Abstract
The issue of environmental pollution has become a hot issue in today's world. Environmental pollution, mainly caused by toxic chemicals, includes air, water, and soil pollution. This pollution results not only in the destruction of biodiversity, but also the degradation of human health. Pollution levels that are increasing day by day need better developments or technological discoveries immediately. Nanotechnology offers many advantages to improve existing environmental technologies and create new technology that is better than current technology. In this sense, nanotechnology has three main capabilities that can be applied in the fields of environment, including the cleanup (remediation) and purification, the detection of contaminants (sensing and detection), and the pollution prevention.
1. Introduction
In today's world where industries have been modernized and advanced, our environment is filled with various types of pollutants emitted from human activities or industrial processes. Examples of these pollutants are carbon monoxide (CO), chlorofluorocarbons (CFCs), heavy metals (arsenic, chromium, lead, cadmium, mercury and zinc), hydrocarbons, nitrogen oxides, organic compounds (volatile organic compounds and dioxins), sulfur dioxide and particulates. Human activities, such as oil, coal and gas combustion, have significant potential to change emissions from natural sources Citation1. In addition to air pollution, there is also water pollution caused by various factors, including waste disposal, oil spills, leakage of fertilizers, herbicides and pesticides, by-products of industrial processes and combustion and extraction of fossil fuels Citation2.
Contaminants are mostly found mixed in the air, water and soil. Thus, we need a technology that is able to monitor, detect and, if possible, clean the contaminants from the air, water and soil. In this context, nanotechnology offers a wide range of capabilities and technologies to improve the quality of existing environment.
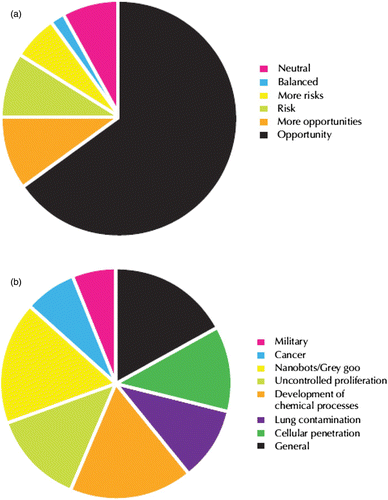
Nanotechnology offers the ability to control matter at the nanoscale and create materials that have specific properties with a specific function Citation3. Surveys from selected European Union (EU) media show relatively high optimism with respect to the chances/risk ratio associated with nanotechnology (), where most of them have been attributed to the prospect of improvement in the quality of life and health Citation4.
Figure 1. European Union (EU) result of people survey: (a) balance between perceptual opportunities and risks of nanotechnology and (b) hypothetical risks of nanotechnology development Citation4.
Nanomaterial is very small and the ratio of surface area to volume ratio is high so that it can be used to detect very sensitive contaminants Citation5. Nanotechnology is also used to prevent the formation of pollutants or contaminants by applying the material technology, industrial processes and others. Thus, three major applications of nanotechnology in the fields of environment can be classified, namely (1) restoration (remediation) and purification of contaminated material, (2) pollution detection (sensing and detection) and (3) pollution prevention. With rapid increment of pollutant species and concentration, the development of instruments that able to treat and prevent it is necessary Citation6–8.
2. Nanotechnology for clean water
Only 30% of all water on the Earth is not trapped in the ice or glaciers and only 0.08% of it is clean water Citation2, an analogy of 1 teaspoon of water versus a 5 litre container of water. In recent years, water has become an important issue, and it is quite difficult to solve the associated problems. The development of nanotechnology can be used to improve water quality. Several methods that can use nanotechnology use reactive media for separation and filtration, bioremediation and disinfection Citation9–11.
Remediation is the process to remove, minimize or neutralize the water contaminants that can damage human health or ecosystems. Remediation technologies can be divided into three categories, namely (1) thermal, (2) physicochemical and (3) biological methods. Most traditional methods such as extraction, adsorption and oxidation are less effective, expensive and time-consuming, whereas the more environmentally friendly biological degradation is inexpensive, but very time-consuming. An advanced method that can be used is nanomaterials, with enhanced affinity, capacity and selectivity for heavy metals and other contaminants. The advantages of using nanomaterials are their higher reactivity, larger surface contact and better disposal capability. There are several examples of nanoparticles and nanomaterials that can be used for remediation of water, e.g. zeolites, carbon nanotubes (CNTs) Citation12, self-assembled monolayers on mesoporous supports, biopolymers, single-enzyme nanoparticles, nanoparticles of zero valent iron (ZVI), among others.
2.1. Water remediation with iron nanomaterial
A common system that has been developed over the years to remediate water is known as a ‘pump and treat’ system Citation13. The system (as shown in ) is meant to pump water from the soil to the surface, to handle it and then to inject it back into the ground. Until 1998, the pump and treat system was still used as a way to remediate water. Another way to remediate water is by using permeable reactive barrier (PRB). PRB cleans subsurface groundwater and remediate without the need to bring the water to the surface (see ). This treatment can be used to clean up pollutants such as chlorinated hydrocarbons, aromatic nitro compounds, polychlorinated biphenyls (PCBs), pesticides and chromate compounds.
Figure 2. A schematic diagram of (a) pump and treat system and (b) permeable reactive barrier (PRB) application made with millimeter-sized construction-grade granular iron Citation13.
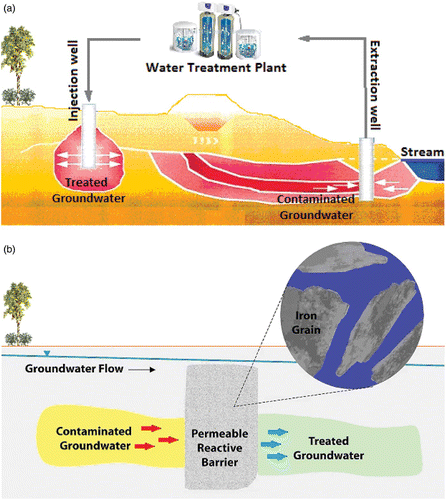
The PRB method (), which is expected to replace the pump and treat method, has some disadvantages, such as its cost (it is very expensive) and there is no definite time of replacement. Sometimes the reactivity of iron is reduced due to the presence of impurities in the form of metal hydroxide and metal carbonate compounds. Several alternative methods have been done to overcome these weaknesses. In the early 1990s, it was found that some zero-valent metals such as iron (ZVI), proposed as a filter material of PBR, can handle or reduce dangerous contaminants in the water in large quantities Citation14. The small particle size makes nano-iron capable of multifunctional use for remediation purposes.
In general, ZVI is classified into two types: (1) nanoscale ZVI (nZVI) and (2) reactive nanoscale iron product (RNIP). They are made using the basic techniques of nanotechnology. nZVI particles have a diameter of 100–200 nm composed of iron (Fe) with a valence of zero, whereas RNIP particles consist of 50/50 wt% Fe and Fe3O4. Although real application data is not yet produced, the potential application of ZVI is good. Miehr et al. Citation15 have identified that ZVI has high reactivity to a large number of contaminants, including Cu2+, chlorinated hydrocarbons, and NO3−.
