinase bind calcium with different affinities and conformational changes. Biochem J 1997; 321(Pt 1):59-64; PMID:9003401
- Sanjuan MA, Jones DR, Izquierdo M, Mérida I et al. Role of diacylglycerol kinase α in the attenuation of receptor signaling. J Cell Biol 2001; 153(1):207-20; PMID:11285286; http://dx.doi.org/10.1083/jcb.153.1.207
- Nagaya H, Wada I, Jia YJ, Kanoh H et al. Diacylglycerol kinase delta suppresses ER-to-Golgi traffic via its SAM and PH domains. Mol Biol Cell 2002; 13(1):302-16; PMID:11809841; http://dx.doi.org/10.1091/mbc.01-05-0255
- Shulga YV, Topham MK, Epand RM. Substrate specificity of diacylglycerol kinase-epsilon and the phosphatidylinositol cycle. FEBS Lett 2011; 585(24):4025-8; PMID:22108654; http://dx.doi.org/10.1016/j.febslet.2011.11.016
- Jennings W, Doshi S, D'Souza K, Epand RM et al. Molecular properties of diacylglycerol kinase-epsilon in relation to function. Chem Phys Lipids 2015; PMID:26134136
- Topham MK, Bunting M, Zimmerman GA, McIntyre TM, Blackshear PJ, Prescott SM et al. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature 1998; 394(6694):697-700; PMID:9716136; http://dx.doi.org/10.1038/29337
- Hogan A, Shepherd L, Chabot J, Quenneville S, Prescott SM, Topham MK, Gee SH et al. Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta. Regulation of nuclear localization by PDZ interactions. J Biol Chem 2001; 276(28):26526-33; PMID:11352924; http://dx.doi.org/10.1074/jbc.M104156200
- Shirai Y, Kouzuki T, Kakefuda K, Moriguchi S, Oyagi A, Horie K, Morita SY, Shimazawa M, Fukunaga K, Takeda J et al. Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKbeta in neurite spine formation, contributing to cognitive function. PLoS One 2010; 5(7):e11602; PMID:20657643; http://dx.doi.org/10.1371/journal.pone.0011602
- Sakai H, Sakane F. Recent progress on type II diacylglycerol kinases: the physiological functions of diacylglycerol kinase delta, eta and kappa and their involvement in disease. J Biochem 2012; 152(5):397-406; PMID:22984004; http://dx.doi.org/10.1093/jb/mvs104
- Chibalin AV, Leng Y, Vieira E, Krook A, Björnholm M, Long YC, Kotova O, Zhong Z, Sakane F, Steiler T et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 2008; 132(3):375-86; PMID:18267070; http://dx.doi.org/10.1016/j.cell.2007.12.035
- Goto K, Kondo H. A 104-kDa diacylglycerol kinase containing ankyrin-like repeats localizes in the cell nucleus. Proc Natl Acad Sci U S A 1996; 93(20):11196-201; PMID:8855332; http://dx.doi.org/; http://dx.doi.org/10.1073/pnas.93.20.11196
- Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF et al. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-α. Nat Immunol 2006; 7(11):1166-73; PMID:17028589; http://dx.doi.org/10.1038/ni1394
- Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA et al., Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol 2003; 4(9):882-90; PMID:12883552; http://dx.doi.org/10.1038/ni958
- Kakefuda K, Oyagi A, Ishisaka M, Tsuruma K, Shimazawa M, Yokota K, Shirai Y, Horie K, Saito N, Takeda J et al. Diacylglycerol kinase β knockout mice exhibit lithium-sensitive behavioral abnormalities. PLoS One 2010; 5(10):e13447; PMID:20976192; http://dx.doi.org/10.1371/journal.pone.0013447
- Mukherjee S, Zeitouni S, Cavarsan CF, Shapiro LA et al. Increased seizure susceptibility in mice 30 days after fluid percussion injury. Front Neurol 2013; 4:28; PMID:23519723; http://dx.doi.org/10.3389/fneur.2013.00028
