ABSTRACT
Inhalation of aerosols containing Legionella pneumophila, a water-borne bacteria commonly found in natural and manmade water systems, is the main causative agent of Legionnaires’ disease (LD). Approximately 10–15% of all reported cases of LD result in fatality, with susceptibility to the disease being higher in immunosuppressed patients, men over 45 years of age, alcoholics, smokers and individuals with underlying diseases. The World Health Organisation (WHO), European Centre for Disease Prevention and Control (ECDC) and The United Kingdom (UK) Health and Safety Executive (HSE) have implemented a strict code of practice and guidelines to minimise the risk of the public from contracting LD. This paper provides a critical review of these three published guidelines. Evidence suggests that the current detection methods for Legionella, by culture and quantitative polymerase chain reaction (qPCR), show large disparities in the detection and quantification of bacteria in water samples, raising concerns about the reliability of measures needed to safeguard public health. Moreover, a survey of 20 residential building complexes in different London boroughs highlights the need for a review of remedial action recommendations and a more inclusive risk assessment strategy that protects ‘at risk’ people in society.
GRAPHICAL ABSTRACT
Introduction
Legionnaires’ disease (LD) is a serious form of pneumonia caused by the bacterium Legionella pneumophila, which naturally occurs in fresh water habitats such as rivers, lakes and wet soil [Citation1]. Importantly, Legionella is increasingly detected at high levels in the manmade environment, with contamination of domestic hot and cold water storage systems, showerheads, cooling towers, spa pools, humidifiers, water closet (WC) cisterns, water fountains, water features and irrigation systems being reported [Citation2]. More than 60 Legionella species, encompassing at least 70 serogroups (approximately half of which have been isolated from, or detected in, clinical specimens), have been identified so far, with the most common species in the United Kingdom (UK), European Union (EU) and United States of America (USA) being L. pneumophila serogroup-1 [Citation3–6]. Legionella is capable of surviving at temperatures ranging from <0°C to 60°C (there is some evidence for survival up to even higher temperatures [Citation7]), and can reproduce at temperatures between 20°C and 45°C, with maximum virulence at ∼37°C.
Allegra et al. [Citation8] proposed that Legionella bacteria can become heat resistant, as some strains of Legionella submitted to superheating in the environment for a long time develop resistance to high temperatures as demonstrated by the high proportion of culturable cells and viable but non-culturable (VBNC) cells still present after a 30-min treatment at 70°C. In a separate study, a 30-min heat-shock treatment at 70°C performed twice in a test loop was unable to remove the biofilm [Citation9]. The results showed that although Legionella diversity was reduced, pathogenic Legionella species (Legionella pneumophila and Legionella anisa) remained after the heat shock and also after chemical treatments. The biofilm was not removed, and the bacterial community structure was transitorily affected by the treatments. It was concluded that eradication of Legionella requires a better understanding of the ecology of bacterial and eukaryal species associated with Legionella-containing biofilms.
Factors positively associated with Legionella proliferation in manmade systems include water stagnation, water pH (between 6.0 and 8.0), the existence of sludge, scale and corrosion products (possibly acting as nutrients), and the presence of biofilms including protozoa (within which it can persist during extreme conditions) [Citation10]. LD is contracted by inhaling or aspirating aerosols (the importance of the aspiration route is often underappreciated) containing the bacteria, and once inside the lungs the normal incubation period is typically 2–16 days and occasionally even longer, after which the disease can be fatal if not diagnosed and treated appropriately [Citation11,Citation12]. Approximately 10–15% of the reported cases of LD are fatal, and this figure can be higher in certain groups including the immunosuppressed (especially patients in hospitals), men over 45 years of age, alcoholics, smokers, and those with kidney disease, respiratory problems, cancer and diabetics [Citation13]. Legionella can also cause two non-fatal illnesses known as Pontiac fever and the synonymous Lochgoilhead fever, both showing milder flu-like symptoms [Citation14].
Legionella can actively colonise and proliferate within domestic hot and cold water systems, and this presents one way for humans to come into contact with Legionella thereby increasing their risk of contracting the disease [Citation15]. The World Health Organisation (WHO), European Centre for Disease Prevention and Control (ECDC), European Legionnaires’ Disease Surveillance Network (ELDSNet) and UK Health and Safety executive (HSE) have published guidelines used to manage the risk of contracting Legionella, thereby protecting public health [Citation15–18]. However, a number of inadequacies were identified in these guidelines. The latest HSE guidance on the legal requirements to control Legionella bacteria in domestic hot and cold water systems HSG274 Part 2 (published in 2014) has addressed many of the critical areas for practical Legionella control that were missing in the WHO and ECDC guidelines. Despite the valuable role of the HSE ACOP L8 [Citation19] and HSG 274 Part 2 [Citation20] in managing the risks of exposure to Legionella, there are still concerns and shortcomings that must be addressed and planned for. This article looks critically at the HSE ACOP L8 [Citation19] and HSG 274 Part 2 [Citation20] compared with the WHO guidelines ‘LEGIONELLA and the prevention of legionellosis’ [Citation16] and ECDC TECHNICAL DOCUMENT European Legionnaires’ Disease Surveillance Network (ELDSNet) Operating procedures (2011) from a practitioner’s perspective, and identifies limitations in the current detection and management of Legionella, in order to promote greater awareness, dialogue and research in key areas.