In addition to the use in PRB, nano-iron can also be used via direct injection into the soil, sediment or solid waste. The trick is to mix the nanoparticles with water to form slurry. Once injected, the particles will remain in the form of a suspension and a treatment zone will be formed. Another way is to attach the nanoparticles to a solid matrix such as activated carbon which has proven quite effective.
Nano-iron could be substituted with other metals. Metals such as zinc and tin have the ability to reduce contaminants such as iron. Two metal alloys such as iron and iron–nickel–copper have been employed to degrade trichloroethene and trichloroethane Citation16. The commonly used metals are palladium, silver, platinum, cobalt, copper and gold, while aluminum is used as an inert.
2.2. Water remediation with ferritin
Ferritin is an iron-containing protein that is able of controlling the formation of mineralized structures. Ferritin can be found in animals and plants and its function is to store iron. Ferritin is formed when 24 polypeptides that are structurally similar to each other form a cage-like protein structure Citation17. Once the cage is formed, the iron molecules can enter the cavity through the protein shell, where the mineralization process transforms iron molecules into ferrihydrite nanoparticles.
Researchers have discovered the ability of ferritin to remediate toxic metals and chlorocarbon under visible light or solar radiation Citation18. The advantages of ferritin over ordinary iron catalyst are: (1) ferritin does not react under photoreduction; and (2) it is also more stable.
One obvious application of ferritin which has been proven in the laboratory is to change chromium Cr(VI) into Cr(III) (see Citation19). Cr(VI) is carcinogenic pollutant that is generally contained in the industrial waste, while Cr(III) is formed naturally as a Cr compound, which is less poisonous and insoluble in water Citation20.
2.3. Air remediation using nanosize semiconductor photocatalyst
Some materials such as titanium dioxide (TiO2), zinc oxide (ZnO), iron (III) oxide (Fe2O3) and tungsten oxide (WO3) may serve as photocatalysts. This photocatalyst has many uses, including as a white pigment which gives colour to paper and paint, ultraviolet light-absorbing material on the sunscreen, protective antimicrobials and automatic cleaners. In relation to the environment and water remediation, photocatalysts are able to oxidize organic pollutants into nontoxic materials. In general, the use of TiO2 in advanced methods of photochemical oxidation for the remediation of water is due to its low levels of toxicity, high photoconductivity, high photostability, and that it is an easily available and inexpensive material.
Using the principle of a semiconductor, organic molecules can be oxidized by light. At a sufficient level of light, the charge transfer process will occur from the valence band to the conduction band causing the surrounding substance to be oxidized. Through the development of nanotechnology, semiconductor photocatalysts are modified in terms of reactivity and selectivity.
One semiconductor photocatalyst has been applied for water remediation under the United States Environmental Protection Agent (US EPA) SITE program Citation19. The photocatalyst is able to remove contaminants from ground water containing 1,1-dichloroethane, cis-1,2-dischloroethane, 1,1,1-threechloroehtane, xylenes and toluene. In a pilot scale, it was also found that TiO2 was capable of eliminating benzene, toluene, ethylbenzene and xylene (BTEX) contents from groundwater.
The surface of TiO2 catalysts which can be developed using nanotubes is shown to be more effective at eliminating the material in comparison with the usual structure of TiO2 powder Citation21. In addition to the use of TiO2, which is already commonly used in industry, ZnO photocatalysts are currently being developed as well. As a concept, ZnO is expected to have two functions, namely to detect and remediate contaminants. During laboratory experiments, a ZnO photocatalyst was successfully used to detect and eliminate 4-chlorocatechol Citation22.
2.4. Water remediation using polymer nanoparticles
Polymer nanoparticles have various uses, including water treatment and sunscreen. Using a similar principle as surfactant micelles, polymeric nanoparticles have amphiphilic properties, where each molecule has hydrophobic and hydrophilic parts. When water is available, the polymer will form a polymer cell with a diameter of several nanometres inside the hydrophobic part, while the hydrophilic part is outside. On polymer nanoparticles, crosslink occurs prior to the aggregation of particles so that their stability is maintained. Amphiphilic polyurethane (APU) nanoparticles have good prospects as a remediation agent. Tungittiplakorn et al. Citation23 used a polyurethane acrylate anionomer (UAA) and poly(ethylene glycol)-modified urethane acrylate (PMUA) as the reactant/precursor chains.
In the application, polymeric nanoparticles offer a solution for commonly used conventional surfactants to enhance remediation of hydrophobic organic contaminants using a pump and treat system. These contaminants are usually classified into nonaqueous-phase liquid which sticks very firmly to the ground so that it is difficult to cleanse, leading the remediation process to be less and less effective. Therefore, a surfactant is needed to clean up these contaminants.
To date, the use of polymeric nanoparticles is still in the research phase Citation19. Several things that need to be studied before these ideas are applied include material suitability for the soil type, recovery and recycling processes of the particles.
2.5. Bioactive nanoparticles for water disinfection
Nanotechnology provides an alternative solution to clean germs in water, a problem that has been getting worse due to the population explosion, growing need for clean water and emergence of additional pollutants. One of the alternatives offered is antimicrobial nanotechnology Citation24 Citation25. Li et al. Citation24 stated that several nanomaterials showed strong antimicrobial properties through diverse mechanisms, such as (1) photocatalytic production of reactive oxygen species that damage cell components and viruses (e.g. TiO2, ZnO and fullerol), (2) compromising the bacterial cell envelope (e.g. peptides, chitosan, carboxyfullerene, CNTs, ZnO and silver nanoparticles), (3) interruption of energy transduction (e.g. Ag and aqueous fullerene nanoparticles) and (4) inhibition of enzyme activity and DNA synthesis (e.g. chitosan). Among all materials, TiO2 has been proposed to be the best candidate as it is stable in water, nontoxic when ingested and low cost.
2.6. Nanofibres and nanobiocides for water purification
Nanofibres and nanobiocides provide a possibility to improve the quality of water filtration membranes Citation26. For membrane fouling caused by bacteria in the water which reduce the quality of water, inhibition of these bacteria can be caused by the surface-modified nanofibres. Based on du Plessis’ result, both polyvinyl alcohol (PVA) and polyacrylonitrile (PAN) nanofibres containing silver nanoparticles have excellent antimicrobial activity, with PVA nanofibres reducing between 91% and 99% of bacteria in a contaminated water sample and PAN nanofibres killing 100%. Neither PVA nor PAN nanofibres leached silver into the water, os it was concluded that PVA is a nontoxic and biodegradable synthetic polymer and PVA–silver nanofibres have excellent antimicrobial activity Citation26.
2.7. Nanofiltration
Nanofiltration is one type of filtration that uses pressure as the driving force. Nanofiltration membranes provide higher thrust or rejection of multivalent ions, pesticides and heavy metals compared with conventional treatment methods. Currently, this technology has become the newest and most leading-edge technology in water treatment and is now available for practical use in your home, business or manufacturing facility. Some reports have been published regarding the performance of nanofiltration membrane Citation27 Citation28. Depending on the requirement, some manufacturers offer nanofiltration membranes to target different molecules based on their molecular weight. As an example, Dow Filmtec offers a nanofiltration membrane with the capability to remove molecules higher than 90, 200 or 270 g/mol Citation25. This provides consumers with many options for applications.
