?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Biodegradable polymers received a lot of interest lately as a possible solution to the grave issue of post-consumer textile waste (PCTW) accumulation and to support sustainable textile consumption. Despite several possible methods to recycle and reuse, most PCTW often ends up in landfills due to the lack of viable options. The complex separation process in blended textiles, low-quality products, higher recycling costs compared to virgin materials, volume, collection, sorting, and transportation costs involved are to name a few of the many issues causing landfilling of PCTW. Understanding how these wastes interact with the environment and impact our ecosystem is important. This multidisciplinary literature review focuses to investigate textile polymer biodegradation as a possible pathway for PCTW accumulation. The majority of the studies published in the polymer biodegradation area focused specifically on synthetic polymers, the plastic industry, and biopolymers. While this review provides a comprehensive understanding of broader textile polymer biodegradation. In their manufacturing process, the textiles (both natural and synthetic origin) go through several chemical processes that can tremendously affect their biodegradation rates and environmental impact. Therefore, it is important to understand the mechanism of biodegradation, factors affecting the rate of biodegradation, and possible barriers to the biodegradation of textile polymers without a narrow focus on merely petroleum-based synthetic polymers. Contemporary technologies such as torrefaction, pyrolysis, enzymatic/biological catalysis, and depolymerization to recycle PCTW have also been discussed in the paper.
1. Introduction
The excessive consumption of resources has led to several issues including but not limited to post-consumer waste accumulation, climate change, air and water pollution, declining ecosystem health, and microplastic pollution [Citation1,Citation2]. This can be commonly observed among fashion consumers as they are often influenced by short-lived trends that are getting even shorter since the dawn of TikTok [Citation3]. Fast fashion consumers swiftly discard clothing (in many cases after single-use) that is no longer in trend leading to the accumulation of post-consumer textile waste (PCTW). The average lifetime of textile products has been estimated to be <5 years [Citation4]. With the current business-as-is approach, there will be an estimated accumulation of >4.5 gigatonnes of textile waste (TW) by 2040 [Citation4,Citation5]. Existing TW processing techniques such as incineration and recycling can process only small amounts of waste and the majority of the PCTW accumulates in landfills [Citation6]. According to the United States Environmental Protection Agency (USEPA) 2018 report, approximately 17.03 million tons of TW are generated annually in the United States alone. Out of which 2.51 million tons (14.73%) are recycled, 3.22 million tons (18.90%) are incinerated, and 11.3 (66.35%) million tons are eventually disposed into landfills annually [Citation7] leading up to the issue of PCTW accumulation.
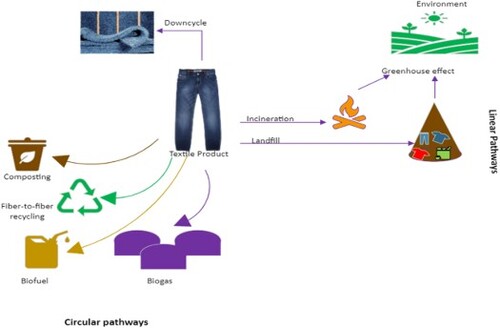
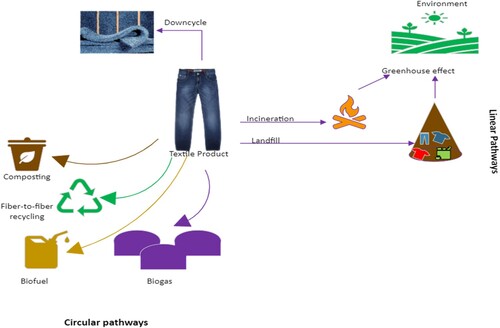
With such a considerable proportion of PCTW ending up in landfills, strategies are needed to handle this graving issue effectively and to reduce its environmental impact by transforming the linear consumption of textiles into a circular economy (). The immense variety of consumer textile products in the market presents a great challenge to find a one-size-fits-all solution to tackle this issue. The fashion industry has a very large and complex global supply chain, which uses a wide range of fibre blends with complex polymer morphology, chemical dyes, performance and comfort finishes, trims, and embellishments. The complex separation process in blended textiles, low-quality products, higher recycling costs compared to virgin materials, lower recycling throughputs, high volume of waste, lack of viable upcycling options, collection, sorting, and transportation costs involved in the recycling process lead to discarding the PCTW in landfills.
Figure 1. Schematic representation of possible linear and circular pathways for post-consumer textile waste.
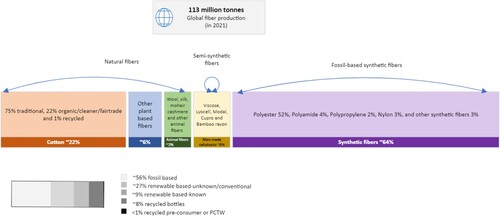
Textile products are mostly composed of fibres that are made up of natural or synthetic polymers. Both these categories of fibres have different issues related to their biodegradation. Natural fibres are organically renewable and biodegradable, and their PCTW should not linger in the biosphere for a long time. Nonetheless, their biodegradability is often affected by the presence of synthetic dyes, finishes, and other treatments that these textiles receive during their manufacturing process for improved functionality and performance. Also, their degradation in natural soil and compost under biotic and abiotic conditions varies by their structure, morphology, time of exposure to soil medium, moisture, temperature, and pH [Citation8,Citation9]. On the other hand, synthetic fibres of petrochemical origin dominate the current global textile fibre market, producing around 113 million tons of fibres annually [Citation10] (). They play a necessary role in the textile industry due to their high performance and functionality, which many natural fibres lack. Polyethylene terephthalate (PET) also called polyester shares 54% of the textile market, while, cotton and other plant-based fibres make 22% and 6% respectively of the total market share, which ultimately also generates end-of-life TW in those proportions [Citation10]. In general, synthetic polymers are resistant to biodegradation because of their high crystallinity and strength, which contributes to the accumulation of overall (macro and micro) plastic waste [Citation11]. A large number of textiles used in the apparel industry are often blends of two or more synthetic polymers and/or natural and synthetic polymers making it difficult to recycle them as their degradation and recycling pathways vary chiefly. Thus, due to a lack of economically and structurally viable recycling options, most of the PCTW ends up in landfills.
Biodegradation presents an opportunity to deflect waste from landfills and use alternative approaches such as composting, anaerobic digestion, or bio-catalytically enhanced recycling processes to tackle the PCTW. In simple terms, biodegradation largely converts complex materials into simple and smaller compounds that can be recycled in the biosphere through enzymatic digestion. However, in reality, this simple-sounding phenomenon is often presented with highly complex challenges as the biodegradation occurring in large-scale waste management environments such as landfills, compost, and anaerobic digestor widely differ from the degradation occurring in natural soil and freshwater systems. Therefore, it is essential to study this phenomenon broadly as well as in-depth. There is a dearth of literature discussing these complex mechanisms, particularly in the context of post-consumer textile waste management. The majority of the literature on polymer degradation focuses precisely on the plastics industry waste. This might be due to the predominant concerns related to the non-biodegradability and waste accumulation of synthetic polymers. This review paper aims at presenting the multidisciplinary knowledge pertaining to textile biodegradation and presenting biodegradation as a possible route for improved textile sustainability. Biodegradation mechanism in general, different phases of the process, factors affecting the rate of biodegradation (biotic and abiotic), and relating these concepts to textile fibre biodegradability (natural, synthetic, and biopolymers), with a culmination to recent advances in TW management practices have been discussed in the following sections of the paper.