Critical review of the HSE ACOP L8 Citation2013 and HSG 274 Part 2
Defining those who are most at risk
Legionella bacteria remain a continuous hazard to human health due to their specialised characteristics [Citation21]. In the latest HSE guideline HSG274 Part 2 (published in 2014), special consideration has been given to healthcare premises and care homes. This includes remedial action, such as investigation, for any detection of Legionella between 1 and 100 colony forming units (cfu)/L in domestic water systems, whereas no action is recommended for the same level of Legionella detection in other premises such as residential complexes with communal hot and cold water systems, considered to be low-risk areas. Healthcare premises (such as hospitals and care homes) are deemed ‘high-risk’ areas due to the high proportion of vulnerable groups with increased susceptibility to LD due to existing illness and/or impaired immune system [Citation20]. (an excerpt of HSG274 Part 2) compares the guidance on remedial action in the event of a positive Legionella result in both high-risk areas (hospitals and care homes) and low-risk areas (residential homes). According to this guidance, a greater level of remedial action or investigation is necessary if Legionella is detected below 100 cfu/L in healthcare premises. However, in non-healthcare premises, the same result would require no remedial action or intervention of any kind [Citation22].
Table 1. Comparison of recommended actions following Legionella detection in water samples taken from hot and cold water systems in healthcare and non-healthcare premises according to HSG274 Part 2.
WHO guidelines [Citation16] state a target level of <1000 cfu/L Legionella spp. for patients with classical individual risk factors and <50 cfu/L Legionella spp. in some areas for high-risk patients in healthcare premises, which is arguably too high (). However, similar to the HSE guideline HSG274 Part 2, there are no guidelines given for Legionella detection in non-healthcare premises. Furthermore, European guidelines recommend remedial action only between 1000 and 10,000 cfu/L () regardless of whether these are detected in healthcare or non-healthcare premises [Citation18,Citation23]. This variability in the stringency of control measures may further accelerate the number of community-acquired pneumonia (CAP) cases. A study carried out on the available European data collated between 2005 and 2012 indicated CAP to cost society around €10 billion annually due to hospitalisation and lost working days [Citation24]. Campese et al. [Citation25] have reviewed the current knowledge of LD in France illustrated by the epidemiological situation in 2013. In the United States, the Environmental Protection Agency (EPA) also provides guidance on the control of Legionella bacteria in water systems. However, updated guidelines for the control of Legionella in Western Pennsylvania published in October 2014 state that ‘in the absence of more definitive evidence or explicit US federal guidance, guidelines from UK and EU can be considered’ [Citation26].
Table 2. WHO guidelines used for verification and corrective action for Legionella detected in samples taken from hot and cold water systems.
Table 3. EU guidelines for required action following Legionella detection in hot and cold water systems.
The US Waterborne Disease and Outbreak Surveillance System (WBDOSS) has reported the increasing importance of LD contracted from individual residential water systems since monitoring commenced in 2001 [Citation27]. A domestic hot water system was also shown to be responsible for an outbreak of LD originating from a residential block of flats in Copenhagen (Denmark) [Citation28]. In Germany, 93.9% of samples (n = 452) taken from domestic water systems of single family residences supplied with treated groundwater were positive for Legionella pneumophila (71.8% serogroup-1), and 12% samples showed the maximum Legionella count of 100,000 cfu/100 mL [Citation29,Citation78]. These studies illustrate that Legionella can occur at high levels in private residential homes, and highlight the need to manage the risks of exposure of susceptible individuals in society to Legionella in non-healthcare premises.
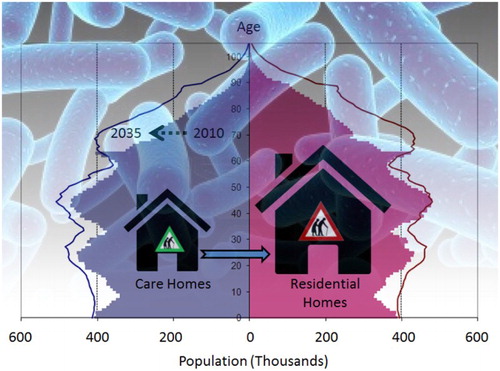
Major contributing factors affecting susceptibility to Legionella bacteria are a weakened immune system, being over 45 years of age, and suffering from existing illness such as respiratory problems, kidney disease, diabetes and cancer [Citation15,Citation30]. Currently, one in six people (17%) living in the UK are aged 65 and above, and it is estimated that this figure will be one in four (25%) by 2050 [Citation31]. Data from Eurostat on population structure and ageing clearly show an increasing trend for an ageing population within EU countries (). By 2080, the total proportion of people over 65 years of age residing in the EU is expected to reach 28.7% compared to 18.9% in 2015 [Citation32]. In 2009, 23% of the total population of Japan was over 65 years of age, and this is expected to rise above 33% by 2030 [Citation33]. Also, in the USA, people aged 65 and above is expected to reach 20% of the total population by 2030 compared to 13% in 2010, with further increases by 2050 [Citation34]. In a recent report by the WHO, it states that ‘as people get older, they become less active and the overall evidence for adults aged 65 years and above showed that they become more susceptible to disease compared to active individuals’ [Citation35]. However, once men reach 45 years they are known to be at higher risk of contracting LD [Citation36], and this risk increases with age up to 65 years of age and above. All these projections indicate the necessity of precautionary measures to be implemented to protect the health of this potential vulnerable population in terms of CAP. Among the over 65-year olds, diabetes and chronic kidney diseases (CKD) are the most common risk factors for LD [Citation37], and about 40% of individuals aged 65 and above living in the UK suffer from long-standing illnesses [Citation38]. In 2013, 11.1 million people in the UK were reported to be over 65 years old, and forecasts suggest that this figure will rise to approximately 16.9 million by 2035 [Citation31]. As revealed by the 2011 Census Analysis, only 3% of over 65-year olds reside in care homes across England and Wales, and 2.5% in London [Citation39]. A recent estimate suggests that there are 5153 nursing homes and 12,525 residential homes in the UK and approximately 405,000 people aged 65 and above live in these homes [Citation38]. By inference, approximately 10.7 million over 65-year olds in the UK, therefore, reside within their own flats and apartments of residential estates sharing communal hot and cold water facilities.