2.8. Other nanotechnologies for water remediation
In addition to the examples which have been described above, there are several other examples of nanotechnology applications in water remediation, among others:
| • | self-assembled monolayers on mesoporous silica (SAMMS); | ||||
| • | dendrimers or dendritic polymers; | ||||
| • | single nanoparticle enzyme (SEN); | ||||
| • | tunable biopolymers; | ||||
| • | nanocrystalline zeolites, etc. | ||||
3. Nanotechnology for the adsorption of toxic gases
Water remediation is not the only application for nanotechnology: toxic gases in the ambient air can also be cleaned by nanotechnology. An example nanotechnology application in toxic gas cleaning is the process of CNTs and gold particles adsorption. CNTs consist of a hexagonal arrangement of carbon atoms in graphene sheets that surround the tube axis. There is a strong interaction between the two benzene rings of dioxin and the surface of CNTs. In addition, dioxin molecules interact with the entire surface of nanotubes with a porous wall, i.e. 2.9 nm, and the possibility of overlapping events that increase the adsorption potential inside the pores. Strong oxidation resistance of CNTs has also been beneficial for the regeneration of the adsorbent at high temperatures.
CNTs, as both single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs), are unique macromolecules that have a one-dimensional structure, thermal stability and exceptional chemical properties. These nanomaterials have shown to have good potential as superior adsorbents to remove various types of organic and inorganic pollutants, both in air streams and in an aqueous environment. The adsorption capacity of pollutants by CNTs is mainly caused by the pore structure and the existence of a broad spectrum of surface functional groups of CNTs that can be achieved by modifying the chemical or thermal treatment to tune the CNTs to have optimal performance for a particular purpose.
Their unique electronic properties and structures have attracted the interest of researchers in enhancing the potential applications of SWNTs and MWNTs. For example, SWNTs have been reported to be a chemical sensor for NO2 and NH3. After being exposed to NO2 or NH3 gas, the electrical resistance of SWNTs was found to change significantly, either up or down. SWNTs and MWNTs can also be used as hydrogen storage. In addition, CNTs have been used as quantum nanowires, electron field emitters, catalyst supports, etc.
3.1. Adsorption of dioxins
Dioxin and related compounds (e.g. polychlorinated dibenzofuran and polychlorinated biphenyls) are stable and highly toxic pollutants. Dibenzo-p-dioxins are a family of compounds consisting of two benzene rings which are joined by two oxygen atoms. It has zero to eight chlorine atoms attached to the ring. Dibenzofuran is a similar yet different compound in which only one of the ties between two benzene rings are bridged by oxygen. The toxicity of dioxins varies depending on the number of chloro atoms. The dioxins having no or a single chloro atom are not toxic, while the dioxins having more than one chloro atom are toxic. 2,3,7,8-Tetraklorodibenzo-p-dioxin (TCDD) is a compound that is known to be carcinogenic to humans. Dioxins also affect the immune and endocrine systems and foetal development.
Dioxin compounds are mainly generated from the combustion of organic compounds in waste incineration. Dioxin compounds formed from combustion have concentrations in the range of 10–500 ng/m3. Regulations on dioxin emissions are complex and vary across countries. Nevertheless, it is generally necessary to reduce dioxin concentrations to below 1 ng/m3. Two critical reviews have been published that discuss the prevention and reduction of dioxin Citation29 Citation30.
Since 1991, adsorption using activated carbon has been widely adopted to eliminate dioxins from waste incinerators in Europe and Japan. Dioxin removal efficiency using an activated carbon adsorbent is much higher than other adsorbents because the bond energy between dioxin and activated carbon is higher than with other adsorbents, such as clay, γ-Al2O3 and zeolites Citation31.
Owing to the extreme toxicity of dioxin, a more efficient adsorbent than activated carbon is required so that the dioxin emissions can be reduced to a lower level. In this case, Long and Yang Citation32 have found that the interaction of dioxin with CNTs is nearly three times stronger than the interaction of dioxin with activated carbon. Although not directly mentioned, the results showed that CNTs were significantly better than activated carbon and γ-Al2O3 for removing dioxins. This improvement is probably due to the nanotube curved surface compared with those for flat sheets that gives stronger interaction forces between dioxin and CNTs Citation33.
3.2. NO x adsorption
There has been a major effort in the development of technologies to eliminate the emissions of NO x (mixture of NO and NO2) from fossil fuel combustion. Common adsorbent used to remove NO x at low temperatures include ion exchange zeolites, activated carbon and FeOOH dispersed on active carbon fibre. NO can be effectively adsorbed to activated carbon due to the reactivity of surface functional groups, although the amount of adsorbed species is still not significant.
Long and Yang Citation34 found that CNTs could be used as an adsorbent for the removal of NO. Uptake rate of NO x , SO2 and CO2 on the CNTs at room temperature is shown in . The amount of NO x absorption was approximately 78 mg/g CNTs.
Figure 3. Adsorption/desorption profile of CO2, NO x , SO2 on carbon nanotubes at 25°C. (Adapted from Citation34.)
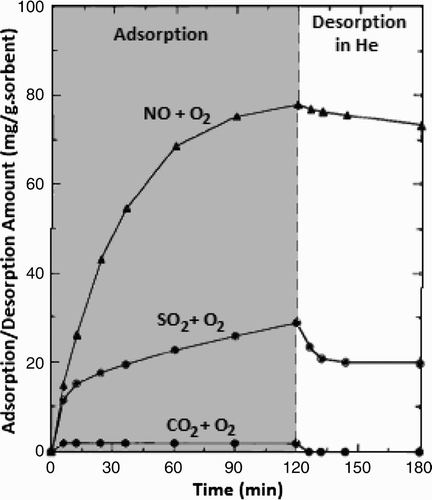
NO x adsorption may be related to the unique structures, electronic properties and surface functional groups of CNTs. When NO and O2 pass through CNTs, NO is oxidized to NO2 and then adsorbed on the surface of nitrate species. This idea was supported by Mochida et al. Citation35 who reported the oxidation of NO to NO2 at room temperature on activated carbon fibre. Compared with NO or NO2, SO2 can also be adsorbed on CNTs, even though the adsorption rate is not promising while CO2 is much less adsorbed on CNTs.
3.3. CO 2 capture
The capture and storage of carbon dioxide (CO2) produced from fossil-fuelled power plants have received significant attention since the Kyoto Protocol came into force on 16 February 2005. Various CO2 capture technologies including absorption, adsorption, cryogenic, membrane and others have been investigated Citation36 Citation37. Among these technologies, the adsorption–regeneration technology has been recognized as the most developed process. It is the process of amine-based absorption or ammonia absorption process.