- Rodriguez de Turco EB, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, Taketomi A, Prescott SM, Bazan NG et al. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl- inositol lipid signaling. Proc Natl Acad Sci U S A 2001; 98(8):4740-5; PMID:11287665; http://dx.doi.org/10.1073/pnas.081536298
- Besterman JM, Pollenz RS, Booker EL Jr, Cuatrecasas P et al. Diacylglycerol-induced translocation of diacylglycerol kinase: use of affinity-purified enzyme in a reconstitution system. Proc Natl Acad Sci U S A 1986; 83(24):9378-82; PMID:3025839; http://dx.doi.org/10.1073/pnas.83.24.9378
- Cipres A, Carrasco S, Merino E, Díaz E, Krishna UM, Falck JR, Martínez AC, Mérida I et al. Regulation of diacylglycerol kinase α by phosphoinositide 3-kinase lipid products. J Biol Chem 2003; 278(37):35629-35; PMID:12832407; http://dx.doi.org/10.1074/jbc.M305635200
- Cutrupi S, Baldanzi G, Gramaglia D, Maffè A, Schaap D, Giraudo E, van Blitterswijk W, Bussolino F, Comoglio PM, Graziani A et al. Src-mediated activation of α-diacylglycerol kinase is required for hepatocyte growth factor-induced cell motility. EMBO J 2000; 19(17):4614-22; PMID:10970854; http://dx.doi.org/10.1093/emboj/19.17.4614
- Yamaguchi Y, Shirai Y, Matsubara T, Sanse K, Kuriyama M, Oshiro N, Yoshino K, Yonezawa K, Ono Y, Saito N et al. Phosphorylation and upregulation of diacylglycerol kinase gamma via its interaction with protein kinase C gamma. J Biol Chem 2006; 281(42):31627-37; PMID:16905533; http://dx.doi.org/10.1074/jbc.M606992200
- Flores I, Casaseca T, Martinez A C, Kanoh H, Merida I et al. Phosphatidic acid generation through interleukin 2 (IL-2)-induced α-diacylglycerol kinase activation is an essential step in IL-2-mediated lymphocyte proliferation. J Biol Chem 1996; 271(17):10334-40; PMID:8626603; http://dx.doi.org/10.1074/jbc.271.17.10334
- Schaap D, van der Wal J, van Blitterswijk WJ, van der Bend RL, Ploegh HL et al. Diacylglycerol kinase is phosphorylated in vivo upon stimulation of the epidermal growth factor receptor and serine/threonine kinases, including protein kinase C-epsilon. Biochem J 1993; 289(Pt 3):875-81; PMID:7679574
- Chianale F, Rainero E, Cianflone C, Bettio V, Pighini A, Porporato PE, Filigheddu N, Serini G, Sinigaglia F, Baldanzi G et al. Diacylglycerol kinase α mediates HGF-induced Rac activation and membrane ruffling by regulating atypical PKC and RhoGDI. Proc Natl Acad Sci U S A 2010; 107(9): 4182-7; PMID:20160093; http://dx.doi.org/10.1073/pnas.0908326107
- Maeda Y, Beznoussenko GV, Van Lint J, Mironov AA, Malhotra V et al. Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. EMBO J 2001; 20(21):5982-90; PMID:11689438; http://dx.doi.org/10.1093/emboj/20.21.5982
- Diaz Anel AM, Malhotra V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol 2005; 169(1):83-91; PMID:15824133; http://dx.doi.org/10.1083/jcb.200412089
- Simon JP, Ivanov IE, Adesnik M, Sabatini DD et al. The production of post-Golgi vesicles requires a protein kinase C-like molecule, but not its phosphorylating activity. J Cell Biol 1996; 135(2):355-70; PMID:8896594; http://dx.doi.org/10.1083/jcb.135.2.355
- Vicinanza M, D'Angelo G, Di Campli A, De Matteis MA et al. Function and dysfunction of the PtdIns system in membrane trafficking. EMBO J 2008; 27(19):2457-70; PMID:18784754; http://dx.doi.org/10.1038/emboj.2008.169
- Clapham DE. Calcium signaling. Cell 2007; 131(6):1047-58; PMID:18083096; http://dx.doi.org/10.1016/j.cell.2007.11.028
- Kooijman EE, Chupin V, de Kruijff B, Burger KN et al. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic 2003; 4(3):162-74; PMID:12656989; http://dx.doi.org/10.1034/j.1600-0854.2003.00086.x