2. Biodegradation mechanism
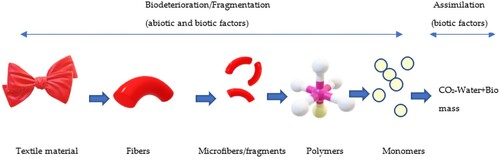
The American Society of Testing and Materials (ASTM) defines degradation as ‘an irreversible process leading to a significant change in the structure of the material typically characterized by a loss of its mechanical properties and fragmentation.’ [Citation12]. It occurs over some time and the rate of degradation of the material is impacted by the environmental factors it is surrounded with. The process of biodegradation is completed in three main phases: 1) Biodeterioration, 2) Assimilation, and 3) Mineralization. Each of these three phases is carried out by the microorganism. Nevertheless, at the very beginning, abiotic factors play a big role in the degradation of textile polymers. Therefore, biodeterioration is often initiated by abiotic factors such as sunlight, air, dust, moisture, heat, etc [Citation13]. The material surface starts to deteriorate due to exposure to abiotic factors. However, the chemical finishes or coatings on the textile surface can act as a barrier at this step. They resist microorganisms and prevent initial breakage of the material. When the external surface layer deteriorates, it becomes suitable for microorganisms to interact with the polymer [Citation14]. As a result, the polymer starts losing its strength, durability, and resistance during the biodeterioration phase [Citation15] (). The biodeterioration step involves the disintegration of the fabric into small fragments that continue to depolymerize into lower molecular weight units and monomers which are consumed and digested by microbes as an energy source. Fragments of relatively low molecular weight can be easily absorbed by the cell wall of the microbes in the next phase which is called assimilation.
The polymer biodegradation process supports microbial growth as the degraded compound is utilized as a source of nutrition and energy by the microbes [Citation16]. All microorganisms need energy for cellular processes and carbon for making new biomass. Macronutrients such as carbon, nitrogen, phosphorus, and sulfur constitute more than 90% of the dry mass of living cells and are structural components of biological molecules such as carbohydrates, proteins, lipids, and nucleic acids [Citation17]. Among all macromolecules, carbon is needed in the greatest amount. Micronutrients such as iron, cobalt, zinc, copper, manganese, and molybdenum are needed in lesser amounts and often serve as structural components of specific enzymes in bacterial cells [Citation8,Citation9,Citation11,Citation16,Citation17]. Thus, for heterotopic bacteria, the need to obtain carbon for biosynthesis is the main driving force for polymer biodegradation. Organic compounds are used as electron donors in the microbial respiration process to generate energy in the form of an energy-rich product called Nicotinamide Adenine Dinucleotide (NADH) [Citation18].
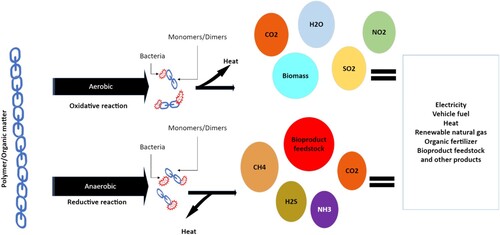
Microbes release exo-enzymes which break the main chain to form low molecular fragments. The process is called fragmentation which starts at the last step of the biodeterioration phase and prior to the second phase of the process called assimilation. Assimilation is the metabolic conversion inside a microbial cell into biomass and simple byproducts [Citation19] Low-weight and short-chained oligomers and monomers are then transported into the cell and assimilated into the internal environment. Assimilation produces energy and different byproducts like CO2, H2O, and CH4 which depends on the presence of oxygen at the time of the assimilation process. (). Enzymes are often selective to the substrate. For example, the cellulase enzyme works to degrade cellulose and lignocellulose-based materials whereas acetyl esterase can degrade cellulose acetate polymers [Citation20]. The conversion of molecules by specific enzymes takes place either in the presence of oxygen or the absence of oxygen which leads to two different pathways. So, it is important to understand the pathways and enzymatic function for further clarification. The quicker the assimilation step is completed, the faster the biodegradability rate would be, and the continuation of the process eventually reaches the mineralization step.
Mineralization is the action of converting organic carbon into new biomass. During aerobic (presence of oxygen) biodegradation, CO2 is produced, and CH4 is produced in the anaerobic (absence of oxygen) biodegradation process. In this phase, the biomass is converted to gases such as CO2, CH4, nitrogen compounds, water, salts, and minerals [Citation21]. The mineralization phase is complete when all the biomass is completely consumed and all the carbon is converted into CO2, and CH4 [Citation22]. A polymer can be called biodegradable if all its organic content can be mineralized into inorganic byproducts. There are several factors including but not limited to chemical structure, mechanical properties, and morphology of the fibre that affect the rate and extent of biodegradation and are discussed in the following sections of the paper.
3. Factors affecting the rate of biodegradation
The polymeric characteristics such as molecular composition, presence of functional groups, crystallinity, morphology, level of orientation, hydrophilicity, intermolecular interaction, and molecular weight in conjunction with environmental conditions such as temperature, air, moisture, and sunlight determine the rate of biodegradation of polymers. Optimum environmental conditions help generate suitable enzymes and assimilate and metabolize the polymer [Citation23]. For instance, biodegradation rates of polymers vary at different temperatures. As an example, Polylactic acid (PLA) fibre degrades faster in the compost condition rather than in natural soil due to higher temperature and concentration of microbial colonies [Citation24] in the compost. Research has shown [Citation25] that PLA can begin losing its tensile strength after 48 days under compost conditions, whereas no degradation of PLA was observed in the natural soil. This could be due to the presence of a higher concentration of microorganisms in the compost condition, thereby presenting a more suitable environment for the polymer to biodegrade.
3.1. Role of polymeric characteristics in biodegradation
Crystallinity is an important factor that affects the rate of biodegradation. The hydrophobicity and crystallinity of synthetic fibres inhibit their biodegradation in natural environmental conditions. On the other hand, natural fibres are hydrophilic and have a relatively larger amorphous region in their morphology that favours interactions with microbial enzymes and eventually biodegradation. The amorphous regions degrade quicker chemically and enzymatically compared to the crystalline region. This is because molecules are packed more closely and uniformly in the crystalline region than in the amorphous region, leaving little to no space for microbes to build colonies and decompose the material. The molecules in the amorphous region are more susceptible to hydrolases followed by the crystalline region of a polymer. The crystallinity of the polymer can be measured by x-ray diffraction, solid-state 13C NMR, infrared diffraction, Raman spectroscopy, and differential scanning calorimetry [Citation26]. Mechanical (particle size reduction), thermal, and chemical treatments to disrupt the crystallinity and bond cleavage of the polymers can improve the degradation rate of the textile polymers and must be investigated.
