 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Contamination of pet food with Salmonella is a serious public health concern, and several disease outbreaks have recently occurred due to human exposure to Salmonella tainted pet food. The problem is especially challenging for raw pet foods (which include raw meats, seafood, fruits, and vegetables). These foods are becoming increasingly popular because of their nutritional qualities, but they are also more difficult to maintain Salmonella-free because they lack heat-treatment. Among various methods examined to improve the safety of pet foods (including raw pet food), one intriguing approach is to use bacteriophages to specifically kill Salmonella serotypes. At least 2 phage preparations (SalmoFresh® and Salmonelex™) targeting Salmonella are already FDA cleared for commercial applications to improve the safety of human foods. However, similar preparations are not yet available for pet food applications. Here, we report the results of evaluating one such preparation (SalmoLyse®) in reducing Salmonella levels in various raw pet food ingredients (chicken, tuna, turkey, cantaloupe, and lettuce). Application of SalmoLyse® in low (ca. 2–4×106 PFU/g) and standard (ca. 9×106 PFU/g) concentrations significantly (P < 0.01) reduced (by 60–92%) Salmonella contamination in all raw foods examined compared to control treatments. When SalmoLyse®-treated (ca. 2×107 PFU/g) dry pet food was fed to cats and dogs, it did not trigger any deleterious side effects in the pets. Our data suggest that the bacteriophage cocktail lytic for Salmonella can significantly and safely reduce Salmonella contamination in various raw pet food ingredients.
Introduction
The raw pet food diet is becoming increasingly popular among pet owners in the USA, with sales of various raw foods increasing by 32% to $69 million in just one year (2014 to 2015).Citation1 Commercially available raw pet foods contain a variety of meats (e.g., chicken), seafood (e.g., tuna), fruits and vegetables (e.g., lettuce) – and many raw pet foods contain more than one of these ingredients combined (e.g., Northwest Natural Chicken pet food “chicken recipe” contains a mixture of more than a dozen ingredients, including chicken, cantaloupe, carrots, broccoli, romaine lettuce, blueberry, and cranberry).Citation2 Raw pet foods offer many potential nutritional benefits for pets; however, because of their nature (raw, not heat treated), they also present an increased risk of bacterial contamination, particularly with Salmonella, which is a common contaminant of various food ingredients included in raw foods. In this context, several disease outbreaks in canines have occurred since the early 1990s; e.g., a salmonellosis outbreak in racing greyhounds was shown to be caused by raw pet food, with tests showing that over 66% of the food sampled was contaminated with Salmonella Schwarzengrund.Citation3 In another study, 80% of tested raw pet diets based on “Bones and Raw Food (BARF)” were contaminated with various Salmonella including S. Schwarzengrund and S. Hadar.Citation4 While raw pet foods are at a greater risk of contamination because they do not undergo rigorous heating and sterlization processs, dry processed foods may also be contaminated with bacteria after heat-treatment, as demonstrated by a 3-year multistate outbreak of S. Schwarzengrund.Citation5 Even with the use of high hydrostatic pressure, which is a non-thermal method to reduce bacteria in raw foods, bacterial species including some strains of Salmonella may persist.Citation6 Salmonellosis is responsible for up to 35% of sick pets visiting veterinary clinics and Salmonella contaminated pet food has been directly linked to human cases of Salmonellosis (including S. Typhimurium).Citation7,8 In addition to pet sickness, which is both unnerving and costly to pet owners, contamination of pet food imposes a significance economic burden to the pet food industry.Citation7,8 For example, a single contamination event originating in China has led to over $24 million dollars in restitution damages and $56 million loss of revenue to various North American pet food companies in 2007.Citation9
Pet owners, especially those interested in natural raw pet foods, are averse to artificial and chemical additions to their pets' diet, preferring natural interventions to reduce potential bacterial contamination. One natural method is the addition of bacteriophages, or phages. Phages are naturally found in every environment/ecosystem on Earth, and they have been commonly isolated from the human intestines, skin, oral cavity, as well as from the oceans, soils, potable tap water and animal feed.Citation9-13 Interest in applying phage technology has recently expanded from disease therapy to food safety, and several phage-based preparations have been recently approved by the FDA for human food applications, including ListShield™, Listex™, SalmoFresh™, Salmonelex™, and EcoShield™.Citation14 During this study, we used the Salmonella-specific phage preparation SalmoLyse® (previously approved for human food applications under the tradename SalmoFresh™).Citation14 SalmoLyse® is composed of 6 lytic phages effective against Salmonella strains belonging to some of the most common and highly pathogenic serotypes, including Typhimurium, Enteritidis, Heidelberg, Newport, Hadar, Kentucky, Thompson, Georgia, Agona, Grampian, Senftenberg, Alachua, Infantis, Reading, and Schwarzengrund.Citation14,15 During previous studies, SalmoLyse reduced Salmonella by ca. 1 log in dry kibble.Citation15 The present study is a follow-up investigation which was carried out to (i) determine the efficacy of the same phage preparation in various raw pet food ingredients (raw chicken, turkey, tuna, cantaloupe and lettuce) and (ii) elucidate its safety for pets (dogs and cats).