- Rainero E, Caswell PT, Muller PA, Grindlay J, McCaffrey MW, Zhang Q, Wakelam MJ, Vousden KH, Graziani A, Norman JC et al. Diacylglycerol kinase α controls RCP-dependent integrin trafficking to promote invasive migration. J Cell Biol 2012; 196(2):277-95; PMID:22270919; http://dx.doi.org/10.1083/jcb.201109112
- Giridharan SS, Cai B, Vitale N, Naslavsky N, Caplan S et al. Cooperation of MICAL-L1, syndapin2, and phosphatidic acid in tubular recycling endosome biogenesis. Mol Biol Cell 2013; 24(11):1776-90, S1-15; PMID:23596323; http://dx.doi.org/10.1091/mbc.E13-01-0026
- Rincon E, Santos T, Avila-Flores A, Albar JP, Lalioti V, Lei C, Hong W, Mérida I et al. Proteomics identification of sorting nexin 27 as a diacylglycerol kinase zeta-associated protein: new diacylglycerol kinase roles in endocytic recycling. Mol Cell Proteomics 2007; 6(6):1073-87; PMID:17351151; http://dx.doi.org/10.1074/mcp.M700047-MCP200
- Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life 2007; 59(4-5):286-92; PMID:17505967; http://dx.doi.org/10.1080/15216540701222872
- Roldan ER, Harrison RA. The role of diacylglycerol in the exocytosis of the sperm acrosome. Studies using diacylglycerol lipase and diacylglycerol kinase inhibitors and exogenous diacylglycerols. Biochem J 1992; 281(Pt 3):767-73; PMID:1311174
- Lopez CI, Pelletán LE, Suhaiman L, De Blas GA, Vitale N, Mayorga LS, Belmonte SA et al. Diacylglycerol stimulates acrosomal exocytosis by feeding into a PKC- and PLD1-dependent positive loop that continuously supplies phosphatidylinositol 4,5-bisphosphate. Biochim Biophys Acta 2012; 1821(9):1186-99; PMID:; PMID:22609963; http://dx.doi.org/10.1016/j.bbalip.2012.05.001
- Huang CC, Yang DM, Lin CC, Kao LS et al. Involvement of Rab3A in vesicle priming during exocytosis: interaction with Munc13-1 and Munc18-1. Traffic 2011; 12(10):1356-70; PMID:21689256; http://dx.doi.org/10.1111/j.1600-0854.2011.01237.x
- Burger KN. Greasing membrane fusion and fission machineries. Traffic 2000; 1(8):605-13; PMID:11208148; http://dx.doi.org/10.1034/j.1600-0854.2000.010804.x
- Holden NJ, Savage CO, Young SP, Wakelam MJ, Harper L, Williams JM et al. A dual role for diacylglycerol kinase generated phosphatidic acid in autoantibody-induced neutrophil exocytosis. Mol Med 2011; 17(11-12):1242-52; PMID:21833457; http://dx.doi.org/10.2119/molmed.2011.00028
- Siddiqui RA, English D. Phosphatidic acid binding to human neutrophils: effects on tyrosine kinase-regulated intracellular Ca2+ mobilization. Cell Signal 1996; 8(5):349-54; PMID:8911683; http://dx.doi.org/10.1016/0898-6568(96)00072-1
- Dickey A, Faller R. Examining the contributions of lipid shape and headgroup charge on bilayer behavior. Biophys J 2008; 95(6):2636-46; PMID:18515396; http://dx.doi.org/10.1529/biophysj.107.128074
- Williams JM, Pettitt TR, Powell W, Grove J, Savage CO, Wakelam MJ et al. Antineutrophil cytoplasm antibody-stimulated neutrophil adhesion depends on diacylglycerol kinase-catalyzed phosphatidic acid formation. J Am Soc Nephrol 2007; 18(4):1112-20; PMID:17360949; http://dx.doi.org/10.1681/ASN.2006090973
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 2012; 28:337-62; PMID:22831642; http://dx.doi.org/10.1146/annurev-cellbio-092910-154152
- Alonso R, Rodríguez MC, Pindado J, Merino E, Mérida I, Izquierdo M et al. Diacylglycerol kinase α regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J Biol Chem 2005; 280(31):28439-50; PMID:15870081 http://dx.doi.org/10.1074/jbc.M501112200