However, other technologies are currently being researched all over the world because energy required for the absorption process is still too high. The Intergovernmental Panel on Climate Change (IPCC) concluded that the design of a large-scale adsorption process might be feasible and the development of a new generation of material which is capable of adsorbing CO2 efficiently will undoubtedly enhance the competitiveness of adsorptive separation in a flue gas application Citation38. Those adsorbents include activated carbon, zeolite, silica adsorbents, SWNTs and nanoporous silica-based molecular baskets.
The chemical modification of CNTs will have a good potential to capture the greenhouse gas CO2. shows CO2 adsorption efficiency (q e ) with various modified CNTs.
Figure 4. The number of adsorption equilibrium q e 10% of CO2 adsorbed on raw and modified CNTs Citation34.
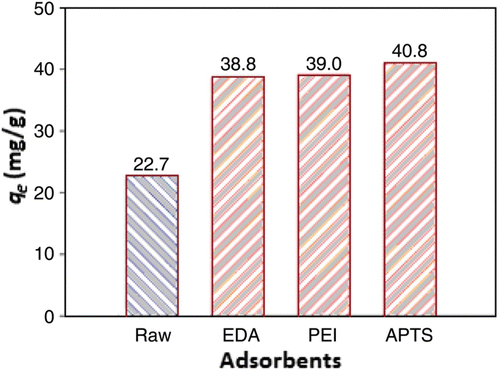
The values of q e increased after the CNT was modified/combined with other chemical solutions, such as ethylene diamine (EDA), polyethyleneimine (PEI) and 3-aminopropyltriethoxysilane (APTS). The solution contains amine groups which can react with CO2 to form carbamate in the absence of water thus boosting the value of q e . APTS-modified CNTs increased q e by a greater amount than the EDA- and PEI-modified CNTs. In general, the performance of CO2 adsorption on modified CNTs increases with the increase in relative humidity, however it decreases with the increase in temperature.
3.4. Removal of volatile organic compounds from air
In addition to nitrogen oxides and sulfur oxides, many chemicals are formed by atmospheric reactions, such as soot Citation39, nitrous acid Citation40, polyaromatic compounds Citation41–43 and volatile organic compounds (VOCs). Clean air regulations have become increasingly stringent as those particles are potentially damaging to human health. Most modern air purification systems are based on photocatalysts, adsorbents such as activated carbon or ozonolysis. However, conventional systems are not very good at getting rid of organic pollutants at room temperature. Japanese researchers have now developed a new material that is very effective for removing VOCs, nitrogen and sulfur oxides from air at room temperature Citation44. It involves highly porous manganese oxide with gold nanoparticles that are grown into it.
To prove the effectiveness of this catalyst, Sinha and Suzuki Citation44 performed tests using three major components of organic indoor air pollutants: acetaldehyde, toluene and hexane. The results showed that all three pollutants in the air were very effectively removed and degraded by this catalyst compared with the conventional catalyst systems.
One reason for the success is porous manganese oxide which has a much larger surface area than all previously known compounds. This large surface area causes better adsorption of volatile molecules. In addition, the adsorbed pollutants are decomposed effectively. Degradation on the surface is very effective because of the presence of free radicals. The presence of gold nanoparticles helps to reduce the barrier of radical formation that is usually very high. This process has opened the possibility for other nano-metal components to be applied.
3.5. Isopropyl alcohol adsorption
In addition to being used as a solvent, isopropyl alcohol (IPA) is often used in the manufacture of optoelectronic devices and semiconductors. Owing to the lack of air pollution control, IPA vapour is released into the atmosphere without any treatment. The release of IPA vapour can cause harm to human health as it is irritating and carcinogenic.
Hsu and Lu Citation45 conducted a study of SWNTs oxidized by a solution of HNO3 and NaClO that was used as an adsorbent to adsorb IPA vapour. Physicochemical properties of SWNTs were improved after being oxidized by HCl, HNO3 and NaClO solution leading to a pore size reduction whereas the surface area of micro-pores, the surface of functional groups and the active surface of the base increased. Consequently, SWCNTs are able to adsorb more IPA vapour from the air stream. SWNTs/NaClO had the best performance to adsorb IPA followed by SWNTs/HNO3. shows the physical and chemical adsorption capacity of IPA by the aforementioned adsorbents above.
Figure 5. The capacity of physical and chemical adsorption of the adsorbent of IPA Citation45.
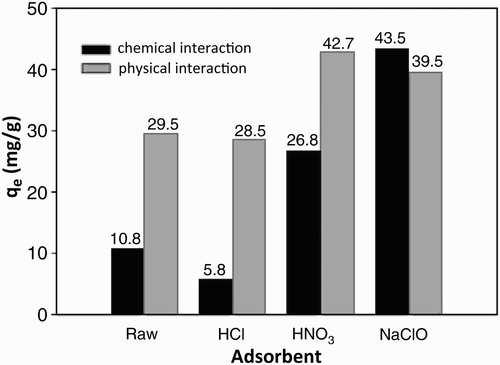
During the adsorption process, IPA has been attracted by physical and chemical interaction. Physical adsorption is due to van der Waals forces between adsorbates and adsorbent while chemical adsorption occurs due to the chemical interaction between adsorbate molecules and adsorbent surface functional groups. The distinction between these two processes is very useful for understanding the factors that affect the rate of adsorption. shows the physical (q ep ) and chemical (q ec ) IPA adsorption capacity of adsorbent with IPA inlet concentration of 500 ppmv. After the SWNTs were oxidized by HNO3 solution and NaClO, the q ep value increased from 29.5 mg/g to 42.7 and 39.5 mg/g, respectively, and q ec increased from 10.8 mg/g to 26.8 and 43.5 mg/g, respectively. The improvement in q ep can be attributed to the pore size of SWNTs which decreased to nearly the size of IPA molecules, thus increasing the physical strength of the bond between SWNTs and IPA vapour. In addition, the increased surface area of micro-pores can increase the bond strength. The improvement in q ec could be due to an increase in the basic surface sites. For relatively low inlet concentration (C in ) of IPA, the IPA vapour adsorption mechanism on SWNTs and SWNTs/NaClO is produced mainly by physical force, whereas for relatively high C in , the IPA vapour adsorption mechanism on SWNTs/NaClO can be generated either by physical or chemical forces.
4. Nanotechnology for sensors and detectors of pollution
It has long been understood that long-term exposure to particulate matter and heavy metal pollution is a significant leading factor in causing health problems in the form of heart conditions, lung cancer and other problems. In urban areas, particulate sizes are typically in the range of 100–300 nm in diameter Citation46 while heavy metals could be found in various ranges of concentration. In addition, heavy metals cannot be broken down by microorganisms (i.e. they are not biodegradable). A high degree of difficulty in the recovery of heavy-metal-contaminated land raises pressure in developing onsite sensors that can detect heavy metal ions before their concentration reaches dangerous levels Citation48.
Rapid and precise sensors able to detect pollutants at the molecular level may enhance the human ability to protect the sustainability of human health and the environment. Large increases in process control, ecosystem monitoring and environmental-based decision-making can occur if the available contaminant detection technology is more sensitive and less expensive. One of the desired technologies is a continuous monitoring tool that is able to provide information, especially information of pollutants in very short analysis time Citation47.