Results
The efficacy studies were performed by contaminating food ingredients with either a single Salmonella strain (turkey meat) or a mixture of 3 Salmonella strains (raw chicken, tuna, cantaloupe and lettuce) and treating some samples with Salmonella phages (test group) or with sterile water or sterile PBS (control group). The efficacy of the phage treatment was determined by comparing Salmonella levels recovered from phage treated vs. control samples.
Phage efficacy on raw foods
SalmoLyse® was applied, via spraying, to the raw foods experimentally contaminated with one or more Salmonella strains, as described in Methods. Phage application significantly (P < 0.01) reduced Salmonella contamination compared to corresponding controls in all examined raw pet food ingredients by 60% to 92% (0.4 to 1.1 log), (, ). More specifically, for turkey meat, reductions were on average (for all treatments, compared to controls) 77%, ranging from 68% to 86%. For chicken, the mean reductions for all 3 treatments were 73%, ranging from 60% for the lowest phage concentration examined (ca. 2×106 PFU/g), to 88% for the standard phage concentration (ca. 9×106 PFU/g). Salmonella reduction in lettuce was comparable to the poultry, with a total mean reduction of 74%, with values also ranging from 69% for the lowest phage treatment, to 80% for the standard treatment compared to controls. There was also significant reduction of Salmonella in cantaloupe, with percent reductions ranging from 80–89%, averaging at 85% for both treatment levels. Although statistically similar to the reductions observed for other food ingredients, the greatest overall Salmonella spp. reduction was on tuna (86% mean and ranged from 80% to 92%). Results for all food ingredients examined are summarized in and ; the latter also includes an estimated multiplicity of infection (MOI, ratio of phages/bacteria) for each experiment.
Figure 1. SalmoLyse® reduces Salmonella contamination on various food surfaces: Mean and standard error bars shown. Statistical analyses were carried out for each food group independently. Asterisks denote significant reduction from corresponding controls based on one-way ANOVA with Tukey's post-hoc tests for multiple corrections: ** denotes P < 0.01, while *** denotes P < 0.001 compared to the corresponding controls. There was significant reduction in Salmonella on all food surfaces with the addition of SalmoLyse® compared to the controls; the mean percent reductions from the control are noted in the boxes above treatment bars. CFU/g = colony forming units per gram. Each letter denotes a food group that was tested with SalmoLyse® and compared to a control: A= chicken; B= lettuce; C= tuna; D= cantaloupe; E = ground turkey.
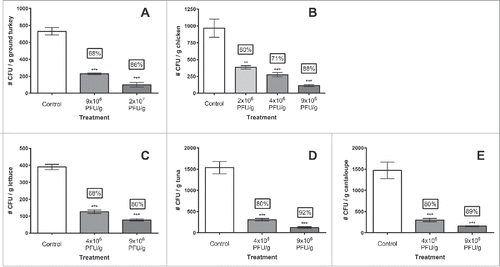
Table 1. Summary of the raw pet food ingredient experiments on turkey trim, chicken, tuna, cantaloupe, and lettuce. All phage treatments are significantly (P < 0.01) reduced from their corresponding control.