- Quann EJ, Merino E, Furuta T, Huse M et al. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol 2009; 10(6):627-35; PMID:19430478; http://dx.doi.org/10.1038/ni.1734
- Izquierdo M, Ruiz-Ruiz MC, Lopez-Rivas A. Stimulation of phosphatidylinositol turnover is a key event for Fas-dependent, activation-induced apoptosis in human T lymphocytes. J Immunol 1996; 157(1):21-8; PMID:8683117
- Alonso R, Mazzeo C, Mérida I, Izquierdo M et al. A new role of diacylglycerol kinase α on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Biochimie 2007; 89(2):213-21; PMID:16989932; http://dx.doi.org/10.1016/j.biochi.2006.07.018
- Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med 1999; 5(1):90-6; PMID:9883845; http://dx.doi.org/10.1038/4779
- Jiang Y, Sakane F, Kanoh H, Walsh JP et al. Selectivity of the diacylglycerol kinase inhibitor 3-[2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl]-2, 3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochem Pharmacol 2000; 59(7):763-72; PMID:10718334; http://dx.doi.org/10.1016/S0006-2952(99)00395-0
- Alonso R, Mazzeo C, Rodriguez MC, Marsh M, Fraile-Ramos A, Calvo V, Avila-Flores A, Merida I, Izquierdo M et al. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ 2011; 18(7):1161-73; PMID:21252909; http://dx.doi.org/10.1038/cdd.2010.184
- Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol 2006; 22:439-55; PMID:16824007; http://dx.doi.org/10.1146/annurev.cellbio.21.012704.133126
- De Matteis MA, Santini G, Kahn RA, Di Tullio G, Luini A et al. Receptor and protein kinase C-mediated regulation of ARF binding to the Golgi complex. Nature 1993; 364(6440):818-21; PMID:7689177; http://dx.doi.org/10.1038/364818a0
- Benjamin JJ, Poon PP, Lewis SM, Auger A, Wong TA, Singer RA, Johnston GC et al. The yeast Arf GTPase-activating protein Age1 is regulated by phospholipase D for post-Golgi vesicular transport. J Biol Chem 2011; 286(7):5187-96; PMID:21135091; http://dx.doi.org/10.1074/jbc.M110.185108
- Poon PP, Nothwehr SF, Singer RA, Johnston GC et al. The Gcs1 and Age2 ArfGAP proteins provide overlapping essential function for transport from the yeast trans-Golgi network. J Cell Biol 2001; 155(7):1239-50; PMID:11756474; http://dx.doi.org/10.1083/jcb.200108075
- Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 2002; 295(5553):325-8; PMID:11729268; http://dx.doi.org/10.1126/science.1066759
- Shemesh T, Luini A, Malhotra V, Burger KN, Kozlov MM et al. Prefission constriction of Golgi tubular carriers driven by local lipid metabolism: a theoretical model. Biophys J 2003; 85(6):3813-27; PMID:14645071; http://dx.doi.org/10.1016/S0006-3495(03)74796-1
- McMaster CR. Lipid metabolism and vesicle trafficking: more than just greasing the transport machinery. Biochem Cell Biol 2001; 79(6):681-92; PMID:11800009; http://dx.doi.org/10.1139/o01-139
- Roth MG. Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol 1999; 9(5):174-9; PMID:10322451; http://dx.doi.org/10.1016/S0962-8924(99)01535-4
- Huijbregts RP, Topalof L, Bankaitis VA. Lipid metabolism and regulation of membrane trafficking. Traffic 2000; 1(3):195-202; PMID:11208102; http://dx.doi.org/10.1034/j.1600-0854.2000.010301.x
- Shirai Y, Segawa S, Kuriyama M, Goto K, Sakai N, Saito N et al. Subtype-specific translocation of diacylglycerol kinase α and gamma and its correlation with protein kinase C. J Biol Chem 2000; 275(32):24760-6; PMID:10827086; http://dx.doi.org/10.1074/jbc.M003151200