Table 4. Ageing population trend in Europe during the period 2005–2015 taken from Eurostat.
Exposure of people over 65 years of age to Legionella in their homes, even at relatively low concentrations, and at levels that are considered to be acceptable according to HSG274 Part 2, raises serious concerns about possible health implications [Citation40]. There are already clear warnings in the literature about the increasing burden of age-related disease in the UK society, with more than 6 million people aged 65 and above with long-standing serious illness expected by 2030 based on the current trends [Citation38].
In order to verify this concern, we conducted a survey of 20 residential building complexes in different London boroughs (). These buildings consisted of flats or apartments of varying size from 64 to 645 units. On average, 18% of the residents were found to be 65 years of age or over (confirming Government statistics), and most reported suffering from impaired immune systems or underlying diseases such as diabetes, cancer, respiratory problems and kidney diseases making them susceptible to Legionella infection. Importantly, the ageing population tend to prefer these shared dwellings over detached or semidetached houses due to the increased security, variety of communal facilities (such as leisure centres, water features and gardens) which they can enjoy without responsibility for the day-to-day maintenance [Citation41]. However, the ageing residents within these complexes are as vulnerable to Legionella infection as care home residents due to continuous use of domestic hot and cold water systems [Citation42]. Therefore, it is our opinion that residential complexes should also be categorised as ‘high-risk’ settings for LD based on the percentage of the elderly and vulnerable population inhabiting each building.
Table 5. Survey report of 5924 residents in 20 residential complexes from different boroughs in London for Legionella risk factors, including age and pre-existing long-term illness.
Quantifying Legionella to inform remedial actions
HGS274 recommends that water sample analysis for Legionella should only be performed in United Kingdom Accreditation Service (UKAS)-accredited laboratories and most of these laboratories currently use culture methods as their standard analysis method [Citation43,Citation44]. Quantitative data presented in the form of colony forming units per litre water (cfu/L) then form the basis for remedial action, if any, according to . Culture methods using Buffered Charcoal-Yeast Extract (BCYE) agar is still the ‘gold standard’ diagnostic procedure used [Citation45]. It can take up to 11 days for the full diagnosis and report to become available using the culture method, thereby increasing the likelihood of exposure to Legionella in contaminated buildings following delays in taking preventative actions [Citation44,Citation46]. Recent studies comparing culture methods to molecular biology approaches (quantitative polymerase chain reaction, qPCR) report large differences in Legionella count, with the BYCE Agar culture method often underestimating the presence of Legionella in around 50% of cases [Citation47]. Indeed, a sample recently analysed by ALcontrol Laboratories for Aqua Technologies produced a culture result <100 cfu/L whilst the molecular determination by PCR reported 2448 genomic unit per litre (GU/L) (2015 personal observation of the authors; unreferenced, data not shown). Thus, significant challenges exist even today in reliably quantifying Legionella bacteria using culture methods due to the growth of other microorganisms and the presence of ‘VBNC’ forms [Citation43,Citation48]. Other often overlooked concerns are the sampling protocol, the associated sample preservation procedure and sample transport conditions to the receiving laboratory carrying out the analysis. If a sample is taken in a way that makes it unfit for purpose (e.g. without immediate neutralisation of any biocides present), then the analysis result used for regulatory action will be unfit for purpose. This also applies to ensuring fit for purpose sample preservation and transport. Typically, sample bottles of 500–1000 mL should be suitable. Sampling guidance is available [Citation49,Citation50]. Ultimately, imprecision in the quantification of Legionella will negatively impact the effectiveness of enforcing authorities to recommend sensible practical guidelines for interpreting monitoring results.
A comparative study of 3967 environmental samples analysed for Legionella using both culture methods and qPCR found large differences, with only 34% (n = 1331) of samples testing positive with culture methods compared to 72% (n = 2856) using qPCR [Citation47]. Culture methods may therefore (i) underestimate the presence of Legionella in samples [Citation51], (ii) struggle to detect low concentrations of bacteria in environmental samples that fall within the regulatory framework, and (iii) introduce delays between monitoring and remedial action [Citation46]. Inaccurate quantification of Legionella (especially at concentrations below 1000 cfu/L) and underestimation of viable bacteria in water samples (used for informing remedial action) raise important questions about the appropriateness of measures used in certain settings to protect human health, as well as the appropriateness of ‘no action’ in non-healthcare premises where Legionella counts below 100 cfu/L are detected [Citation51,Citation52].