SalmoLyse® applied at the standard (i.e., recommended for typical commercial applications) concentration (ca. 9×106 PFU/g) was effective at removing, on average, 87 ± 2 % (0.91 ± 0 .07 log) Salmonella across all foods tested. With the application of the lower concentration SalmoLyse® (ca. 4×106 PFU/g), the average reduction of Salmonella was 74 ± 3 % (0.55 ± 0 .05 log) across all foods. In all food ingredients, with all phage concentrations examined, the levels of Salmonella recovered in the phage treated foods were significantly lower (P < 0.01) than in their respective controls (water or PBS) (). However, there were no significant differences between the levels of Salmonella recovered when different concentrations of phage were applied (P > 0.05 for all), regardless of food type (i.e., reductions observed between standard, low, and lowest phage concentrations, while numerically different, were statistically identical within each food category examined).
Feeding study
Cats: All cats completed the study, and there were no noticeable observed behaviors signifying distress or negative health effects for any cat (n = 12). Measured health parameters, such as weight change, intake of food or BCS (n = 12, Supplemental Table 1) did not vary from before, during or after phage consumption. In addition, fecal scores were “ideal” (score = 4) for 93.7% (n = 158) of the total feces examined for cats (Supplemental Table 2). No cat received a fecal score of less than 3 for any bowel movement. Hair is commonly found in cat stools and hair was found in 93.9% of stool samples. Mucus was not found in any individually collected feces. However, during group collections, where cats share a single litter box, mucus was observed 11 times (0.07%).
Dogs: All dogs completed the study. One dog during the study had one episode of a low fecal score (score = 1) on the afternoon of day 3. However, this event coincided with warmer (94°F/34.4°C) than usual weather and was consistent with an event that occurred prior to the study with the same dog. The dog recovered quickly, and was not removed from the study. Body weight, BCS and food intake did not differ from baseline and post study conditions (Supplemental Table 1). The primary fecal score was an ideal fecal quality (score 4) for 89% of the collections (Supplemental Table 2). Secondary fecal scores were assigned in less than 10% of the evaluated stools. Most of these secondary scores (79%) were characteristics of softer feces. When both scores (primary and secondary) were taken in account to calculate a weighed score, 84% of the stools received an ideal score of 4 (Supplemental Table 2).
Discussion
Lytic bacteriophages are becoming increasingly utilized for improving the safety of human food supply, yet their application in the pet food industry is still very limited (reviewed inCitation14,16). In a previous study, we reported the application of the same Salmonella phage preparation significantly reduced Salmonella contamination of dry pet food. Citation15 The current study provides additional experimental evidence that lytic bacteriophages could also effectively reduce Salmonella contamination in various raw pet food ingredients. The foods tested in this study were representative of common food ingredients found in various raw pet food diets. The level of Salmonella reduction as the result of phage treatment compared to the controls was similar (60–90%) for all foods examined in our study (cantaloupe, lettuce, and raw tuna, chicken, and turkey) regardless of differences in their surface types, topography, tension viscosity, and moisture levels. In addition, despite different Salmonella culturing methods employed in the turkey trim experiment, the results were consistent across the food groups. This observation suggests that phage application could be effective across a variety of raw pet food ingredients, potentially including those that were not specifically tested during our study.
Several previous studies have suggested that efficacy of phage application is concentration-dependent; i.e., higher phage concentrations result in more effective reduction in the levels of the targeted bacterial cells.Citation17,18 Our data are in agreement with those earlier observations; i.e., we also observed a greater reduction in the Salmonella levels with our “standard” (ca. 9×106 PFU/g) concentration vs. our “low” (ca. 4×106 PFU/g) or “very low” (or “lowest”) (ca. 2×106 PFU/g) phage concentrations (). The difference in phage concentrations we examined during our studies were relatively small (on the order of ca. Two-fold each vs. more typical log-scale differences; ). The goal of these studies was not to determine the minimal effective dose for our phage preparation (in which case, log-differences may have been more appropriate); rather, we wanted to determine how small variations in phage concentrations (e.g., those that might occur in real life settings due to minor human error during preparing phage solution) might impact the outcome of the phage treatment. For each food type, the differences between the levels of Salmonella recovered in the phage treated groups were not very large (and were statistically not different; P > 0.05) irrespective of the phage numbers applied per gram of food (), suggesting that relatively small variations in phage concentrations are not likely to impact the efficacy of treatment.