Lower molecular weight is another factor that assists in the degradation of a polymer. Depolymerization occurs faster among polymers with low molecular weight and high amorphous regions [Citation27]. Both in the case of synthetics [Citation28–35] and natural fibres [Citation36,Citation37] it was observed that crystallinity and molecular weight play a significant role in biodegradation. In a study [Citation36] focusing on the comparative analysis of cotton, ramie, and viscose biodegradability, it was found that viscose has a faster rate of biodegradation compared to amorphous cotton followed by ramie. This can be attributed to cotton’s higher molecular weight and crystallinity. Natural fibres are formed of parallel cellulose molecules, however, regenerated fibres such as viscose are made of antiparallel cellulose arrangement creating a weaker structure and assisting in breaking down. A similar trend is observed in composting conditions as well [Citation37].
Moisture diffusion is another important factor that affects the rate of textile biodegradation. For example, the rate of degradation for cellulosic acetate is much lower than for cotton and viscose rayon due to the hydrophobicity of the fibre blocking the moisture diffusion in the fibre matrix. Cellulosic fibres have high moisture absorbency and regain due to the presence of synsynt hydroxyl groups and the moisture absorbency increases further with lower crystallinity in the polymeric structure. Regenerated cellulosic fibres have higher moisture regain compared to cotton, therefore, their biodegradation rate is relatively higher. Since the rate of biodegradation is affected by several factors, it's important to consider them concurrently. The addition of cellulosic natural fibres to polymeric composites enhances their biodegradation as the filler disrupts the polymer structure and improves water absorption and swelling. Lastly, the temperature is an important factor. Exposure of the material close to the glass transition (Tg) temperature of the polymer further assists in the quicker breaking down of the polymer, thereby facilitating the biodegradation process. Other factors that affect biodegradability are the chemical structure, degree of polymerization, molecular mass, carbon content, hydrophobicity, time, and pH [Citation8].
3.2. Environmental factors affecting the rate of biodegradation
Environmental factors do not completely biodegrade the polymers but assist in the initial breaking down of polymeric material. The action of temperature, sunlight, humidity, rain, wind, etc. can assist in the initial deterioration of polymeric materials, which can be assessed in the form of a change in the mechanical and chemical properties of the material. The soil environment also plays a significant role in microbial activity. The soil's physical characteristics (such as texture, density, and porosity) and chemical characteristics (such as pH, cation exchange capacity, and organic matter) play an important role in polymer biodegradation ().
Oxidation and hydrolysis are two important processes associated with the biodegradation of polymeric materials due to environmental factors. The oxygen and ozone present in the atmosphere release free radicals that can attack the polymer bonds causing deterioration. Similarly, in the process of hydrolysis, the water content in the atmosphere assists in the disintegration of hydrolyzable covalent bonds of the polymer. Once broken down into smaller fragments, microorganisms can start degrading the textile polymers by aerobic or anaerobic digestion depending on the availability of oxygen in the local environment. In aerobic biodegradation, the microbes utilize oxygen as the.
terminal electron acceptor to obtain carbon and energy. On the other hand, there is an absence of oxygen in anaerobic conditions for biodegradation.
Sunlight is another environmental factor that assists in the biodegradation process and ultraviolet (UV) blocking finish on some textiles can inhibit this process. Some natural fibres such as hemp and chitosan can be good alternatives for this function as they have the desired ultraviolet protection factor (UPF) in them without the need for a chemical finishing treatment and they also biodegrade naturally. To impart functional properties like improved flame resistance and UV resistance without hampering the degradation of the polymer, a recent study [Citation38] stressed the use of bio-based materials like chitosan and phytic acid to develop nanocomposites with PLA. Phytic acid and chitosan nanoparticles (NP) were developed. These nanocomposites showed a higher Limited Oxygen Index (LOI) pass in the U-94 vertical test and showed improved degradability with the incorporation of the nanoparticles. For 120 days of soil burial test, some cracks appeared on the neat PLA surface under scanning electron microscopy. On the contrary, the cracks on the NP composite surface were visible to the naked eye depicting a higher rate of biodegradation. Likewise, the molecular weight loss of the NP composite surface was reported to be higher than the neat PLA [Citation38].
4. Textile polymer biodegradation (natural/synthetic/bio-polymers)
Textile products have a high level of structural complexity. Fabrics are 2D planar structures that are either woven, knitted, or nonwoven. Depending on the fabric construction method, these can be made from either yarn (that are twisted strands made of fibres) in the case of woven and knitted fabrics or fibres in the case of nonwoven fabrics. Fibres can be further classified as natural or synthetic depending on their source of origin. Natural textile polymers can be further classified into 3 main categories: cellulosic, protein, and mineral. Cellulose is one of the most abundant natural polymers in the world and is derived mainly from a plant source. For instance, plant-based fibres such as cotton, linen, jute, hemp, flax, sisal, etc. are cellulosic fibres. The fibre is considered the most fundamental unit of a fabric’s structure and is composed of polymers assembled into semicrystalline substructures.
Both natural and synthetic fibres are composed of crystalline and amorphous regions that give toughness and strength to the fibre. The amorphous regions are relatively more susceptible to the initial disintegration of the fibre as the molecular arrangement in these is loose and disorderly. These ‘free spaces’ in the amorphous regions are readily available for disintegration caused by moisture and microbial colonies [Citation39], facilitating the fragmentation of the fibre into microfibers and its further breakage as explained in section 2 of this paper. However, the crystalline regions in the textile polymers usually inhibit the initial degradation of the polymers leading to their accumulation for a longer duration. The synthetic fibres that morphologically have higher crystalline regions take a longer time to decompose or in many cases do not decompose at all for several years. A recent review on key findings of textile biodegradation has been presented in .
Table 1. Recent review papers published on textile biodegradability.
Zambarano et al. [Citation51] studied the biodegradation of textile fibres in the aquatic environment. The authors found that 100% cotton degrades faster than cotton/polyester blend, and then 100% polyester. In another study, Niu et al, Citation2012 [Citation36] concluded that cotton biodegraded faster than rayon despite its slightly higher crystallinity, which shows that a small difference in the amount of crystallinity barely plays any role in fibres’ biodegradation.
Furthermore, the degradation of cellulosic fibres is carried out by the action of hydrolysis and oxidation processes [Citation9]. The initial breakdown in the cellulosic fibres first begins with oxidation to open up the structure, followed by hydrolysis of the glycosidic bonds and ultimately the breakdown of the covalent (strongest) bonds in the fibres. In general, natural fibres degrade faster compared to petroleum-based synthetic fibres. However, even the natural fibres that are treated with synthetic pigments and/or dyes and processed with chemical finishes (for improved function) can persist in the environment for longer periods. Typically, natural fibres such as cotton, flax, hemp, jute, and wool show biodeterioration in less than 1 year (See ), however, petroleum-based synthetic fibres such as Polypropylene (PP) and Polyethylene terephthalate (PET) exhibit insignificant degradation even after several years [Citation40–45]. This can be attributed to their highly ordered morphological structure and hydrophobic behaviour that creates a blockage for water and microbes to react with the fibre. Biodegradation is a complex process that can be accelerated or slowed down by a combination of biotic and abiotic factors. Therefore, it is critical to make biodegradability claims carefully.
Table 2. Expected textile fibres biodegradability based on previous studies.