An alternative rapid and sensitive testing method for quantifying Legionella bacteria in water is qPCR [Citation53]. However, qPCR often overestimates the concentration of Legionella as this approach detects both living and non-living Legionella cells [Citation46]. Another reported disadvantage of qPCR is that the amplification reactions can be inhibited by certain substances found in the environmental samples thereby producing inaccurate results [Citation54]. Calcium ions, rust, bile salts, urea, phenol, ethanol, polysaccharides, sodium dodecyl sulphate, humic acids, tannic acid, melanin, different proteins such as collagen, myoglobin, haemoglobin, lactoferrin, immunoglobin G (IgG) and proteinases are some of the inhibitors possibly present in environmental samples [Citation55,Citation56]. The problem of environmental inhibitors can be addressed by simply measuring them in water samples prior to analysis, and if present, diluting the DNA extract a further 10-fold prior to qPCR [Citation57,Citation58]. However, regardless of the approach used, the variability in measures to quantify Legionella in environmental samples is a barrier to effective monitoring and protection of public health. More accurate, reliable and rapid standard testing methods are therefore needed for the effective control of Legionella bacteria in domestic water systems [Citation52]. As stated above, appropriate robust sampling protocols need to be applied. Finally, laboratory clients should request long-term proficiency test results of their testing laboratory. Also, it is worth bearing in mind that in the authors’ opinion most proficiency samples reporting tend to be ‘best case’ examples.
Future perspectives – climate change
A study carried out by the ELDSNet found that 10,582 out of 11,836 suspected cases of LD were confirmed between 2009 and 2010 in the EU. Out of the 10,582 confirmed cases, 71% (n = 7397) were community acquired. Healthcare-related cases were only 8% (n = 893) and the remaining 20% (n = 2187) were travel-related. The UK Health Technical Memorandum for Safe water in healthcare premises – Part B also confirms that ‘the incidence of healthcare associated waterborne illness, including Legionnaires’ disease, is relatively low’ [Citation59]. Forty-three per cent of total reported cases (n = 5100) were found in individuals aged 65 and above [Citation60]. This study also confirmed a significant increase of reported cases in 2010 (n = 775) possibly as a result of the exceptionally warm summer that year [Citation61]. Long-term climate predictions indicate that EU and UK temperatures and precipitation are likely to increase in the near future as a part of global warming [Citation62,Citation63] and environmental conditions are likely to become increasingly favourable to the proliferation of Legionella bacteria in water systems [Citation64]. Indeed, warmer domestic cold water temperatures would enhance Legionella survival and replication, and the likelihood of exposure could be exacerbated further by increased humidity which may prolong L. pneumophila survival in aerosols [Citation65,Citation66]. Investigations carried out in Netherlands and the UK confirm that warm and wet weather conditions can lead to a greater incidence of community-acquired LD due to changes in the rate of Legionella proliferation [Citation67–69], suggesting possible increases in community-acquired LD cases in EU and in the UK, and more widely, as a result of predicted global warming.
Understanding the real extent of LD in society
The significant number of people classed as vulnerable/more susceptible/high risk residing in non-healthcare premises is not presently adequately protected by HSE ACOP L8 [Citation19] and HSG 274 Part 2, combined with the possible role of changing external factors (e.g. climate change) in the UK, clearly indicates that a greater number of people are likely to be exposed to Legionella than presently thought. The latest report from the British Lung Foundation showed that 0.5–1.1% of adults in the UK get pneumonia each year [Citation70], and 0.1% of UK adults receive treatment for pneumonia every year by the National Health Service (NHS) [Citation71]. However, in most instances, these cases are treated primarily based on their symptoms, and a specific diagnosis of the cause of the illness is not routinely made. Therefore, it is likely that the 306 cases of Legionella reported annually [Citation72] are not a reflection of the true extent of LD in the England and Wales. These findings can further be supported by a study in Germany led by The Competence Network for Community Acquired Pneumonia (CAPNETZ); thus reported that Legionella species was the causative pathogens in 3.8% of CAP cases but only 3.7% in hospitalised patient-related cases. Legionella pneumophila was the predominant species in both community-acquired and hospitalised cases. According to a recent clinical review of the diagnosis and management of pneumonia in the UK, 2–8% of all CAP cases are caused by Legionella pneumophila [Citation73]. It is vital to understand that this 2–8% reported is excluding pneumonia in immunocompromised groups or pneumonia as a pre-terminal event. This reported percentage of LD cases would be much higher if immunocompromised groups also included. Reports indicate that 261,000 cases of CAP have been diagnosed annually during 1992–1993 in the UK, costing £4407 million to the NHS based on 1992/1993 prices [Citation74]. Furthermore, two large-scale studies investigating the incidence of pneumonia in hospitals in England reported significant increases in admissions between 1998–2014 and 2002–2009, respectively [Citation22]. Moreover, 2012 NHS reports showed that the costs of pneumonia-related admissions in hospitals account for £1,700–£5,100 per each admitted patient [Citation73]. It is likely, therefore, that the current financial burden on the NHS, related to CAP, is much higher than the 1992/1993 cost of £440.7 million [Citation75]. If cases of pneumonia are treated with normal antibiotics without proper diagnosis for LD, the health consequences can be extremely serious, leading to long-term morbidity, and creating additional cost burdens to the NHS [Citation74,Citation76]. Indeed, a long-term study carried out of 122 survivors of LD in the Netherlands found persistent symptoms of fatigue (in 75% of patients), neurologic symptoms (in 66%) and neuromuscular symptoms (in 63%) after 17 months despite the original diagnosis of LD [Citation77].