Lytic phages are ubiquitous in the environment and are consumed by animals (including humans) daily in the foods they eat and water they drink.Citation9,11,12,19,20-22 Phages are also naturally present in many, if not all, commercial pet foods.Citation12 Thus, their general safety profile is excellent, and several phage preparations have been granted GRAS (Generally Recognized As Safe) status by the FDA for human food applications.Citation14 However, to the best of our knowledge, no studies have been performed in live animals in which consumption of phage-treated pet food was evaluated for safety, although several studies are available showing that direct oral administration of lytic phages is (i) safe for various animals, and (ii) does not deleteriously alter their normal gut microflora.Citation23-25 Due to the generally accepted safety of well-purified lytic phages, the feeding studies (rather than formal toxicology studies) were performed based on FDA recommendations. The criteria examined included standard metrics typically evaluated during all feeding studies, such as weight loss, body composition scores, fecal scores, appetite, and any signs of distress. During the studies presented in this report, we showed (for the first time) that eating phage-treated pet food was safe for cats and dogs. This feeding safety study used dry kibble treated with phages in the concentrations expected to be used in the real life commercial settings; while we have not specifically examined the impact of “wet” pet food in a similar feeding study, the amount of phage delivered to the animals' GI tract with dry vs. wet pet food is expected to be essentially identical (assuming the same level of treatment) and we therefore do not anticipate any variation in tolerance of phages with different pet food types. In summary, our data continue to suggest that bacteriophages lytic for Salmonella offer an additional safe and effective strategy (as part of a multi-hurdle approach) for reducing Salmonella contamination in various pet foods and food ingredients.
Methods
Phage efficacy raw foods
Bacteriophage preparation
The bacteriophage product, SalmoLyse®, used in our studies is comprised of 6 lytic monophages: SBA-1781, SPT-1, SSE-121, STML-198, STML-13-1, and SKML-39. The phages have been described in more detail previously.Citation15 The following SalmoLyse® lots (batches) were used: Lot 0213H050123 (chicken); Lot 0211C150168 (tuna and cantaloupe); Lot 0212H200172 (lettuce); Lot #02TestSample (turkey).
Bacterial isolates
Salmonella contamination was simulated in the laboratory by adding 3 different strains of Salmonella to foods. The following strains were used during the study: (1) S.E900: A nalidixic acid resistant mutant developed from S.E660 (also known as ATCC13076, Salmonella enterica serotype Enteritidis), (2) S.Ty901: A nalidixic acid resistant mutant developed from S.Ty653 (also known as ATCC6539, Salmonella enterica serotype Typhi), and (3) S.He902: A nalidixic acid resistant mutant developed from S.He899 (also known as ATCC8326, Salmonella enterica serotype Heidelberg). The strains were selected for nalidixic acid resistance by serially passaging the original isolates on Luria-Bertani (LB) agar plates supplemented with increasing concentrations of nalidixic acid. The strains underwent ≤8 serial passages before they were determined to be nalidixic acid-resistant at a concentration of 25 μg/ml. After passaging, the above-noted Intralytix strain designations were assigned (i.e., S.E900, S.Ty901, S.He902). The strains were stored at −80°C, at Intralytix, in 70% LB broth/30% glycerol supplemented with 25 μg/ml nalidixic acid.