4.1. Biopolymers
Biopolymers present a suitable alternative for fossil-based synthetic polymers in terms of both function and are engineered for relatively faster biodegradation. Biopolymers such as PLA (the most successful commercially available) and PBAT or Poly(butylene adipate-co-terephthalate) present suitable replacements for some conventional synthetic fibres to combat the PCTW issue. Both PLA and PBAT are compostable. The textile industry is looking at biopolymers as a sustainable solution especially for serving the sports and outdoor industry. These biopolymers are developed with an expectation to provide the eco-friendliness of natural fibres and mimic the functionality of petroleum-based textiles. The mechanical properties of PBAT are comparable to PP and have applications in the rope industry and for garment interfacing and lining. It is also being used as a biodegradable alternative to low-density polyethylene (LDPE) geotextiles and organic waste bags [Citation55].
On the other hand, poly(3-hydroxybutyrate-co-3-hydroxy hexanoate) or PHBH can be used to produce fibres by melt spinning technology, however, due to slow crystallization rate and reported lower strength (156 MPa) they are unsuitable for the apparel industry [Citation56]. The copolymer aliphatic ester linkages in PBAT and PHBH are more accessible for breakage in the environment compared to conventional synthetic polymers such as PET and PP. Similarly, bioplastics such as polycaprolactone (PCL) are also industrially compostable and degrade in anaerobic conditions under higher temperatures compared to the regular climate.
PLA is one of the most promising polymers that have sustainable bio-based origin and successful application in the apparel industry [Citation46]. It provides the necessary function and has the ability to biodegrade with the industrial composting method. In contrast to biodegradable polymers that go through continuous surface deterioration by microbes, PLA degrades in a two-step process. In the first step, its chemical bond cleavage occurs due to humidity in the environment which leads to the initial disintegration of the polymer. This process accelerates especially at temperatures > 60 oC [Citation57]. It also lowers the molecular weight to 10 kDa, followed by interaction with the microbes and ultimately assimilation of the fragment residues in the second step of the process [Citation58]. It can degrade completely under thermophilic aerobic conditions in industrial compost, however, only partial degradation of PLA occurs in mesophilic ambient conditions [Citation55,Citation58].
The biggest challenge with alternative polyesters such as PHA, PBAT, PHBH, PCL, etc. that their mechanical properties are unsuitable for the apparel industry. However, their successful applications in nonwoven agrotextiles, geotextiles, and medical textiles make these alternative materials replace traditional petroleum-based plastics in several other industries. Given the strong desire in producing the new alternative and sustainable textile fibres, researchers are exploring different combinations of these alternative polyesters with other polymers producing blended or composite fibre materials with improved fibre properties [Citation59]. For example, biobased polymers have been presented as an alternative to petroleum-based fibres to overcome the PCTW accumulation issue. However, their biodegradability assumptions lack empirical evidence and the potential release of toxic catalysts that may be used in biopolymer manufacturing is poorly understood [Citation60,Citation61]. Likewise, the scaling up the quantities of these alternative fibres to meet the high consumer demand and be cost-effective with conventional materials is yet to be figured out.
5. Current challenges with the textile biodegradation research
Historically, textile biodegradation has been an area of interest for museum curators, archeologists, and forensic studies researchers [Citation62]. However, from a sustainability perspective, there is very little literature on measuring the persistence of PCTW in the biosphere. It is a relatively new area and currently missing specific regulatory requirements, policies, and laws. Numerous factors such as chemical composition, degree of orientation, hydrophilicity, crystallinity, degree of polymerization, presence of dyes, and chemical finishes [Citation63] contribute to a textile material’s ability to biodegrade as discussed in the above sections of this paper. Due to so many variables in play, it is important that the biodegradation claims are made and understood carefully. Many studies use cotton as reference material to study the biodegradation behaviour of the material in question. It meets the biodegradability standards originally developed for plastics, in the absence of chemical finishes and dyes. However, the presence of dyes and finishes can inhibit or at least prolong the biodegradation process, therefore, more studies are needed to understand the effect of additives on the biodegradation behaviour of natural textile fibres.
5.1. Lack of standard methods designed specifically for textile biodegradation
There are some standards for testing the biodegradation of plastics that textile researchers have been using to assess the biodegradation of textile materials however, these studies are vexed with several issues. For example, not all textile fibres are thermoplastic, therefore, the testing conditions of these standard tests may not apply to non-thermoplastic fibres. Moreover, the testing conditions used in such studies vary widely, and the physical form of the substrate (fabric swatch, film, pulverized materials, yarn, fibre, etc.) varies. Also, the duration of testing often diverges from the standard methods. For example, studies involving the measurement of natural fibres’ biodegradation often conclude sooner than those that deal with thermoplastics. Many studies are time-limited and do not provide a complete understanding of the biodegradation process. Lastly, the majority of the studies collect data pertaining to biodegradation only till the biodeterioration phase and skip studying the assimilation and mineralization phases of the process.
Natural fibres are commonly blended with synthetics for improved function and performance. It is often blended in different ratios with polyester for improved durability, wrinkle resistance, and easy care. It is very challenging to separate the two fibres that were once blended and constructed into a yarn and then fabric. If such a fabric ends up in a landfill, it may not degrade completely in a reasonable time as both cotton and polyester fibres have different rates of biodegradation. Depending on the percentage of synthetic fibres in the blend, it may or may not even qualify to be called biodegradable as per the conditions of the standard method. For instance, as per the ASTM D6691-17 standard, a polymeric material that degrades more than 70% under seawater within a duration of 90 days is considered biodegradable. Any other materials that do not meet the percent degradation and/or timeline threshold will not be considered biodegradable as per the standard. These partially biodegradable materials are often confused with broader categories of ‘biodegradable’, ‘semi-biodegradable’ or ‘non-biodegradable’ polymers in the literature. Further research is needed for clarification on this grey area terminology.
5.2. Impact of chemical additives on textile biodegradation
Another factor that presents a challenge in the biodegradation process of textile materials is the chemical additives in the form of dyes, pigments, and finishing treatments at the manufacturing stage. Fabrics are treated with several chemical finishes to enhance their function, aesthetics, comfort, and durability. The use of such treatments is even more in the case of high-performance functional textiles for various industrial applications. For instance, antimicrobial finishes for medical textile applications are often coated with biocidal nanoparticles. The presence of these finishing chemicals inhibits the biodegradation process [Citation24,Citation34]. In a study, the cotton sample treated with silver and titanium dioxide (TiO2) nanoparticles (NP) was found unchanged within the first 25 days, whereas the untreated cotton fabric showed a faster rate of degradation by more than 50% [Citation17] in the same amount of time. The use of silver nanoparticles as antimicrobial agents can resist microbial attack and delay the biodegradation of the treated fabric.