A nanocontact sensor has been developed and this sensor has the potential to detect some metal ions without preconcentration required. In particular, this sensor is suitable for the onsite detection of heavy metal ions, including radioactive elements. Nanocontact sensors can be made in miniature size and automatic mode so that they are easy to use onsite or taken to the land. In addition, the use of these sensors is also inexpensive (cost-effective) because they ate made with conventional microelectronics manufacturing equipment using simple electrochemical techniques Citation49.
4.1. Nanotechnology-based biosensors
Nanotechnology-based biosensors that employ biomaterials have been developed Citation50. Liu et al. Citation51 have found a way to increase the nanoprobe sensitivity of the test strips which will allow the creation of a portable biosensor. Portable biosensors are able to quickly detect people who have been exposed to chemicals. In the Pacific Northwest National Laboratory (PNNL), Wang et al. Citation52 produced a nanoparticle ‘label’ that is able to enhance the ability of sensors to detect and interpret biomarker signals. The approach is based on the electrochemical immunoassay method. This method involves the use of specific antibodies to attract the biomarkers of disease. The provision of ‘label’ on the second antibody with a nanoparticle amplifies the biomarker signals. This raises the level of sensitivity which will improve the precision of the detector to identify the concentration of biomarkers in biological samples Citation53.
4.2. Nanowires and nanotube-based sensors
Nanowires or nanotubes offer tremendous capabilities as materials for chemical and biological sensors Citation54. SWNTs have shown a faster response and higher sensitivity than the conventional probes that are currently used in the detection of gas molecules such as NO2 and NH3. In this case, gas molecules are directly bonded to the surface of SWNTs and influencing the electrical resistance of the sensor. Another advantage of SWNTs as sensors is the ability to achieve high sensing sensitivity at room temperature. In general, conventional solid sensors are operated at temperatures of 200–600°C.
Although SWNTs are highly promising alternatives to nanosensors, SWNTs also have some limitations. First, the current method of the SWNT synthesis produces a mixture of metallic and semiconducting nanotubes, and nanotube is the only material that can be used as a sensor. Second, in order to detect various chemical and biological species, the surface of nanotubes needs to be modified with a specific functional chemical group. Moreover, the flexibility of the chemical detection relies of the type of functional group doped on the nanotube surface. In contrast, some nano-semiconductors such as Si nanowires (SiNWs) do not have this kind of limitation. Boron-doped SiNWs have been used for protein and antibody detection in real-time electrical detection. The small size and the ability of semiconductor nanowire to detect many types of analytes in real-time sensors can be used to develop detectors of chemical and biological agents that are pathogenic in the air, water and food.
4.3. Cantilever sensors
A cantilever sensor is a device made of a silicon cantilever array coated with nano-coating that is sensitive to specific pollutants Citation55. A cantilever is typically 10–500 μm in length, but it has a thickness of less than several micrometres. Interactions between pollutants with the nano-coated cantilever array causes the array to bend as a result of changes in surface pressure. The small bending will be measured by a laser beam which can result in the quantitative measurement of the detected mass of pollutants. Cantilever sensors have been developed to detect VOCs, heavy metals, pesticides and harmful bacteria such as salmonella. shows a schematic diagram of cantilever-based biosensors.
Figure 6. Schematic diagram of how cantilever-based biosensors work: (a) before and (b) after interaction between target molecule and probe. (Picture courtesy of www.nmji.in).
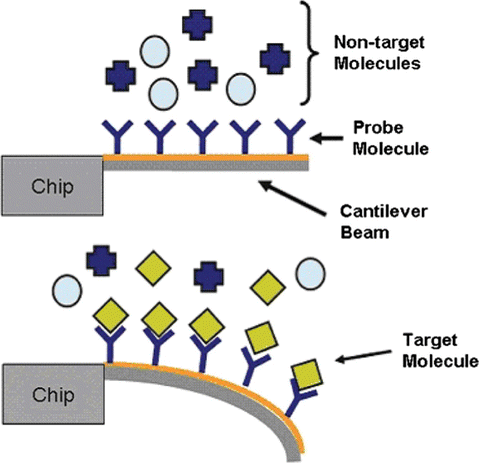
In , the molecular probe (probe) is only appropriate for the target molecule. When the target molecule is attached, the cantilever arm will bend and react. Thus, the reaction will detect and signal the presence of target molecules.
4.4. Other advances in nanotechnology sensors
Some other advances in sensors, detectors and nano-based monitoring technologies are listed as follows Citation56:
| • | Functionalized-tetraphenylsilole nanoparticles are able to form anionic oxidants bonding thereby allowing the detection of carcinogenic substances at very low concentrations. Examples of the detectable substances are Cr(VI) and Ar(V). | ||||
| • | Peptide nanoelectrodes have been employed based on the concept of thermocouple. In a ‘nano-distance’ separation gap, a peptide molecule is placed to form a molecular junction. When a specific metal ion is bound to the gap, the electrical current will result conductance in a unique value. Thus, the metal ion will be identified easily. | ||||
| • | Composite electrodes, a mixture of nanotubes and copper, have been created to detect substances such as organophosphorus pesticides, carbohydrates and other woods pathogenic substances in low concentrations. | ||||
| • | Polymer nanospheres have been developed to measure organic contaminants in very low concentrations, i.e. parts per billion concentrations. | ||||
5. Nanotechnology for pollution prevention
Prevention of pollution refers to a reduction in pollution sources and other practices that utilize raw materials, energy, utilities and other resources effectively in order to reduce or eliminate waste generation. Nanotechnology offers many innovative strategies to reduce waste production in various processes such as improving manufacturing processes, reducing hazardous chemicals, reducing greenhouse gas emissions and reducing the use of biodegradable plastics. The discussion below is just a few of many approaches that can be done to reduce environmental pollution. Nanotechnology is actively involved in this sector, both for producing advanced materials that have low pollution levels and improving production efficiency in industrial processes (e.g. nanocatalysts).
5.1. Environmentally friendly materials (environmentally compliant materials)
The application of nanotechnology is able to create an environmentally friendly substance or material, replacing widely used toxic materials. For example, liquid crystalline display (LCD) computer screens that are more energy efficient and less toxic have largely replaced the screen cathode ray tubes (CRTs) which contain many toxic materials. LCDs also do not contain lead and consume less energy compared with CRT computer screens. The use of CNTs in computer screens may further reduce the impact on the environment by eliminating toxic heavy metals, reducing material and energy needs drastically, as well as improving performance according to customer needs. The example of display technology that uses CNTs is field emission displays (FEDs).
In addition, the application of nanotechnology in composite materials has the potential to produce a material with better mechanical and other properties. This is because nanotechnology has the ability to produce structures that are lighter and smaller without degrading the quality of existing properties. The advantage of this technology is the increased robustness, reduced system costs and whole replacement, as well as reduced environmental impact. Examples of environmentally friendly materials that can be produced using nanotechnology are: biodegradable plastics made from polymers with a molecular structure that is easy to decompose; nanocrystalline composite materials that are not toxic to replace the lithium–graphite electrodes in rechargeable batteries; and glass with self-cleaning ability.