Salmonella challenges on turkey
The first study was performed using turkey trims, and it was designed to determine SalmonLyse® activity against a single Salmonella strain. Turkey trims were supplied by a large poultry producer. The test strain S.He902 was thawed and grown in NZCYM broth supplemented with nalidixic acid (25 µg/ml) until it reached approximately 1×109 CFU/mL. The freshly grown culture was diluted 1000-fold before application. The bacterial suspension was applied at ca. 1,250 CFU/g onto the turkey and evenly spread with a hockey stick onto the surfaces. The bacteria were allowed to colonize at room temperature for 60 minutes, at which time either SalmoLyse® or phosphate-buffered saline (PBS) was applied to the contaminated turkey trims. Two different titers of SalmoLyse® were compared: ca. 9×106 PFU/g and ca. 2×107 PFU/g (). Phages were applied to surfaces with an air-atomizer sprayer (Model 200, Badger Air-Brush Co; previously shown to not impact phage activity, data not shown). Samples were incubated at room temperature for 5 min before being ground (#10 meat grinder Kitchener #508313). Triplicate ∼25 g samples were placed in sterile bags with 225 mL of peptone water, hand mushed, then stomached for a minimum of 30 seconds. Aliquots of the mixture (0.5 mL) were plated onto Salmonella/Shigella agar (SSA) supplemented with nalidixic acid (25 mg/mL), and were incubated at 35 ± 2°C for 24 ± 2 h. The CFU/g of sample was calculated after counting colonies as follows:
Salmonella challenges on chicken, tuna, cantaloupe and lettuce
The study was further expanded to mimic conditions where a mixture of Salmonella serotypes may be present in the foods rather than just a single Salmonella strain. Raw foods were purchased in local grocery stores in Maryland. Tuna was sushi grade, and none of the food samples were prewashed. Prior to phage additions, the 3 Salmonella strains were thawed and grown (37 ± 2°C, 16–24 h) in LB broth supplemented with nalidixic acid (25 μg/ml). Equal volumes of the 3 bacterial cultures were then mixed and diluted appropriately for each study. For each experimental test, 100 g portions of each food were contaminated with an approximately equal ratio (1:1:1) of the 3 Salmonella strain suspension. The bacteria were evenly spread with hockey sticks after the Salmonella mixture had been pipetted onto the various food ingredients (ca. 1500 CFU/g on chickens; ca. 2000 CFU/g on tuna/cantaloupe; and ca. 500 CFU/g on lettuce). Bacteria were allowed 60 min to adhere to the food samples at room temperature, and were treated with either the phage solution or water control as described in . After treatment, samples were incubated at room temperature for 5 min. Triplicate ∼25 g samples were removed and placed into sterile bags with 225 mL of peptone water. Bags were manually homogenized and stomached for a minimum of 30 seconds. Aliquots of the stomached mixture were plated onto Hektoen-Enteric Agar (HE) plates supplemented with 25 ug/mL nalidixic acid. Plates were incubated in 35 ± 2°C for 24 ± 2 h. Salmonella levels (CFU/g) were determined as described above for the turkey experiments. Phages were not inactivated before enumeration; previous studies have shown that there are no significant differences among results with and without phage inactivation.Citation26,27
Statistical analysis
Statistical analysis was performed using GraphPad InStat (V3.05 or V3.10) and GraphPad Prism (V4.0 or V6.05) (GraphPhad Software, San Diego, CA). Analysis of Variance (ANOVA) was used to determine differences between food ingredients and their respective experimental control (i.e. each food ingredient was tested separately). Values of P<0.01 were considered significant for post hoc tests (corrected for multiple t-tests). Statistical analyses were not combined for various foods, and were performed for each food category. All pet food ingredient studies were conducted at Intralytix, Inc. (Baltimore, MD).
Feeding safety
Selection of population study: Dog and cat participant selection
Study participants for both the cat and dog studies were subject to the same inclusion and exclusion criteria. To qualify, a dog or cat was required to be an adult at the time of study (≥2 years) and not have any significant health issues. An animal was excluded if it had participated in any other study, had been on antibiotics within 30 d of the study start, and/or had any history of frequent GI upset, including vomiting and/or low fecal scoring (i.e., diarrhea). Criteria for removal from the study was decided a priori. These included symptoms such as developing vomiting/diarrhea for longer than one day, 15% weight change in either direction, 50% food refusal for 5 d or more and any medical condition requiring antibiotic use. No study participant from either the cat or dog study required removal, and all participants finished the study (n = 12 for cats; n = 12 for dogs). All appropriate IACUC approvals were obtained before animal studies were initiated.
Phage application to kibble and the feeding study
Before application to food, SalmoLyse® titers were determined using the agar layer method.Citation28 SalmoLyse® was diluted 1:10 in tap water to achieve the desired concentration of phage cocktail in the kibble and was applied equally to surfaces by spraying. After SalmoLyse® application to kibble, triplicate samples from 2 prepared batches were analyzed for phage concentrations remaining in the food. All cats were fed once daily the same diet consisting of Iams® MultiCat coated with SalmoLyse® at a final concentration of ca. 2×107 PFU/g. Dogs were fed twice daily with Modified Iams® Mini Chunks coated with SalmoLyse® at a concentration of ca. 2×107 PFU/g. All study animals were allowed unrestricted access to drinking water. Salmonella were not added to any of the participants' food.