Furthermore, due to its hydrophilic property, cellulosic fibres tend to crease and form wrinkles. They are often treated with durable press finishes to circumvent the issue of wrinkle formation, which affects the degradation of cellulose by modifying the chemical structure of the fibre and thereby inhibiting the growth of microorganisms [Citation16]. Similarly, the use of crosslinking agents in soil-repellent finishes creates a hydrophobic barrier on the fibre surface that prevents the growth of microbes [Citation64–68], thereby prolonging the biodegradation process. Lack of moisture absorption in such fabrics is a major factor hindering biodegradation. Similarly, flame retardant finishes have a negative impact on the biodegradation of the treated textile as well. Additionally, functional textiles treated with chemicals may release toxic chemicals in some cases that can not only kill microorganisms in the soil [Citation68] but also release bioaccumulative toxins in the environment. Some of the fluorochemical-based (C8 or above) finishes that were once used to achieve a water-repellent function for textiles are now banned by the US EPA due to the perfluoroalkyl sulfonates released during the finishing process [Citation69]. Nevertheless, its smaller chain counterpart (C6) is still being used for finishing fabrics and other materials [Citation70]. Several eco-friendly alternatives are being researched in this area, however, achieving a better or even equivalent function remains a challenge. Another caveat is that the focus of these studies is on achieving the water-repellency function and the impact of the finishing treatment on the biodegradability of the finished material is rarely studied. The outdoor apparel industry uses these water-and-oil repellent finishes heavily which plays a big role in per- and poly-fluoroalkyl substances (PFAS) accumulation in the biosphere. From 2009–2017, approximately 455 PFAS in the form of anions, cations, and zwitterions have been detected in the aquatic environment alone [Citation71]. They are ubiquitously present in sediments, soil, landfills leachate, and now in our food chain as well raising concerns around their toxicity, bioaccumulative and persistent nature [Citation69,Citation70,Citation72]. Therefore, how the post-consumption waste of these treated textiles interacts with the environment is yet to be fully answered.
With so many challenges in the field of textile biodegradation lies several opportunities for improving processes, policies, and regulations. Especially, methods must be developed to measure the residual dyestuff and finishing chemicals in the environment [Citation73]. Although, the textile industry is certainly not the only contributor to the PFAS issue, it does add to this issue significantly. Therefore, green chemistry based solutions are urgently needed to replace the traditional water-and-oil repellent finishes for the textile industry. It is possible to achieve water repellency function through alternate finishes but the desirable oil repellency can only be achieved through fluorochemical finishes which cause the PFAS release. [Citation74] suggested that landfill leachate is the main source of PFAS due to the discardation of consumer products (including textiles) containing PFAS, which ultimately contaminates the neighbouring soil and water systems. The constant imbalance between functional performance and biodegradability of textiles is inevitable. To ascertain and control this imbalance is a critical challenge in the realm of modern textile research.
6. Recent technologies in PCTW management
Given the recent interest in sustainable textile materials, their fate, and lifecycle assessment, it is important to find a way to create a circular economy and break the linear process pathway of PCTW accumulation. Conventionally, most of the PCTW is eventually landfilled, incinerated, or left in the environment. Landfilling of discarded TW means loss of value, and it remains there because the landfills are designed for containment rather than degradation of the waste disposed of in them. Many states in the United States have pledged to ban the disposal of textiles and encourage the reuse and recycling of textiles. For example, the Massachusetts Government has developed a Solid Waste Master Plan, which prohibits the disposal of textile and mattress waste [Citation75]. Due to the high energy content of textile waste, incineration is another widely used method for producing fuel from TW [Citation76]. However, this high-temperature process releases dioxin emissions. Dioxins are bioaccumulative toxins that can be traced back in the food chain and have an adverse effect on human health [Citation77].
New innovative methods are being used to minimize TW and improve the conversion efficiency of the existing waste. Currently, thermochemical, physiochemical, and bioconversion are three widely used approaches to convert textile solid waste into value-added products. Incineration- a commonly used approach to convert solid TW into energy. Solid waste from the textile industry has a high energy content and can be used as a raw material for generating thermal energy. [Citation78] Cotton and polyester blend contains an energy content of ∼16 MJ/kg. Incineration of blended fabric at 700 0C for a few minutes can produce a gas stream with a thermal energy content of 1100 0C at an airflow rate of 819 kg/m2/ h [Citation79].
In the recent past, researchers have also used the pyrolysis and torrefaction [Citation79] approach to produce activated carbon fibre, biochar, bio-oil, and syngas. Pyrolysis is performed at a high temperature in absence of oxygen [Citation80]. Cotton lint pyrolyzed at 550 0C with NaOH [Citation81] and di-ammonium hydrophosphate [Citation82] can produce a higher surface area of activated carbon compared to regular pyrolysis. Several studies also focussed on different physiochemical modifications of the TW to produce concrete, activated carbon, gypsum, ceramics, tiles, bricks, and absorbent materials for soundproofing and construction.
Biochemical conversion by fermentation is also a promising method for utilizing TW. For example, cotton is usually composed of 88–96% cellulose and the rest is composed of proteins, pectins, and waxes. Therefore, it is possible to hydrolyze cotton into glucose enzymatically and ferment it into value-added products [Citation81,Citation82]. Ethanol is a common product generated from TW by the enzymatic hydrolysis method, but due to its low yield, a pretreatment process is often used to increase the yield of ethanol.
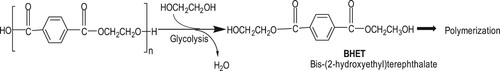
PET is one of the most used synthetic polymers in the textile industry. A biochemical approach to PET’s enzymatic recycling is another area of interest. Instead of making more biodegradable polymers with similar functions and properties to PET, it’s broken down into their monomer constituents through enzymatic hydrolysis and glycolysis (). The monomers are then used to recycle into new polymers. The industrial enzymatic recycling process uses enzymes to depolymerize the polymers into monomers that can be separated, purified, and reused to produce virgin polymers [Citation83,Citation84]. This approach supports the circular production of PET fibres. The process is more efficient on the amorphous PET compared to the polymer grades with higher crystallinity.
7. Key knowledge gaps and concluding remarks
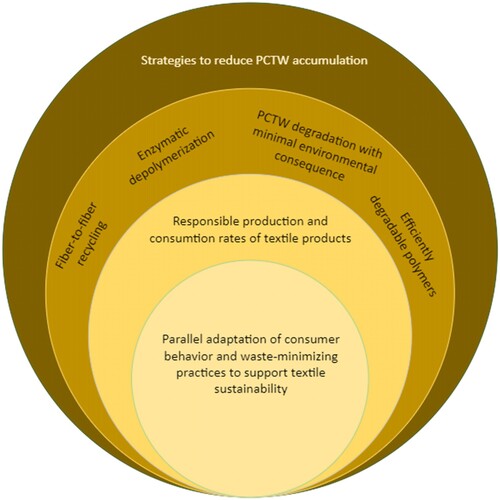
An interest to tackle PCTW with a sustainable approach has amplified tremendously over the last couple of decades. This review provides a state-of-the-science overview of biodegradation as a possible route to enhance the sustainability of the vast and complex textile industry. To advance the textile industry’s sustainability through biodegradation, research efforts in 3 main directions are needed (). First, to create textile polymers capable of efficient degradation in controlled/industrial microbial environments such as anaerobic digestion and composting (which can further be used to derive clean energy). Innovative and renewable biopolymers that deliver the performance and function of synthetic textiles and at the same time have biodegradability of natural fibres can help curb the accumulation of PCTW.