An example of a glass product with self-cleaning capability that has been made widely available in the market is ActivTM Glass, a commercial product from Pilkington. describes how the self-cleaning glass Pilkington ActivTM works.
Figure 7. The self-cleaning mechanism of glass Pilkington ActivTM (Picture courtesy of www.conservatoryland.com).
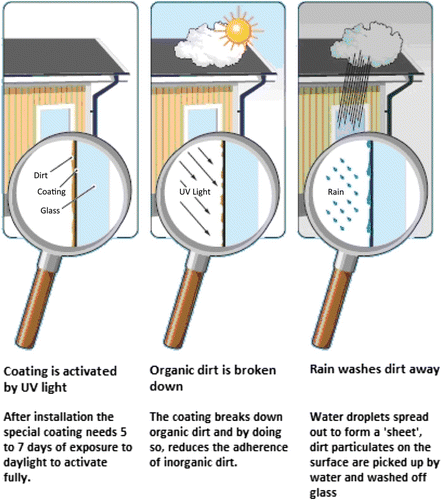
The glass has a layer (coating) made of special TiO2 nanocrystal. When exposed to sunlight, the glass is reacted in two ways. First, the glass decomposes all organic molecules which are deposited on the glass surface. Second, when exposed to rain, the flow of water will bring down the solid pollutants through the glass surface easily. In this product, TiO2 is in the form of a thin film in the range of 2–20 nm applied by high-temperature gas-phase deposition. The film thickness is very important to ensure the maximum photocatalytic activity and transparency.
The surface coating has a hydrophilic property (contact angle with water is 20°, smaller when compared with conventional soda glass with 40° of contact angle of water). When solid pollutants are deposited on the glass surface, the contact angle surface increases then decreases again due to irradiation. Photochemical reactions, which require oxygen, are complex and involve a number of products between the radicals. TiO2 is only acting as a catalyst and is not consumed during the reaction. The reaction will result in the decomposition of organic matter into CO2. Simultaneously, the contact angle surface is reduced further due to irradiation (between 20° to 15°). After irradiation, solid pollutants will be more easily cleaned from the glass surface by rain. Water can be spread effectively by forming a coating on the surface of the glass.
5.2. Textile products and antimicrobial coating
An antimicrobial coating is needed in various applications, such as protecting surfaces and medical equipment or to reduce the attacks from microorganisms. Conventional spray and coating methods for this purpose already exist, but further development is needed in this area because more and more microbes are becoming resistant to widely used antibiotic treatment. To prevent the attachment of bacteria, the topography of the surface of nanocoating with a specific function is shown to be important. Antifouling surface coatings have been investigated for applications in instruments and medical equipment, household appliances and ships. A widely used antimicrobial coating nanomaterial is silver (Ag).
The antibacterial properties of silver are due to the formation of Ag+ ions by bulk metals when they are oxidized. In fact, silverware appliances have antimicrobial activity only if the species is oxidized at the surface. Silver ions affect the oxidative stress in the bacterial cell wall which has an influence on the ability of bacteria to perform respiration. Silver has been proven to be toxic to many types of bacteria, both gram-negative and gram-positive, and fungi.
In recent years, nanoparticles of silver (often called nanosilver) have been added to some consumer products to provide antimicrobial properties. The types of products are very broad, covering household appliances, personal clothing, sports apparel, refrigerators, washing machines, air-filtering equipment, spray disinfectant and cosmetics. Nanosilver is included in the material and equipment through various impregnation techniques (sprayed, painted on the surface of the product, mixed in plastic, etc.). However, broad application of nanosilver is still controversial, especially as in may have an effect on human health Citation57.
5.3. Green manufacturing
The manufacturing process is always accompanied by a wide range of waste production which is harmful to the environment. Ideally, the manufacturing process should be designed to minimize raw material usage, waste production and energy consumption. Green manufacturing is a common name that widely covers methods and technologies to achieve these goals. Green manufacturing includes the development of industrial processes (e.g. water-based processes take precedence over organic solvent-based processes), a reduction in the use of hazardous substances, i.e. metals, the development of green chemicals which are less harmful to the environment and the use of energy-efficient processes.
An example of green nanotechnology is the development of microemulsions (aqueous) as an alternative to VOCs in the cleaning industry. Toxic and carcinogenic compounds, such as chloroform, hexane and perchloroethylene, are commonly used in the cleaning industry, textiles industry and oil extraction. Microemulsions containing nano-sized aggregates can be used as receptors for the extraction of specific molecules at the nanoscale level. Scientists from the University of Oklahoma have synthesized a microemulsion that becomes the connector between water-attractive and water-repellent substances, inserted between the head and tail of the surfactant molecules Citation58. The result is a surfactant that has very low interfacial tension for various oil types. In a test, the microemulsion was able to clean textiles from oil. It was also found that the microemulsion was very competitive to the conventional cleaning compounds, both in the extraction yield and the simplicity of the process.
6. Risk of nanotechnology
Although nanotechnology offers a broad range of potential uses and rapid advances, this technology may also have unintended effects on human health and the environment. shows the potential risk of the application of nanomaterials in our daily life. Materials that are harmless in bulk forms can become highly toxic at the nanoscale, for example, if they enter and build up in drinking water supplies and the food chain, and do not biodegrade. The inhalation of airborne nanoparticles and the impact upon lung disease is a specific concern, with recent studies showing a similar response by the human body to some forms of CNTs as to asbestos particles, if inhaled in sufficient quantities Citation59.
These concerns are exacerbated by the current poor understanding of the fate and behaviour of nanoparticles in humans and the environment. However, it is very early in the development of this technology, and the amount of testing has been relatively limited. Currently many international organizations, such as the Royal Commission on Environmental Pollution (RCEP) Citation37 and European Union Citation4, are aware that laboratory tests on some nanomaterials suggest that they have properties which could cause concern. The understanding of toxicity and potential health risks associated with nanomaterials is extremely limited Citation60.
Nanotechnology risk assessment research for establishing the potential impacts of nanoparticles on human health and the environment is crucial to aid in balancing the technology's benefits and potential unintended consequences Citation7 Citation38. Scientific authorities acknowledge this as a massive challenge, since monitoring the huge volume of diverse nanoparticles being produced and used and their consequent impact is very difficult to track. This strengthens our case for an increase in the amount and type of testing to assess whether these theoretical risks are real, and to monitor their behaviour in the environment.
7. Conclusions
Nanotechnology has been developed to achieve the purpose of maintaining environmental sustainability. In this case, environmental sustainability is not limited to human environmental issues, but also human health problems. Technologies that have been developed include technologies which can enhance and improve the conventional technological capabilities and new technologies which replace the conventional technologies.
The water purification process using nanotechnology can use iron nanoparticles, ferritin, polymeric nanoparticles, nanofibres, nanobiocides, nanoenzymes and nanofiltration techniques. Despite being applied in cleaning and water purification, nanotechnology can also be applied to clean the air from toxic gases such as CO, VOCs and dioxins using CNTs, gold nanoparticles and other adsorbents. Nanoparticles and nanotubes can also be applied as a sensor for toxic substances, particularly substances that are difficult to detect with conventional technology because they have a very small in size and concentration.