- McGee TP, Skinner HB, Whitters EA, Henry SA, Bankaitis VA et al. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol 1994; 124(3):273-87; PMID:8294512; http://dx.doi.org/10.1083/jcb.124.3.273
- Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA et al. A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J Biol Chem 1998; 273(27):16635-8; PMID:9642212; http://dx.doi.org/10.1074/jbc.273.27.16635
- Mousley CJ, Tyeryar KR, Vincent-Pope P, Bankaitis VA et al. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim Biophys Acta 2007; 1771(6):727-36; PMID:17512778; http://dx.doi.org/10.1016/j.bbalip.2007.04.002
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA et al. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature 1997; 387(6628):101-5; PMID:9139830; http://dx.doi.org/10.1038/387101a0
- Johansen J, Ramanathan V, Beh CT. Vesicle trafficking from a lipid perspective: Lipid regulation of exocytosis in Saccharomyces cerevisiae. Cell Logist 2012; 2(3):151-160; PMID:23181198; http://dx.doi.org/10.4161/cl.20490
- Sarri E, Sicart A, Lázaro-Diéguez F, Egea G et al. Phospholipid synthesis participates in the regulation of diacylglycerol required for membrane trafficking at the Golgi complex. J Biol Chem 2011; 286(32):28632-43; PMID:21700701; http://dx.doi.org/10.1074/jbc.M111.267534
- Siddhanta A, Shields D. Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels. J Biol Chem 1998; 273(29):17995-8; PMID:9660750; http://dx.doi.org/10.1074/jbc.273.29.17995
- Asp L, Kartberg F, Fernandez-Rodriguez J, Smedh M, Elsner M, Laporte F, Bárcena M, Jansen KA, Valentijn JA, Koster AJ et al. Early stages of Golgi vesicle and tubule formation require diacylglycerol. Mol Biol Cell 2009; 20(3):780-90; PMID:19037109; http://dx.doi.org/10.1091/mbc.E08-03-0256
- Gutierrez-Martinez E, Fernández-Ulibarri I, Lázaro-Diéguez F, Johannes L, Pyne S, Sarri E, Egea G et al. Lipid phosphate phosphatase 3 participates in transport carrier formation and protein trafficking in the early secretory pathway. J Cell Sci 2013; 126(Pt 12):2641-55; PMID:23591818; http://dx.doi.org/10.1242/jcs.117705
- Fernandez-Ulibarri I, Vilella M, Lázaro-Diéguez F, Sarri E, Martínez SE, Jiménez N, Claro E, Mérida I, Burger KN, Egea G et al. Diacylglycerol is required for the formation of COPI vesicles in the Golgi-to-ER transport pathway. Mol Biol Cell 2007; 18(9):3250-63; PMID:17567948; http://dx.doi.org/10.1091/mbc.E07-04-0334
- Weigert R, Silletta MG, Span∫ S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature 1999; 402(6760):429-33; PMID:10586885; http://dx.doi.org/10.1038/46587
- Schmidt A, Wolde M, Thiele C, Fest W, Kratzin H, Podtelejnikov AV, Witke W, Huttner WB, Söling HD et al. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature 1999; 401(6749):133-41; PMID:10490020; http://dx.doi.org/10.1038/43613
- Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung TC, Frohman MA, Morris AJ, Shields D et al. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol 1997; 138(3): 495-504; PMID:9245781; http://dx.doi.org/10.1083/jcb.138.3.495
- Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG et al. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol 1996; 134(2):295-306; PMID:8707816; http://dx.doi.org/10.1083/jcb.134.2.295
- Bossard C, Bresson D, Polishchuk RS, Malhotra V et al. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J Cell Biol 2007; 179(6):1123-31; PMID:18086912; http://dx.doi.org/10.1083/jcb.200703166