Secondly, to develop bio-based circular manufacturing technologies that use depolymerization techniques to convert textile polymers back to monomers for recycling and circular manufacturing. Researchers are exploring several ways to recycle the PCTW as noted in this review. Nevertheless, fibre-to-fibre recycling, which can transform the current linear textile consumption into truly circular textile manufacturing remains a challenge. Textiles lose their mechanical properties with time and use. With the current technologies, it’s not possible to recycle PCTW into another viable fibre via either chemical or mechanical recycling. Recycled PET fibres currently used in the textile industry often uses plastic bottles as a feedstock to manufacture recycled polyester. More rigorous research efforts are needed to derive solutions for a true fibre-to-fibre recycling.
The third strategy is to minimize the consequence of releasing PCTW into the natural environment. When releasing such wastes in a natural environment, they must be capable of degradation without causing immediate or long-lasting damage to the biosphere. New policies and laws must be in place that reward the circular production and consumption and penalize the resource overuse and pollution caused by textile producers and consumers to minize TW and pollution. This review paper senses an urgent need to frame science-based policies and laws to regulate and minimize the environmental impact of TW.
Innovative techniques such as torrefaction, pyrolysis, and enzymatic hydrolysis present some sort of resolution to manage the PCTW by creating value-added products and biomass. However, it must be noted that these methods are not energy negative. Therefore, it is important to be mindful of textile production and consumption rates. Ideally, the service period of textile products should be increased to control the production and consumption rates, which in turn will also keep the PCTW accumulation in check. Textile products must be designed for longevity and circularity that enables longer garment use times. In the case of products where short-life or single-use is necessary, they must meet the safe incineration and/or biodegradation standards at the end of their consumption period. The new textile material innovation and recycling strategies motivated by the desire for increased sustainability also require parallel adaptation of consumer behaviour and waste-minimizing practices to curtail the accumulation of TW in the first place.
This importunate issue must be researched with a holistic view point and finding more cost-effective, practical, and environmentally friendly solutions to tackle PCTW is a dire need of the hour. The real-world solutions are needed to create circular manufacturing and improve the overall sustainability in the textile industry. In conclusion, it is imperative to find a balance between the three pillars of sustainable development often denoted by the 3Es: Environmental impact, Equity, and Economic gain for a balanced economy, production, and consumption. For that, all the stakeholders including producers, policymakers, governmental bodies, manufacturers, retailers and consumer must work together to achieve a sustainable future for the textile industry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data analyzed for this work are included in the reference list of this published article.
Additional information
Funding
Notes on contributors
Sunidhi Mehta
Sunidhi Mehta is currently serving as an Assistant Professor at West Virginia University, specializing in the field of Textile Science. She earned her Ph.D. in Textile and Apparel Science from Punjab Agricultural University in India. Her research focuses on addressing matters related to human health and environmental sustainability in the textile industry.
References
- Home | Sustainable Development. [cited 2022 Jul 21]. Available from: https://sdgs.un.org/.
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7). doi:10.1126/SCIADV.1700782/SUPPL_FILE/1700782_SM.PDF
- Moreno D. (2022). TikTok’s fashion trends are gateway for overconsumption and cultural appropriation. https://www.highlandernews.org/83624/tiktoks-fashion-trends-are-a-gateway-for-overconsumption-and-cultural-appropriation/.
- Campione C. (2017). Copenhagen fashion summit: how not to make the fashion industry more sustainable. Greenpeace.Org, May, 1. https://www.greenpeace.org/international/story/7575/copenhagen-fashion-summit-how-not-to-make-the-fashion-industry-more-sustainable/https://www.greenpeace.org/international/story/7575/copenhagen-fashion-summit-how-not-to-make-the-fashion-industry-more-sus.
- Jönsson C, Wei R, Biundo A, et al. Biocatalysis in the recycling landscape for synthetic polymers and plastics towards circular textiles. ChemSusChem. 2021;14(19):4028–4040. doi:10.1002/cssc.202002666
- Soyer M, Dittrich K. Sustainable consumer behavior in purchasing, using and disposing of clothes. Sustain. 2021;13(15):8333. doi:10.3390/SU13158333
- USEPA. National Overview: Facts and Figures on Materials, Wastes, and Recycling | US EPA. [cited 2022 Jul 08]. Available from: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials.
- Vaid R, Yildirim E, Pasquinelli MA, et al. Hydrolytic degradation of polylactic acid fibers as a function of PH and exposure time. Molecules. 2021;26(24). doi:10.3390/MOLECULES26247554
- Jung JS, Song KH, Kim SH. Biodegradable acetylated kenaf fiber composites. Fibers Polym. 2021;22(12):3437–3443. doi:10.1007/S12221-021-1237-X
- Textile Exchange. (2022). Prefered materials and materials market Report, 2022. https://textileexchange.org/knowledge-center/reports/preferred-fiber-and-materials/.
- Chen HL, Cluver B, Rode C, et al. Biodegradation and mildew resistance of naturally colored cottons. Text Res J. 2010;80(20):1732–1740. doi:10.1177/0040517510376264
- ASTM. Standard test method for determining aerobic biodegradation of plastic materials in the marine environment by a defined microbial consortium or natural sea water inoculum. [cited 2022 Jul 08). Available from: https://www.astm.org/d6691-17.html.
- Arshad K, Skrifvars M, Vivod V, et al. Biodegradation of natural textile materials in soil. Tekstilec. 2014;57(2):118–132. doi:10.14502/tekstilec2014.57.118-132
- Alshehrei F. Biodegradation of synthetic and natural plastic by microorganisms. J Appl Environ. Microbiol. 2017;5(1):8–19. doi:10.12691/JAEM-5-1-2
- Lee SH, Kim IY, Song WS. Biodegradation of Polylactic Acid (PLA) fibers using different enzymes. Macromol. Res. 2014;22(6):657–663. doi:10.1007/S13233-014-2107-9
- Tomšič B, Klemenčič D, Simončič B, et al. Influence of antimicrobial finishes on the biodeterioration of cotton and cotton/polyester fabrics: leaching versus bio-barrier formation. Polym Degrad Stab. 2011;96(7):1286–1296. doi:10.1016/J.POLYMDEGRADSTAB.2011.04.004
- Milošević M, Krkobabić A, Radoičić M, et al. Biodegradation of cotton and cotton/polyester fabrics impregnated with Ag/TiO 2 nanoparticles in soil. Carbohydr Polym. 2017;158:77–84. doi:10.1016/J.CARBPOL.2016.12.006
- Gentry TJ, Fuhrmann JJ, Zuberer DA. Principles and applications of soil microbiology. Amsterdam, Netherlands: Elsevier; 2021.
- Chiellini E, Corti A, D’antone S. Oxo-biodegradable full carbon backbone polymers e biodegradation behaviour of thermally oxidized polyethylene in an aqueous medium. 2007. doi:10.1016/j.polymdegradstab.2007.03.007.