Shortcomings in the Legionella risk assessment
The first two important steps in Legionella control are (i) to carry out a detailed Legionella risk assessment to identify the possible risk factors and (ii) establish a regular monitoring programme on the basis of the highlighted risks [Citation15]. WHO guidelines, EU guidelines and HSE regulations all require a suitable and sufficient risk assessment and a regular review of the assessment to make necessary changes to keep the risk assessment up to date. There are proper guidelines given in HSG274 Part 2 to consider all aspects of Legionella control while carrying out a risk assessment. However, consideration of the type of population residing in buildings with communal domestic hot and cold water systems is presently missing. HSE ACOP L8 (fourth edition) published in 2013 has provided the guidelines to consider when to carry out a risk assessment review. This includes changes to the water system or its use, changes to the use of the building in which the water system is installed (e.g. a toilet and/or a wash basin is no longer ‘in use’ and rooms containing these items being used as storerooms instead), the availability of new information about risks or control measures, the results of checks indicating that control measures are no longer effective, changes to key site responsible personnel and a case of LD or Legionellosis associated with the system [Citation19]. Within this guideline, we propose that the proportion of individuals in residential buildings with a weakened immune system, and/over 45 years of age and/or suffering from existing illnesses are also important factors to consider. New legislation enabling the collection of health and demographic data on individuals in residential buildings by the Duty Holder for the purposes of risk management of LD is therefore needed.
Recommendations
Based on a comprehensive review of (i) known and emerging risk factors for LD in society and (ii) the international guidelines and regulatory measures currently used to protect the public from Legionella, we offer the following recommendations:
In light of increasing evidence for community-acquired LD, the adequacy of measures used to protect residents occupying non-healthcare premises from exposure to Legionella bacteria should be examined.
In order to protect society at large, we propose greater harmonisation of the Legionella standards that form the basis for regulatory action, in both healthcare and non-healthcare premises.
In order to adapt to ageing populations within society, risk management strategies to prevent LD in residential building complexes are needed that consider the proportion of inhabitants over 65 years of age and vulnerable groups.
The ability to reliably quantify Legionella in water samples forms the basis of risk management. In view of current shortcomings in the quantification of Legionella, we encourage the development and approval of novel rapid test methods for quantifying live Legionella in water samples for use by accredited laboratories.
The potential future impact of global warming on the risk of exposure to Legionella bacteria in society and public health consequences should be investigated.
Conclusions
Legionella bacteria are ubiquitous in the manmade environment and remain a serious threat to public health. Discrepancies in quantification of Legionella, shortfalls in remedial action recommendations, more emphasis by practitioners on the ageing population residing in normal residential complexes when undertaking a risk assessment, and potential long-term issues (such as climate change) means that practitioners must be ever more vigilant in protecting the public against the threat of LD. The requirement of a more accurate, reliable and rapid standard sampling and testing method to quantify viable Legionella, and a review of remedial action recommendations is needed for the effective control of Legionella bacteria in domestic water systems. The present lack of consideration of the ageing population in the Legionella risk assessment guideline highlights the need for a more focussed risk assessment strategy to manage the risk of Legionella infection in vulnerable individuals throughout society at large.
Disclosure statement
No potential conflict of interest was reported by the authors. Survey data are provided in full in the main section of this paper.
ORCID
Aji Peter http://orcid.org/0000-0001-8343-5605
K. Clive Thompson http://orcid.org/0000-0002-1630-9905
Edwin John Routledge http://orcid.org/0000-0001-7695-364X
References
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). 2014. Legionella questions and answers [Internet]. [cited 2016 Oct 12]. Available from: https://www.publichealthontario.ca/en/…/Legionella_Questions_Answers_2014.pdf
- Fields BS, Benson RF, Besser RE. Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev. 2002;15:506–526. doi: 10.1128/CMR.15.3.506-526.2002
- Kirrage D, Reynolds G, Smith GE, et al. Investigation of an outbreak of Legionnaires’ disease: Hereford, UK 2003, for the Hereford Legionnaires Outbreak Control Team. Respir Med. 2007;101:1639–1644. doi: 10.1016/j.rmed.2006.11.026
- Marston BJ, Lipman HB, Breiman RF. Surveillance for Legionnaires’ disease: risk factors for morbidity and mortality. Arch Intern Med. 1994;154:2417–2422. doi: 10.1001/archinte.1994.00420210049006
- Gomez-Valero L, Rusniok C, Rolando M, et al. Comparative analyses of Legionella species identifies genetic features of strains causing Legionnaires’ disease. Genome Biol. 2014;15:505.
- Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev. 2015;28:95–133. doi: 10.1128/CMR.00029-14
- Borella P, Montagna MT, Romano-Spica V, et al. Legionella infection risk from domestic hot water. Emerg Infect Dis. 2004;10:457. doi: 10.3201/eid1003.020707
- Allegra S, Grattard F, Girardot F, et al. Longitudinal evaluation of the efficacy of heat treatment procedures against Legionella spp. in hospital water systems by using a flow cytometric assay. Appl Environ Microbiol. 2011;77:1268–1275. doi: 10.1128/AEM.02225-10
- Farhat M, Moletta-Denat M, Frère J, et al. Effects of disinfection on Legionella spp., eukarya, and biofilms in a hot water system. Appl Environ Microbiol. 2012;78:6850–6858. doi: 10.1128/AEM.00831-12
- Temmerman R, Vervaeren H, Noseda B, et al. Necrotrophic growth of Legionella pneumophila. Appl Environ Microbiol. 2006;72:4323–4328. doi: 10.1128/AEM.00070-06
- Garcia-Fulgueiras A, Navarro C, Fenoll D. Legionnaires’ disease outbreak in Murcia, Spain. Emerg Infect Dis. 2003;9:915–921. doi: 10.3201/eid0908.030337
- Legionnaires’ Disease Outbreak Investigation Toolbox (LDOIT) [Internet]. 2014 . [cited 2017 May 7]. Available from: https://legionnaires.ecdc.europa.eu/?pid=107
- Public Health Wales (PHW). 2016. Legionnaires’ disease [Internet]. [cited 2015 Dec 14]. Available from: www.wales.nhs.uk/sitesplus/888/page/44350
- Kuroda Y, Takeuchi S. Legionnaires’ disease: environmental risk factors. Enc Environ Health. 2011: 448–452. doi: 10.1016/B978-0-444-52272-6.00448-7
- Health and Safety Executive (HSE) [internet]. 2000. [Cited 2016 March 4]. Available from: http://www.hse.gov.uk/pubns/books/l8.htm
- WHO. 2007. Legionella and the prevention of Legionellosis. Available from: www.who.int/water_sanitation_health/emerging/legionella.pdf
- ECDC. 2012. European Centre for Disease Prevention and Control. European Legionnaires’ Disease Surveillance Network (ELDSNet): operating procedures. Stockholm.
- ECDC. 2011. European guidelines for control and prevention of travel associated Legionnaires’ disease. Available from: www.ecdc.europa.eu/en/healthtopics/legionnaires…/EWGLI-Technical-Guidelines.pdf
- HSE ACOP L8. 2013. Legionnaires’ disease, control of Legionella bacteria in water systems. Approved Code of Practice L8 [Internet]. [cited 2016 Feb 20]. Available from: www.hse.gov.uk/pubns/books/l8.htm
- HSG Part2. 2014. The control of legionella bacteria in hot and cold water systems. Available from: http://www.hse.gov.uk/pubns/books/l8.htm
- Borges A, Simoes M, Martinez-Murcia A, et al. Detection of Legionella spp. in natural and man-made water systems using standard guidelines. J Microbiol Res. 2012;2:95–102. doi: 10.5923/j.microbiology.20120204.06
- Quan TP, Fawcett NJ, Wrightson JM, et al. Increasing burden of community-acquired pneumonia leading to hospitalisation, 1998–2014. Thorax. 2016;71:535–542. doi: 10.1136/thoraxjnl-2015-207688
- Legionella France (LF) [Internet]. 2015. [cited 2017 May 12] Available from: http://social-sante.gouv.fr/soins-et-maladies/maladies/maladies-infectieuses/article/legionellose
- Torres A, Peetermans WE, Viegi G, et al. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68:1057–1065. doi: 10.1136/thoraxjnl-2013-204282
- Campese C, Descours G, Lepoutre A, et al. Legionnaires’ disease in France. Med Mal Infect. 2015;45:65–71. doi: 10.1016/j.medmal.2015.01.015
- Updated Guidelines for the Control of Legionella in Western Pennsylvania (UGCLWP) [Internet]. 2014. [cited 2017 Jan 27]. Available from: http://www.achd.net/infectd/pubs/pdf/2014_FINAL_Legionella_Guidelines_for_Western_PA.pdf
- Craun GF, Brunkard JM, Yoder JS, et al. Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clin Microbiol Rev. 2010;23:507–528. doi: 10.1128/CMR.00077-09
- Krojgaard LH, Krogfelt KA, Albrechtsen HJ, et al. Cluster of Legionnaires’ disease in a newly built block of flats, Denmark, December 2008-January 2009. Eurosurveillance (Online Edition). 2011;16:11–17.
- Mathys W, Stanke J, Harmuth M, et al. Occurrence of Legionella in hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating. Int J Hyg Environ Health. 2008;211:179–185. doi: 10.1016/j.ijheh.2007.02.004
- Qin T, Xia J, Ren H, et al. Liver cirrhosis as a predisposing condition for Legionnaires’ disease: a report of four laboratory-confirmed cases from China. J Med Microbiol. 2012;61:1023–1028. doi: 10.1099/jmm.0.040170-0
- Rutherford T. 2012. Population Ageing Statistics. House of Commons Library [Internet]. [cited 2015 Nov 8]. Available from: http://researchbriefings.parliament.uk/ResearchBriefing/Summary/SN03228#fullreport.
- PSA. Population structure and ageing - Statistics Explained. 2016 [Internet]. [cited 2017 Jan 23] Available from: www.ec.europa.eu/eurostat/statistics-explained/index…/Population_structure_and_ageing.