The application of nanotechnology in the environmental field is not limited to the conditions where environmental contamination has occurred. Nanotechnology can also be applied to prevent the creation of pollution. Its applications include the synthesis of green materials, coatings and biocides to prevent the release of hazardous substances into the environment.
Although nanotechnology has many applications in the fields of environmental technology, it needs to be studied further to assess its risk. This is in accordance with the principle that the more sophisticated the technologies, the greater the risks they pose.
Acknowledgements
The author thanks to the Korea Institute of Science and Technology (KIST), Seoul, Korea as some parts of this work have been supported by KIST IRDA Alumni Program.
References
- Environmental Defense Fund . The health risks of burning coal for energy Report, 2006. Available at http://www.edf.org/climate/remaking-energy.
- Krantzberg , G. , Tanik , A. , do Carmo , J. S.A. , Indarto , A. and Ekda , A. 2010 . Advances in water quality control , Scientific Research Publishing .
- Roco , M. C. , Williams , S. and Alivisatos , P. 1999 . Nanotechnology research directions: vision for nanotechnology in the next decade , Washington, DC : IWGN Workshop Report, U.S. National Science and Technology Council .
- European Commission . 2010 . Communicating nanotechnology. Why, to whom, saying what and how? Available at http://cordis.europa.eu/nanotechnology.
- Lu , G. Q. and Zhao , X. S. 2004 . “ Nanoporous Materials - Science and Engineering ” . In Nanoporous Materials-an Overview , Singapore : World Scientific .
- Royal Commission on Environmental Pollution (RCEP) . Novel materials in the environment: the case of nanotechnology 2008. Available at http://www.defra.gov.uk/news/2005/050310a.htm.
- Hyder , M. A.H. 2003 . Nanotechnology and environment: potential application and environmental implications of nanotechnology , Germany : Technical University of Hamburg-Harburg . Master thesis
- Indarto , A. , Choi , J. W. , Lee , H. and Song , H. K. 2008 . Decomposition of greenhouse gases by plasma . Environ. Chem. Lett. , 6 : 215 – 222 . (doi:10.1007/s10311-008-0160-3)
- Zhang , W. 2005 . “ Nanotechnology for water purification and waste treatment ” . In Frontiers in Nanotechnology , Washington, DC : U.S. EPA Millennium Lecture Series .
- Cloete , T. E. , de Kwaadsteniet , M. , Botes , M. and Lopez-Romero , J. M. 2010 . Nanotechnology in water treatment applications , Norfolk : Caister Academic Press .
- Kunze , C. , Schulz , R. , Teunchens , L. and Velzen , L. V. 2010 . Environmental remediation of radioactively contaminated sites , Environmental Radiation Survey and Site Execution Manual (EURSSEM .
- Sharmaa , Y. C. , Srivastava , V. , Singh , V. K. , Kaul , S. N. and Weng , C. H. 2009 . Nano-adsorbents for the removal of metallic pollutants from water and wastewater . Environ. Technol. , 30 : 583 – 609 . (doi:10.1080/09593330902838080)
- Tratnyek , P. G. and Johnson , R. L. 2006 . Nanotechnologies for environmental cleanup . NanoToday , 1 : 44 – 48 .
- Uyttebroek , M. , Baillieul , H. , Vermeiren , N. , Scholiers , R. , Devleeschauwer , P. , Gemoets , J. and Bastiaens , L. In situ remediation of a chlorinated ethene contaminated source zone by injection of zero-valent iron: from lab to field scale . Proceedings of PRB/RZ .
- Miehr , R. , Tratnyek , P. , Bandstra , J. Z. , Scherer , M. M. , Alowitz , M. J. and Bylaska , E. J. 2004 . Diversity of Contaminant reduction reactions by zerovalent iron: role of the reductate . Environ. Sci. Technol. , 38 : 138 – 147 . (doi:10.1021/es034237h)
- O'Carroll , D. , Sleep , B. , Krol , M. , Boparai , H. and Kocur , C. 2012 . Nanoscale zero valent iron and bimetallic particles for contaminated site remediation . Adv. Water Res. , DOI: 10.1016/j.advwatres.2012.02.005.
- Theil , E. 1987 . Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms . Ann. Rev. Biochem. , 56 : 289 – 315 . (doi:10.1146/annurev.bi.56.070187.001445)
- Moretz , P. 2004 . Nanoparticles developed that could clean environment . Temple Times , http://www.temple.edu/temple_times/9-9-04/nanoparticles.html.
- Watlington , K. 2005 . Emerging nanotechnologies for site remediation and wastewater treatment , National Network for Environmental Management Studies Fellow, North Carolina State University .
- U.S. Environmental Protection Agency . 1998 . Toxicological review of trivalent Chromium , Washington, DC : National Center for Environmental Assessment, Office of Research and Development .
- Liang , H. C. , Li , X. Z. and Nowotny , J. 2010 . Photocatalytic properties of TiO2 nanotubes . Solid State Phenom. , 162 : 295 – 328 . (doi:10.4028/www.scientific.net/SSP.162.295)
- Kamat , P. , Huehn , R. and Nicolaescu , R. 2002 . A “Sense and Shoot” approach for photocatalytic degradation of organic contaminants in water . J. Phys. Chem. B , 106 : 788 – 794 . (doi:10.1021/jp013602t)
- Tungittiplakorn , W. , Lion , L. W. , Cohen , C. and Kim , J. Y. 2004 . Engineered polymeric nanoparticles for soil remediation . Environ. Sci. Technol. , 38 : 1605 – 1610 . (doi:10.1021/es0348997)
- Li , Q. , Mahendra , S. , Lyon , D. Y. , Brunet , L. , Liga , M. V. , Li , D. and Alvarez , P. J.J. 2008 . Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications . Water Res. , 42 : 4591 – 4601 . (doi:10.1016/j.watres.2008.08.015)
- Mamadou , D. , Duncan , J. S. , Savage , N. , Street , A. and Sustich , R. C. 2009 . Nanotechnology applications for clean water , Norwich , NY : William Andrew Inc .
- Marguerite du Plessis , D. 2011 . Fabrication and characterization of anti-microbial and biofouling resistant nanofibers with silver nanoparticles and immobilized enzymes for application in water filtration , University of Stellenbosch . Master thesis
- van der Bruggen , B. , Manttari , M. and Nystrom , M. 2008 . Drawbacks of applying nanofiltration and how to avoid them: a review . Separat. Purificat. Technol. , 63 : 251 – 263 . (doi:10.1016/j.seppur.2008.05.010)
- Hilal , N. , Al-Zouhi , Darwish , H. A. , Muhammad , A. W. and Arabi , M. A. 2004 . A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy . Desalination , 170 : 281 – 308 . (doi:10.1016/j.desal.2004.01.007)
- Kulkarni , P. S. , Crespo , J. G. and Afonso , A. M. 2008 . Dioxins sources and current remediation technologies – A review . Environ. Intl , 34 : 139 – 153 . (doi:10.1016/j.envint.2007.07.009)
- Wielgosiński , G. 2010 . The possibilities of reduction of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans emission . Intl J. Chem. Eng. , 2010 article ID 392175.