Testing for safety
Baseline demographic information for all participating animals, including age, gender, food intake, fecal scores, weight and body condition, were recorded before food studies began. Body condition scores were determined by standard veterinary measures for dogs and cats.Citation29 Fecal scores range from 1–5 (1= Absence of feces; 2= Liquid with/without particle matter; 3= Soft, shapeless; 4=Firm, well formed; 5=Extremely dry). Once the study was underway, food intake, fecal scoring and any GI discomfort (emesis, diarrhea, or physical signs of GI-related pain) was determined daily. Fecal scores were taken individually twice a day for dogs, while cat fecal scores were taken individually twice a day Monday through Friday (n = 10) and as a group (sampled from a group litterbox) on weekends (n = 4). Some dogs had uneven stools (>25 <50% uneven) and in these cases were given a weighted score based on following calculation:
In addition to the daily parameters, body weight and body condition scores (BCS) were measured at the end of the study (14 d for dogs and 15 d for cats). Animal feeding studies were conducted at P&G (Mason, OH).
Disclosure of potential conflicts of interest
NS, TA, JW, ML and AS hold an equity stake in Intralytix, Inc., a Maryland corporation developing bacteriophage preparations (including SalmoLyse®) for various applications.
KNAC_A_1220347_Supplementary_tables.zip
Download Zip (160.7 KB)References
- Tarkan L. Raw pet food sales are booking, but are the products safe? Fortune 2015. Accessed online at http://fortune.com/2015/08/06/raw-pet-food-sales-safety/.
- NorthWest Naturals. Chicken. 2016. Accessed online at http://nwnwordpress1.nw-naturals.net/wp/chicken-ingredients.
- Chengappa MM, Staats J, Oberst RD, Gabbert NH, McVey S. Prevalence of Salmonella in raw meat used in diets of racing greyhounds. J Vet Diagn Invest 1993; 5:372-7; PMID:8373850; http://dx.doi.org/10.1177/104063879300500312
- Joffe DJ, Schlesinger DP. Preliminary assessment of the risk of Salmonella infection in dogs fed raw chicken diets. Canadian Vet J 2002; 43:441-2
- Behravesh CB, Ferraro A, Deasy M, Dato V, Moll M, Sandt C, Rea NK, Rickert R, Marriott C, Warren K, et al. Human salmonella infections linked to contaminated dry dog and cat food, 2006–2008. Pediatrics 2010; 126:477-83; PMID:20696725; http://dx.doi.org/10.1542/peds.2009-3273
- Rendueles E, Omer MK, Alvseike O, Alonso-Calleja C, Capita R, Prieto M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT - Food Sci Technol 2011; 44:1251-60; http://dx.doi.org/10.1016/j.lwt.2010.11.001
- Finley R, Reid-Smith R, Weese JS, Angulo FJ. Human health implications of salmonella-contaminated natural pet treats and raw pet food. Clin Infect Dis 2006; 42:686-91; PMID:16447116; http://dx.doi.org/10.1086/500211
- Cavallo SJ, Daly ER, Seiferth J, Nadeau AM, Mahoney J, Finnigan J, Wikoff P, Kiebler CA, Simmons L. Human outbreak of salmonella typhimurium associated with exposure to locally made chicken jerky pet treats, New Hampshire, 2013. Foodborne Pathog Dis 2015; 12:441-6; PMID:25793722; http://dx.doi.org/10.1089/fpd.2014.1889
- Tiwari R, Dhama K, Kumar A, Rahal A, Kapoor S. Bacteriophage therapy for safeguarding animal and human health: a review. Pakistan J Biol Sci 2014; 17:301-15
- Dinsdale EA, Edwards RA, Hall D, Angly F, Breitbart M, Brulc JM, Furlan M, Desnues C, Haynes M, Li L, et al. Functional metagenomic profiling of nine biomes. Nature 2008; 452:629-32; PMID:18337718; http://dx.doi.org/10.1038/nature06810
- Housby JN, Mann NH. Phage therapy. Drug Discov Today 2009; 14:536-40; PMID:19508915; http://dx.doi.org/10.1016/j.drudis.2009.03.006
- Maciorowski KG, Pillai SD, Ricke SC. Presence of bacteriophages in animal feed as indicators of fecal contamination. J Environ Health 2001; 36:699-708; http://dx.doi.org/10.1081/PFC-100106196