- Puls J, Wilson SA, Hölter D. Degradation of cellulose acetate-based materials: a review. J Polym Environ. 2011;19(1):152–165. doi:10.1007/S10924-010-0258-0/FIGURES/11
- Tiwari AK, Gautam M, Maurya HK. Recent development of biodegradation techniques of polymer. Int J Res -Granthaalayah. 2018;6(6):414–452. doi:10.29121/GRANTHAALAYAH.V6.I6.2018.1389
- Gewert B, Plassmann MM, Macleod M. Pathways for degradation of plastic polymers floating in the marine environment. 2015. doi:10.1039/c5em00207a
- Gu JD. Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegradation. 2003;52(2):69–91. doi:10.1016/S0964-8305(02)00177-4
- Primc G, Tomšič B, Vesel A, et al. Biodegradability of oxygen-plasma treated cellulose textile functionalized with ZnO nanoparticles as antibacterial treatment. J Phys D Appl Phys. 2016;49(32):324002), doi:10.1088/0022-3727/49/32/324002
- Karamanlioglu M, Preziosi R, Robson GD. Abiotic and biotic environmental degradation of the bioplastic polymer poly(Lactic Acid): a review. Polym Degrad Stab. 2017;137:122–130. doi:10.1016/J.POLYMDEGRADSTAB.2017.01.009
- Linares AB, Jiménez JC, López P, et al. Biodegradability study by FTIR and DSC of polymers films based on polypropylene and cassava starch. Orbital. 2019;11(2):71–82. doi:10.17807/orbital.v11i2.1360
- Antipova TV, Zhelifonova VP, Zaitsev KV, et al. Biodegradation of poly-ϵ-caprolactones and poly-l-lactides by fungi. J Polym Environ. 2018;26(12):4350–4359. doi:10.1007/S10924-018-1307-3
- Pantani R, Sorrentino A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(Lactic Acid) samples in controlled composting conditions. Polym Degrad Stab. 2013;98(5):1089–1096. doi:10.1016/J.POLYMDEGRADSTAB.2013.01.005
- Gigli M, Lotti N, Gazzano M, et al. Biodegradable aliphatic copolyesters containing PEG-like sequences for sustainable food packaging applications. Polym Degrad Stab. 2014;105(1):96–106. doi:10.1016/J.POLYMDEGRADSTAB.2014.04.006
- Hsu ST, Tan H, Yao YL. Effect of laser-induced crystallinity modification on biodegradation profile of poly(L-Lactic Acid). Int Congr Appl Lasers Electro-Optics. 2018;2012(1):720. doi:10.2351/1.5062531
- Genovese L, Lotti N, Gazzano M, et al. Novel biodegradable aliphatic copolyesters based on poly(Butylene Succinate) containing thioether-linkages for sustainable food packaging applications. Polym Degrad Stab. 2016;132:191–201. doi:10.1016/J.POLYMDEGRADSTAB.2016.02.022
- Prudnikova SV, Vinogradova ON, Trusova MY. Specific character of bacterial biodegradation of polyhydroxyalkanoates with different chemical structure in soil. Dokl Biochem Biophys. 2017;473(1):94–97. doi:10.1134/S1607672917010185
- Wang HT, Wang JM, Wu TM. Synthesis and characterization of biodegradable aliphatic–aromatic nanocomposites fabricated using maleic acid-grafted poly[(Butylene Adipate)-Co-Terephthalate] and organically modified layered zinc phenylphosphonate. Polym Int. 2019;68(8):1531–1537. doi:10.1002/PI.5862
- Li F, Guo Z, Wang N, et al. Biodegradation of poly(3-Hydroxybutyrate)-derived polymers with different 4-hydroxybutyrate fractions by a novel depolymerase from paecilomycessp. 1407. Polym Degrad Stab. 2019;159:107–115. doi:10.1016/J.POLYMDEGRADSTAB.2018.11.016
- Tang TO, Simon GP. Biodegradation of 3D-printed polylactic acid milliprojections under physiological conditions. J Appl Polym Sci. 2020;137(38):49129. doi:10.1002/APP.49129
- Niu J, Zhang X, Pei N, et al. Biodegradability of cellulose fibers and the fabrics in activated sludge. Appl Mech Mater. 2012;217–219:918–922. doi:10.4028/www.SCIENTIFIC.NET/AMM.217-219.918
- Hou HZ, Zhang WX, Li LL, et al. Study on biodegradability of cellulosic fabrics. Acta Polymerica Sinica. 2013;1:30–35. doi:10.3724/SP.J.1105.2013.12116.
- Li Y, Qiu S, Sun J, et al. A new strategy to prepare fully bio-based poly(lactic acid) composite with high flame retardancy, UV resistance, and rapid degradation in soil. Chem Eng J. 2022;428:131979. doi:10.1016/J.CEJ.2021.131979
- Holmberg AL, Reno KH, Wool RP, et al. Biobased building blocks for the rational design of renewable block polymers. Soft Matter. 2014;10:7405–7424. doi:10.1039/c4sm01220h
- Gaytán I, Burelo M, Loza-Tavera H. Current status on the biodegradability of acrylic polymers: microorganisms, enzymes and metabolic pathways involved. Appl Microbiol Biotechnol. 2021;105(3):991–1006. doi:10.1007/S00253-020-11073-1/FIGURES/4
- Wei R, Zimmermann W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: how far are we? Microb Biotechnol. 2017;10(6):1308–1322. doi:10.1111/1751-7915.12710
- Biundo A, Ribitsch D, Guebitz GM. Surface engineering of polyester-degrading enzymes to improve efficiency and tune specificity. Appl Microbiol Biotechnol. 2018;102(8):3551–3559. doi:10.1007/S00253-018-8850-7/FIGURES/7
- Carr CM, Clarke DJ, Dobson ADW. Microbial polyethylene terephthalate hydrolases: current and future perspectives. Front Microbiol. 2020;11:2825. doi:10.3389/FMICB.2020.571265/BIBTEX
- Leitão AL, Enguita FJ. Structural insights into carboxylic polyester-degrading enzymes and their functional depolymerizing neighbors. Int J Mol Sci. 2021;22(5):2332. doi:10.3390/IJMS22052332
- Kawai F, Zhang B, Liu B. The current state of research on PET hydrolyzing enzymes available for biorecycling. Catal. 2021;11(2):206. doi:10.3390/CATAL11020206
- Zaaba NF, Jaafar M. A review on degradation mechanisms of polylactic acid: hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym Eng Sci. 2020;60(9):2061–2075. doi:10.1002/PEN.25511
- Guo C, Li C, Kaplan DL. Enzymatic degradation of bombyx mori silk materials: a review. Biomacromolecules. 2020;21(5):1678–1686. doi:10.1021/ACS.BIOMAC.0C00090/ASSET/IMAGES/LARGE/BM0C00090_0005.JPEG
- Qui J, Wilkens C, Barrett K, et al. Microbial enzymes catalyzing keratin degradation: classification, structure, function. Biotechnol Adv. 2020;44:107607. doi:10.1016/J.BIOTECHADV.2020.107607
- Fu J, Su J, Wang P, et al. Enzymatic processing of protein-based fibers. Appl Microbiol Biotechnol. 2015;99(24):10387–10397. doi:10.1007/S00253-015-6970-X/FIGURES/9
- Yadav N, Hakkarainen M. Degradable or not? cellulose acetate as a model for complicated interplay between structure, environment and degradation. Chemosphere. 2021;265:128731. doi:10.1016/J.CHEMOSPHERE.2020.128731
- Zambrano MC, Pawlak JJ, Daystar J, et al. Aerobic biodegradation in freshwater and marine environments of textile microfbers generated in clothes laundering: efects of cellulose and polyester-based microfbers on the microbiome. Mar Pollut Bull. 2020. doi:10.1016/j.marpolbul.2019.110826
- Ansari IA, East GC, Johnson DJ. Structure–property relationships in natural cellulosic fibres: part IV. Biodegradability Text Fibres. 2009;94(1–2):16–36. doi:10.1080/00405000308630591
- Zumstein MT, Schintlmeister A, Nelson TF, et al. Biodegradation of synthetic polymers in soils: tracking carbon into CO2 and microbial biomass. Sci Adv. 2018;4(7). doi:10.1126/SCIADV.AAS9024/
- Warnock M, Davis K, Wolf D, et al. Soil burial effects on biodegradation and properties of three cellulosic fabrics. AATCC Rev. 2011;11:53–57.