- Statistics Bureau (SB). 2010. Statistics Bureau Portal site of official statistics of Japan, 2010 [Internet]. [cited 2017 Jan 17]. Available from: http://www.e-stat.go.jp/SG1/estat/ListE.do?lid=000001063433
- An Aging Nation (AAN). 2014. The Older Population in the United States [Internet]. [cited 2017 Jan 23]. Available from: https://www.census.gov/prod/2014pubs/p25-1140.pdf
- WHO. 2017. Risk factors of ill health among older people [Internet]. [cited 2017 May 6]. Available from: http://www.euro.who.int/en/health-topics/Life-stages/healthy-ageing/data-and-statistics/risk-factors-of-ill-health-among-older-people
- Farnham A, Alleyne L, Cimini D, et al. Legionnaires’ disease incidence and risk factors, New York, New York, USA, 2002–2011. Emerg Infect Dis. 2014;20:1795. doi: 10.3201/eid2011.131872
- Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third national health and nutrition examination survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007
- Later Life in the United Kingdom (LLUK) [Internet]. 2015 . [cited 2016 Feb 20] Available from: www.ageuk.org.uk/Documents/EN-GB/Factsheets/Later_Life_UK_factsheet.pdf
- Office for National Statistics (ONS). 2014. Changes in the Older Care Home Population at Local Authority Level between 2001 and 2011 [Internet]. [cited 2015 Dec 14] Available from: http://www.ons.gov.uk/ons/dcp171776_388099.pdf
- Ruiz M, Ewing S, Marcos MA. Etiology of community-acquired pneumonia: impact of age, comorbidity and severity. Am J Respir Crit Care Med. 1999;160:397–405. doi: 10.1164/ajrccm.160.2.9808045
- Annear MJ, Cushman G, Gidlow B. Leisure time physical activity differences among older adults from diverse socioeconomic neighbourhoods. Health Place. 2009;15:482–490. doi: 10.1016/j.healthplace.2008.09.005
- Lin YE, Yu VL. 2014. Legionnaires’ disease in LTC facilities: a hidden threat. Institute for the Advancement of Senior Care [Internet]. [cited 2016 Jan 21] Available from: http://www.ltlmagazine.com/article/legionnaires-disease-ltc-facilities-hidden-threat
- Gruas C, Alvarez I, Lara C, et al. Identification of Legionella spp. in environmental water samples by ScanVIT-Legionella Method in Spain. Indian J Microbiol. 2013;53:142–148. doi: 10.1007/s12088-013-0363-6
- Xiao-Yong Z, Chao-Hui H, Qing-Yi Z. Comparative study on sampling methods for monitoring Legionella species in environmental water. Afr J Microbiol. 2014;8:974–985. doi: 10.5897/AJMR2013.6484
- Diederen BMW, Kluytmans JAJW, Vandenbroucke-Grauls CM, et al. Utility of real-time PCR for diagnosis of Legionnaires’ disease in routine clinical practice. J Clin Microbiol. 2008;46:671–677. doi: 10.1128/JCM.01196-07
- Delgado-Viscogliosi P, Solignac L, Delattre JM. Viability PCR, a culture-independent method for rapid and selective quantification of viable Legionella pneumophila cells in environmental water samples. Appl Environ Microbiol. 2009;75:3502–3512. doi: 10.1128/AEM.02878-08
- Whiley H, Taylor M. Legionella detection by culture and qPCR: comparing apples and oranges. Crit Rev Microbiol. 2016;38:1–10.
- Yanez MA, Carrasco-Serrano C, Barbera VM, et al. Quantitative detection of Legionella pneumophila in water samples by immunomagnetic purification and real-time PCR amplification of the dotA gene. Appl Environ Microbiol. 2005;71:3433–3441. doi: 10.1128/AEM.71.7.3433-3441.2005
- ISO 19458 :2006. Water quality -- Sampling for microbiological analysis [Internet]. [cited 2017 Apr 16]. Available from: https://www.iso.org/standard/33845.html
- BS 7592:2008. Sampling for Legionella bacteria in water systems. Code of practice. [Internet]. [cited 2017 Feb 13]. Available from: http://shop.bsigroup.com/ProductDetail?pid=000000000030161148
- Dusserre E, Ginevra C, Hallier-Soulier S, et al. A PCR-based method for monitoring Legionella pneumophila in water samples detects viable but noncultivable Legionella that can recover their cultivability. Appl Environ Microbiol. 2008;74:4817–4824. doi: 10.1128/AEM.02899-07
- Delgado-Viscogliosi P, Simonart T, Parent V, et al. Rapid method for enumeration of viable Legionella pneumophila and other Legionella spp. in water. Appl Environ Microbiol. 2005;71:4086–4096. doi: 10.1128/AEM.71.7.4086-4096.2005
- Joly P, Falconnet PA, Andre J, et al. Quantitative real-time Legionella PCR for environmental water samples: data interpretation. Appl Environ Microbiol. 2006;72:2801–2808. doi: 10.1128/AEM.72.4.2801-2808.2006
- Brooks T, Osicki RA, Springthorpe VS, et al. Detection and identification of Legionella species from groundwaters. J Toxicol Environ Health Part A. 2004;67:1845–1859. doi: 10.1080/15287390490492449
- Schrader C, Schielke A, Ellerbroek L, et al. PCR inhibitors – occurrence, properties and removal. J Appl Microbiol. 2012;113:1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x
- Maiwald MK, Kissel S, Srimuang M, et al. Comparison of polymerase chain reaction and conventional culture for the detection of legionellae in hospital water samples. J Appl Bacteriol.1994;76:216–225. doi: 10.1111/j.1365-2672.1994.tb01619.x
- Miyamoto H, Yamamoto H, Arima K, et al. Development of a new seminested PCR method for detection of Legionella species and its application to surveillance of Legionellae in hospital cooling tower water. Appl Environ Microbiol. 1997;63:2489–2494.