- Haynes M, Rohwer F. The Human virome. In: Nelson KE, ed. Metagenomics of the Human Body: Springer New York, 2011:63-77; http://dx.doi.org/10.1002/9780470015902.a0025962
- Woolston J, Sulakvelidze A. Bacteriophages and Food Safety. In: eLS, ed. Chichester: John Wiley & Sons Ltd, 2015
- Heyse S, Hanna LF, Woolston J, Sulakvelidze A, Charbonneau D. Bacteriophage cocktail for biocontrol of salmonella in dried pet food. J Food Prot 2015; 78:97-103; PMID:25581183; http://dx.doi.org/10.4315/0362-028X.JFP-14-041
- Sulakvelidze A, Barrow PA. Phage therapy in animals and agribusiness. In: Kutter E, Sulakvelidze A, eds. Bacteriophages: Biology and Applications. Boca Raton, FL: CRC Press, 2005:335-80
- Leverentz B, Conway WS, Camp MJ, Janisiewicz WJ, Abuladze T, Yang M, Saftner R, Sulakvelidze A. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl Environ Microbiol 2003; 69:4519-26; PMID:12902237; http://dx.doi.org/10.1128/AEM.69.8.4519-4526.2003
- Hudson J, Billington C, Wilson T, On S. Effect of phage and host concentration on the inactivation of Escherichia coli O157:H7 on cooked and raw beef. Food Sci Technol Int 2013; 21:104-109.
- Willner D, Furlan M, Haynes M, Schmieder R, Angly FE, Silva J, Tammadoni S, Nosrat B, Conrad D, Rohwer F. Metagenomic analysis of respiratory tract dna viral communities in cystic fibrosis and non-cystic fibrosis Individuals. PloS One 2009; 4:e7370; PMID:19816605; http://dx.doi.org/10.1371/journal.pone.0007370
- Pride DT, Salzman J, Haynes M, Rohwer F, Davis-Long C, White RA, Loomer P, Armitage GC, Relman DA. Evidence of a robust resident bacteriophage population revealed through analysis of the human salivary virome. ISME J 2012; 6:915-26; PMID:22158393; http://dx.doi.org/10.1038/ismej.2011.169
- Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, Rohwer F. Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol 2003; 185:6220-3; PMID:14526037; http://dx.doi.org/10.1128/JB.185.20.6220-6223.2003
- Chibani-Chennoufi S, Bruttin A, Dillmann ML, Brussow H. Phage-host interaction: an ecological perspective. J Bacteriol 2004; 186:3677-86; PMID:15175280; http://dx.doi.org/10.1128/JB.186.12.3677-3686.2004
- Mai V, Ukhanova M, Visone L, Abuladze T, Sulakvelidze A. Bacteriophage administration reduces the concentration of listeria monocytogenes in the gastrointestinal tract and its translocation to spleen and liver in experimentally infected mice. Int J Microb 2010; 2010:624234-9; http://dx.doi.org/10.1155/2010/624234
- Mai V, Ukhanova M, Reinhard MK, Li M, Sulakvelidze A. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage 2015; 5:e1088124; PMID:26909243; http://dx.doi.org/10.1080/21597081.2015.1088124
- Bruttin A, Brussow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 2005; 49:2874-8; PMID:15980363; http://dx.doi.org/10.1128/AAC.49.7.2874-2878.2005
- Sharma M, Patel JR, Conway WS, Ferguson S, Sulakvelidze A. Effectiveness of bacteriophages in reducing Escherichia coli O157:H7 on fresh-cut cantaloupes and lettuce. J Food Prot 2009; 72:1481-5; PMID:19681274
- Perera MN, Abuladze T, Li M, Woolston J, Sulakvelidze A. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food Microbiol 2015; 52:42-8; PMID:26338115; http://dx.doi.org/10.1016/j.fm.2015.06.006
- Adams MH. Enumeration of bacteriophage particles. In: Benzer S, Bertani M, Delbruck M, Garen A, Harm W, Herriott M, Hershey AD, Lanni F, Murray RG, Stahl FW, et al., eds. Bacteriophages. London: Interscience Publishers, 1959:29
- German AJ, Holden SL, Moxham GL, Holmes KL, Hackett RM, Rawlings JM. A Simple, reliable tool for owners to assess the body condition of their dog or cat. J Nutri 2006; 136:2031S-3S; PMID:16772488