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N et al. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 1998; 21(1):123-36; PMID:9697857; http://dx.doi.org/10.1016/S0896-6273(00)80520-6
- Yang J, Seo J, Nair R, Han S, Jang S, Kim K, Han K, Paik SK, Choi J, Lee S, et al. DGKiota regulates presynaptic release during mGluR-dependent LTD. EMBO J 2011; 30(1):165-80; PMID:21119615; http://dx.doi.org/10.1038/emboj.2010.286
- Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron 1999; 24(2):323-33; PMID:10571227; http://dx.doi.org/10.1016/S0896-6273(00)80847-8
- Crotty T, Cai J, Sakane F, Taketomi A, Prescott SM, Topham MK et al. Diacylglycerol kinase delta regulates protein kinase C and epidermal growth factor receptor signaling. Proc Natl Acad Sci U S A 2006; 103(42):15485-90; PMID:17021016; http://dx.doi.org/10.1073/pnas.0604104103
- Luo B, Prescott SM, Topham MK. Association of diacylglycerol kinase zeta with protein kinase C α: spatial regulation of diacylglycerol signaling. J Cell Biol 2003; 160(6):929-37; PMID:12629049; http://dx.doi.org/10.1083/jcb.200208120
- Topham MK, Prescott SM. Diacylglycerol kinase zeta regulates Ras activation by a novel mechanism. J Cell Biol 2001; 152(6):1135-43; PMID:11257115; http://dx.doi.org/10.1083/jcb.152.6.1135
- Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J 2008; 409(1):1-18; PMID:18062770; http://dx.doi.org/10.1042/BJ20071040
- Ohno S, Nishizuka Y. Protein kinase C isotypes and their specific functions: prologue. J Biochem 2002; 132(4):509-11; PMID:12359062; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a003249
- Cai J, Crotty TM, Reichert E, Carraway KL 3rd, Stafforini DM, Topham MK et al. Diacylglycerol kinase delta and protein kinase C(α) modulate epidermal growth factor receptor abundance and degradation through ubiquitin-specific protease 8. J Biol Chem 2010; 285(10):6952-9; PMID:20064931; http://dx.doi.org/; http://dx.doi.org/10.1074/jbc.M109.055731
- Becker KP, Hannun YA. cPKC-Dependent sequestration of membrane-recycling components in a subset of recycling endosomes. J Biol Chem 2003; 278(52):52747-54; PMID:14527960; http://dx.doi.org/10.1074/jbc.M305228200
- Idkowiak-Baldys J, Becker KP, Kitatani K, Hannun YA et al. Dynamic sequestration of the recycling compartment by classical protein kinase C. J Biol Chem 2006; 281(31):22321-31; PMID:16751194; http://dx.doi.org/10.1074/jbc.M512540200
- Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC et al. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 2008; 183(1):143-55; PMID:18838556; http://dx.doi.org/10.1083/jcb.200804140
- Xie S, Naslavsky N, Caplan S. Diacylglycerol kinase α regulates tubular recycling endosome biogenesis and major histocompatibility complex Class I recycling. J Biol Chem 2014; 289(46):31914-26; PMID:25248744; http://dx.doi.org/10.1074/jbc.M114.594291
- Carlton J, Bujny M, Rutherford A, Cullen P et al. Sorting nexins—unifying trends and new perspectives. Traffic 2005; 6(2):75-82; PMID:15634208; http://dx.doi.org/10.1111/j.1600-0854.2005.00260.x
- Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol 2002; 3(12):919-31; PMID:12461558; http://dx.doi.org/10.1038/nrm974
- Burger KN, Demel RA, Schmid SL, de Kruijff B et al. Dynamin is membrane-active: lipid insertion is induced by phosphoinositides and phosphatidic acid. Biochemistry 2000; 39(40):12485-93; PMID:11015230; http://dx.doi.org/10.1021/bi000971r
- Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P et al. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 1998; 94(1):131-41; PMID:9674434; http://dx.doi.org/10.1016/S0092-8674(00)81228-3
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M et al. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 2005; 436(7047):78-86; PMID:15889048; http://dx.doi.org/10.1038/nature03571
- Antonescu CN, Danuser G, Schmid SL. Phosphatidic acid plays a regulatory role in clathrin-mediated endocytosis. Mol Biol Cell 2010; 21(16):2944-52; PMID:20573978; http://dx.doi.org/10.1091/mbc.E10-05-0421
- Kawasaki T, Kobayashi T, Ueyama T, Shirai Y, Saito N et al. Regulation of clathrin-dependent endocytosis by diacylglycerol kinase delta: importance of kinase activity and binding to AP2alpha. Biochem J 2008; 409(2):471-9; PMID:17880279; http://dx.doi.org/10.1042/BJ20070755
- Subramanya S, Mensa-Wilmot K. Diacylglycerol-stimulated endocytosis of transferrin in trypanosomatids is dependent on tyrosine kinase activity. PLoS One 2010; 5(1):e8538; PMID:20049089; http://dx.doi.org/10.1371/journal.pone.0008538
- Lennartz MR. Phospholipases and phagocytosis: the role of phospholipid-derived second messengers in phagocytosis. Int J Biochem Cell Biol 1999; 31(3-4):415-30; PMID:10224668; http://dx.doi.org/10.1016/S1357-2725(98)00108-3
- Fallman M, Lew DP, Stendahl O, Andersson T et al. Receptor-mediated phagocytosis in human neutrophils is associated with increased formation of inositol phosphates and diacylglycerol. Elevation in cytosolic free calcium and formation of inositol phosphates can be dissociated from accumulation of diacylglycerol. J Clin Invest 1989; 84(3):886-91; PMID:2527254; http://dx.doi.org/10.1172/JCI114249
- Schlam D, Bohdanowicz M, Chatgilialoglu A, Steinberg BE, Ueyama T, Du G, Grinstein S, Fairn GD et al. Diacylglycerol kinases terminate diacylglycerol signaling during the respiratory burst leading to heterogeneous phagosomal NADPH oxidase activation. J Biol Chem 2013; 288(32):23090-104; PMID:23814057; http://dx.doi.org/10.1074/jbc.M113.457606
- Ueyama T, Lennartz MR, Noda Y, Kobayashi T, Shirai Y, Rikitake K, Yamasaki T, Hayashi S, Sakai N, Seguchi H et al. Superoxide production at phagosomal cup/phagosome through β I protein kinase C during Fc gamma R-mediated phagocytosis in microglia. J Immunol 2004; 173(7):4582-9; PMID:15383592; http://dx.doi.org/10.4049/jimmunol.173.7.4582
- Bohdanowicz M, Schlam D, Hermansson M, Rizzuti D, Fairn GD, Ueyama T, Somerharju P, Du G, Grinstein S et al. Phosphatidic acid is required for the constitutive ruffling and macropinocytosis of phagocytes. Mol Biol Cell 2013; 24(11):1700-12, S1-7; PMID:23576545; http://dx.doi.org/10.1091/mbc.E12-11-0789
- Corrotte M, Chasserot-Golaz S, Huang P, Du G, Ktistakis NT, Frohman MA, Vitale N, Bader MF, Grant NJ et al. Dynamics and function of phospholipase D and phosphatidic acid during phagocytosis. Traffic 2006; 7(3):365-77; PMID:16497229; http://dx.doi.org/10.1111/j.1600-0854.2006.00389.x
- Okada M, Hozumi Y, Iwazaki K, Misaki K, Yanagida M, Araki Y, Watanabe T, Yagisawa H, Topham MK, Kaibuchi K et al. DGKzeta is involved in LPS-activated phagocytosis through IQGAP1/Rac1 pathway. Biochem Biophys Res Commun 2012; 420(2):479-84; PMID:22450320; http://dx.doi.org/10.1016/j.bbrc.2012.03.057
- Abramovici H, Gee SH. Morphological changes and spatial regulation of diacylglycerol kinase-zeta, syntrophins, and Rac1 during myoblast fusion. Cell Motil Cytoskeleton 2007; 64(7):549-67; PMID:17410543; http://dx.doi.org/10.1002/cm.20204