- Ballard AL, Fry NK, Chan L, et al. Detection of Legionella pneumophila using a real-time PCR hybridization assay. J Clin Microbiol. 2000;38:4215–4218.
- Health Technical Memorandum (HTM). 2016. Safe water in healthcare premises – Part B: operational management [Internet]. [cited 2016 Oct 6]. Available from: https://www.gov.uk/government/uploads/…/DH_HTM_0401_PART_B_acc.pdf
- Beaute J, Zucs P, de-Jong B, on behalf of the European Legionnaires’ Disease Surveillance Network. 2013. Legionnaires’ disease in Europe, 2009–2010 [Internet]. [cited 2016 Mar 3] Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20417
- Barriopedro D, Fischer EM, Luterbacher J, et al. The hot summer of 2010: redrawing the temperature record map of Europe. Science.2011;332:220–224. doi: 10.1126/science.1201224
- Ekstroma M, Fowlerb HJ, Kilsbyb CG, et al. New estimates of future changes in extreme rainfall across the UK using regional climate model integrations. 2. Future estimates and use in impact studies. J Hydrol. 2005;300:234–251. doi: 10.1016/j.jhydrol.2004.06.019
- Dobney K, Baker CJ, Chapman L, et al. The future cost to the United Kingdom’s railway network of heat-related delays and buckles caused by the predicted increase in high summer temperatures owing to climate change. J Rail Rapid Transit. 2010;224:25–34. doi: 10.1243/09544097JRRT292
- Sakamoto R. 2015. Legionnaire’s disease, weather and climate [Internet]. [cited 2016 Nov 7]. Available from: http://www.who.int/bulletin/volumes/93/6/14–142299/en/
- Dennis PJ, Lee JV. Differences in aerosol survival between pathogenic and non-pathogenic strains of Legionella pneumophila serogroup 1. J Appl Bacteriol. 1988;65:135–141. doi: 10.1111/j.1365-2672.1988.tb01501.x
- Berendt RF. Survival of Legionella pneumophila in aerosols: effect of relative humidity. J Infect Dis. 1980;141:689–689. doi: 10.1093/infdis/141.5.689
- Karagiannis I, Brandsema P, Van der Sande M. Warm, wet weather associated with increased Legionnaires’ disease incidence in the Netherlands. Epidemiol Infect. 2009;137:181–187. doi: 10.1017/S095026880800099X
- Ricketts KD, Charlett A, Gelb D, et al. Weather patterns and Legionnaires’ disease: a meteorological study. Epidemiol Infect. 2009;137:1003–1012. doi: 10.1017/S095026880800157X
- Halsby KD, Joseph CA, Lee JV, et al. The relationship between meteorological variables and sporadic cases of Legionnaires’ disease in residents of England and Wales. Epidemiol Infect. 2014;142:2352–2359. doi: 10.1017/S0950268813003294
- British Lung Foundation (BLF). 2014. Pneumonia [Internet]. [cited 2016 Feb 23]. Available from: http://shop.blf.org.uk/collections/lung-health-information/products/pneumonia.
- NHS UK. 2014. Pneumonia [Internet]. [cited 2016 Jan 21]. Available from: http://www.nhs.uk/conditions/pneumonia/Pages/Introduction.aspx.
- Public Health England (PHE) [Internet]. 2014 . [cited 2015 Dec 17]. Available from: https://www.gov.uk/government/news/latest-legionnaires-disease-data-published-for-england-and-wales
- CRCAP. 2012. Clinical Review - Community-acquired pneumonia By Dr Simon Barry [Internet]. [cited 2017 May 12]. Available from: http://www.gponline.com/clinical-review-community-acquired-pneumonia/respiratory-system/copd/article/1126412
- Guest JF, Morris A. Community-acquired pneumonia: the annual cost to the National Health Service in the UK. Official Sci J ERS. 1997;10:1530–1534.
- Feldman C, Anderson R. Community-acquired pneumonia: still a major burden of disease. Curr Opin Crit Care. 2016;22:477–484. doi: 10.1097/MCC.0000000000000340
- Ott SR, Hauptmeier BM, Ernen C, et al. Treatment failure in pneumonia: impact of antibiotic treatment and cost analysis. Eur Respir J.2012;39:611–618. doi: 10.1183/09031936.00098411
- Lettinga KD, Verbon A, Nieuwkerk PT, et al. Health-related quality of life and posttraumatic stress disorder among survivors of an outbreak of Legionnaires disease. J Clin. Infect Dis. 2002;35:11–17. doi: 10.1086/340738
- Von Baum H, Ewig S, Marre R, et al. 2008. Competence Network for Community Acquired Pneumonia Study Group. (2008). Community-acquired Legionella pneumonia: new insights from the German competence network for community acquired pneumonia. J Clin Infect Dis. 46: 1356–1364. doi: 10.1086/586741