- Cudahy , J. J. and Helsel , R. W. 2000 . Removal of products of incomplete combustion with carbon . Waste Manag. , 20 : 339 – 345 . (doi:10.1016/S0956-053X(99)00335-9)
- Long , R. Q. and Yang , R. T. 2001 . Carbon nanotubes as a superior sorbent for removal dioxine . J. Amer. Chem. Soc. , 123 : 2058 – 2059 . (doi:10.1021/ja003830l)
- Bhushan , B. 2010 . Springer Handbook of Nanotechnology , 3 , New York : Springer .
- Long , R. Q. and Yang , R. T. 2001 . Carbon nanotubes as a superior sorbent for nitrogen oxides . Ind. Eng. Chem. Res. , 40 : 4288 – 4291 . (doi:10.1021/ie000976k)
- Mochida , I. , Kawabuchi , Y. , Kawano , S. , Matsumura , Y. and Yoshikawa , M. 1997 . High catalytic activity of pitch-based activated carbon fibers of moderate surface area for oxidation of NO to NO2 at room temperature . Fuel , 76 : 543 – 548 . (doi:10.1016/S0016-2361(96)00223-2)
- White , C. M. , Strazisar , B. R. , Granite , E. J. , Hoffman , J. S. and Pennline , H. W. 2003 . Separation and capture of CO2 from large stationary sources and sequestration in geological formations-coalbeds and deep saline aquifers . J. Air Waste Manag. Assoc. , 53 : 645 – 715 . (doi:10.1080/10473289.2003.10466206)
- Aaron , D. and Tsouris , D. 2005 . Separation of CO2 from flue gases: a review . Separat. Sci. Technol. , 40 : 321 – 348 . (doi:10.1081/SS-200042244)
- Metz , B. , Davidson , O. , de Coninck , H. , Loos , M. and Meyer , L. 2005 . Carbon dioxide capture and storage , Cambridge : Cambridge University Press .
- Indarto , A. 2009 . Soot growing mechanism from polyynes: a review . Environ. Eng. Sci. , 26 : 251 – 257 . (doi:10.1089/ees.2007.0325)
- Indarto , A. 2012 . Heterogeneous reactions of HONO formation from NO2 and HNO3: a review . Res. Chem Interned. , 38 : 1029 – 1041 . (doi:10.1007/s11164-011-0439-z)
- Santiago , R. M. and Indarto , A. 2008 . A density functional theory study of phenyl formation initiated by ethynyl radical (C2H·) and ethyne (C2H2) . J. Mol. Model , 14 : 1203 – 1208 . (doi:10.1007/s00894-008-0376-y)
- Natalia , D. and Indarto , A. 2008 . Aromatic formation from vinyl radical and acetylene. A mechanistic study . Bull. Korean Chem. Soc. , 29 : 319 – 322 . (doi:10.5012/bkcs.2008.29.2.319)
- Indarto , A. , Giordana , A. , Ghigo , G. and Tonachini , G. 2009 . Formation of PAHs and soot platelets: multiconfiguration theoretical study of the key step in the ring closure-radical breeding polyyne-based mechanism . J. Phys. Org. Chem. , 23 : 400 – 410 .
- Sinha , A. K. and Suzuki , K. 2007 . Novel mesoporous chromium oxide for VOCs elimination . Appl. Catal. B: Environ. , 70 : 417 – 422 . (doi:10.1016/j.apcatb.2005.10.035)
- Hsu , S. and Lu , C. 2007 . Modification of single-walled carbon nanotubes for enhancing isopropyl alcohol vapor adsorption from water streams . Separat. Sci. Technol. , 42 : 2751 – 2766 . (doi:10.1080/01496390701515060)
- Johnston , M. V. Real-time measurement of chemical composition of fine and ultrafine airborne particles . Proceedings of EPA Nanotechnology and the Environment: Applications and Implications STAR Progress Review Workshop . pp. 23 – 24 .
- Masciangioli , T. and Zhang , W. X. 2011 . Environmental technologies at the nanoscale . Environ. Sci. Technol. , 37 : 102A – 108A . (doi:10.1021/es0323998)
- Choi , K. Ethical issues of nanotechnology in the Asia-Pacific region . pp. 341 – 342 . Bangkok : Regional Meeting on Ethics of Science and Technology .
- Tao , N. A nanocontact sensor for heavy metal ion detection . Proceedings EPA Nanotechnology and the Environment: Applications and Implications STAR Progress Review Workshop . pp. 28 – 29 .
- Staiano , M. , Baldassarre , M. , Esposito , M. , Apicella , E. , Vitale , R. , Aurilia , V. and D'Auri , S. 2010 . New trends in bio/nanotechnology: stable proteins as advanced molecular tools for health and environment . Environ. Technol. , 31 : 935 – 942 . (doi:10.1080/09593331003639575)
- Liu , G. , Lin , Y. Y. , Wang , J. , Wu , H. , Wai , C. M. and Lin , Y. 2007 . Disposable electrochemical immunosensor diagnosis device based on nanoparticle probe and immunochromatographic strip . Anal. Chem. , 79 : 7644 – 7653 . (doi:10.1021/ac070691i)
- Wang , J. , Liu , G. and Lin , Y. 2007 . Immunosensors based on functional nanoparticle labels . ECS Transactions , 2 : 1 – 7 . (doi:10.1149/1.2408982)
- Lin , Y. and Gavaskar , A. 2004 . Environmental benefits of nanotechnology applications through technology: nano-based biosensors . Nanoscience Batelle ,
- Smart , S. K. , Cassady , A. I. , Lu , G. Q. and Martin , D. J. 2006 . The biocompatibility of carbon nanotubes . Carbon , 44 : 1034 – 1047 . (doi:10.1016/j.carbon.2005.10.011)
- Filipponi , L. and Sutherland , D. 2010 . “ NANOYOU teachers training kit in nanotechnologies ” . Interdisciplinary Nanoscience Centre, iNANO, Aarhus University .
- Berger , M. 2008 . Nanotechnology and water treatment . Available at http://www.nanowerk.com/spotlight/spotid=4662.php.
- Burke , M. 2012 . “ Nanosilver in consumer goods under the spotlight ” . Chemistryworld .
- Sabatini , D. A. , Do , L. D. and Phan , T. T. Microemulsions with extended-surfactants: characterization and applications . 101st AOCS Annual Meeting and Expo . Arizona .
- Buzea , C. , Blandino , I. I.P. and Robbie , K. 2007 . Nanomaterials and nanoparticles: Sources and toxicity . Biointerphases. , 2 : MR17 – MR172 . (doi:10.1116/1.2815690)
- Wickson , F. , Nielsen , K. N. and Quist , D. 2011 . “ Nano and the environment: potential risks, real uncertainties and urgent issues ” . GenØk Biosafety Brief 2011/01 .