- Lunt J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym Degrad Stab. 1998;59(1–3):145–152. doi:10.1016/S0141-3910(97)00148-1
- Qin Q, Takarada W, Kikutani T. Fiber structure development of PHBH through stress-induced crystallization in high-speed melt spinning process. J Fiber Sci Technol. 2017;73(2):49–60. doi:10.2115/FIBERST.2017-0007
- Gorrasi G, Pantani R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: assessment of structural modification and kinetic parameters. Polym Degrad Stab. 2013;98(5):1006–1014. doi:10.1016/J.POLYMDEGRADSTAB.2013.02.005
- Mochizuki M. Textile applications. In: Auras RA, Selke SE, Lim L-T, etal, editors. Poly(lactic acid): synthesis, structures, properties, processing, and applications, vol 1. Hoboken, NJ: John Wiley & Sons Inc, p. 469–476.
- Castro-Aguirre E, Iñiguez-Franco F, Samsudin H, et al. Poly(lactic acid)—mass production, processing, industrial applications, and end of life. Adv Drug Delivery Rev. 2016;107:333–366. doi:10.1016/J.ADDR.2016.03.010
- Shruti VC, Kutralam-Muniasamy G. Bioplastics: missing link in the era of microplastics. Sci Total Environ. 2019;697:134139. doi:10.1016/J.SCITOTENV.2019.134139
- Bhagwat G, Gray K, Wilson SP, et al. Benchmarking bioplastics: a natural step towards a sustainable future. J Polym Environ. 2020;28(12):3055–3075. doi:10.1007/S10924-020-01830-8/METRICS
- Schmatz DA, Da Silva Uebel L, Kuntzler SG, et al. Scaffolds containing spirulina Sp. LEB 18 biomass: development, characterization and evaluation of in vitro biodegradation. J Nanosci Nanotechnol. 2016;16(1):1050–1059. doi:10.1166/JNN.2016.12331
- Gómez EF, Michel FC. Biodegradability of conventional and bio-based plastics and natural fiber composites during composting, anaerobic digestion and long-term soil incubation. Polym Degrad Stab. 2013;98(12):2583–2591. doi:10.1016/J.POLYMDEGRADSTAB.2013.09.018
- Lykaki M, Zhang YQ, Markiewicz M, et al. The influence of textile finishing agents on the biodegradability of shed fibres. Green Chem. 2021;23(14):5212–5221. doi:10.1039/d1gc00883h
- Poznyak TI, Chairez Oria I, Poznyak AS. Biodegradation. Ozonation Biodegrad Environ Eng. 2019: 353–388. doi:10.1016/B978-0-12-812847-3.00023-8
- Ghosh M, Rao GV, Chakrabarti SK, et al. Biodegradability study to develop longer life jute geotextiles for road applications. 2019;89(19–20):4162–4172. doi:10.1177/0040517519828985
- Renouard S, Hano C, Ouagne P, et al. Protection of flax fiber-based yarns against natural soil degradation by chitosan. Mater Lett. 2014;137:269–273. doi:10.1016/J.MATLET.2014.09.030
- Podgornik BB, Šandric S, Kert M. Microencapsulation for functional textile coatings with emphasis on biodegradability—a systematic review. Coatings. 2021;11(11). doi:10.3390/coatings11111371
- Mehta S. Optimization of fluorochemical finish concentration for liquid repellency treatment of 100% cotton fabric and resulting physical properties. AATCC J Res. 2008;5(5):15–22. doi:10.14504/ajr.5.5.3
- Mehta S. An experimental investigation on optimizing liquid repellency of fluorochemical urethane finish and its effect on the physical properties of polyester/cotton blended fabric. Fibers. 2020;8:72. doi:10.3390/fib8120072
- Xiao F. Emerging poly- and perfluoroalkyl substances in the aquatic environment: a review of current literature. Water Res. 2017;124:482–495. doi:10.1016/J.WATRES.2017.07.024
- Conder JM, Hoke RA, de Wolf W, et al. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol. 2008;42(4):995–1003. doi:10.1021/ES070895G/SUPPL_FILE/ES070895G-FILE001.PDF
- Feng C, Sui X, Ankeny MA, et al. Identifcation and quantifcation of CI reactive blue 19 dye degradation product in soil. Color Technol. 2021;137:251–258. doi:10.1111/cote.12527
- Wei Z, Xu T, Zhao D. Treatment of per- and polyfluoroalkyl substances in landfill leachate: status, chemistry and prospects. Environ Sci: Water Res Technol. 2019;5(11):1814–1835. doi:10.1039/C9EW00645A
- Solid Waste Master Plan | Mass.gov. [cited 2022 Jul 28]. Available from: https://www.mass.gov/guides/solid-waste-master-plan.
- Tojo N, Kogg B, Kiørboe N, et al. Prevention of Textile Waste. 2012. doi:10.6027/TN2012-545.
- Dioxins and their effects on human health. [cited 2022 Aug 05]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health.
- Ryu C, Phan AN, Sharifi VN, et al. Combustion of textile residues in a packed Bed. Exp Therm Fluid Sci. 2007;31(8):887–895. doi:10.1016/J.EXPTHERMFLUSCI.2006.09.004
- Dong C, Zhang H, Pang Z, et al. Sulfonated modification of cotton linter and its application as adsorbent for high-efficiency removal of lead(II) in effluent. Bioresour Technol. 2013;146:512–518. doi:10.1016/J.BIORTECH.2013.07.108
- Nahil MA, Williams PT. Surface chemistry and porosity of nitrogen-containing activated carbons produced from acrylic textile waste. Chem Eng J. 2012;184:228–237. doi:10.1016/J.CEJ.2012.01.047
- Jeihanipour A, Aslanzadeh S, Rajendran K, et al. High-rate biogas production from waste textiles using a two-stage process. Renew Energy. 2013;52:128–135. doi:10.1016/J.RENENE.2012.10.042
- Gholamzad E, Karimi K, Masoomi M. Effective conversion of waste polyester–cotton textile to ethanol and recovery of polyester by alkaline pretreatment. Chem Eng J. 2014;253:40–45. doi:10.1016/J.CEJ.2014.04.109
- Carniel A, Gomes AdC, Coelho MAZ, et al. Process strategies to improve biocatalytic depolymerization of post-consumer PET packages in bioreactors, and investigation on consumables cost reduction. Bioprocess Biosyst Eng. 2021;44(3):507–516. doi:10.1007/S00449-020-02461-Y/METRICS
- Barth M, Honak A, Oeser T, et al. A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol J. 2016;11(8):1082–1087. doi:10.1002/BIOT.201600008