Abstract
Attention-deficit hyperactivity disorder (ADHD) is a common chronic neurodevelopmental disorder characterized by symptoms of inattention, overactivity, and/or impulsiveness. The prevalence of ADHD varies in different settings and there have been voices raised to call for more objective measures in order to avoid over- and underdiagnosing of ADHD. Auditory Brainstem Response (ABR) is a method where click shaped sounds evoke potentials that are recorder from electrodes on the skull of a patient. The aim of this study was to explore possible alterations in the ABR of 29 patients with ADHD compared to 39 healthy controls. We used a forward masked sound. We found differences in ABR that correspond to the thalamic area. The thalamus seems to play an active role in regulation of activity level in ADHD. More research is needed to draw any further conclusions on using ABR as an objective measurement to detect ADHD.
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterized by symptoms of inattention, overactivity, and/or impulsiveness that are age-inappropriate, persistent, and pervasive (Diagnostic and Statistical Manual of Mental Disorders, 5th Edition [DSM-5]; American Psychiatric Association, Citation2013; Faraone & Biederman, Citation2005) with a worldwide prevalence of child ADHD is estimated at 5%, adult ADHD prevalence rates 2.5% (Chauhan et al., Citation2022; Polanczyk & Rohde, Citation2007; Willcutt Citation2012). Figures vary depending on the diagnostic setting and culture (Bruchmüller et al., Citation2012; Fayyad et al., Citation2017; Thomas et al., Citation2015). ADHD is most frequently diagnosed during the school years but affects individuals across the lifespan (Polanczyk & Rohde, Citation2007). Well-studied comorbidities of ADHD include oppositional defiant disorder (ODD), conduct disorder, depressive disorders, anxiety disorders, and sleep disorders. Speech and learning disorders are the most common (Chauhan et al., Citation2022; Sciberras et al., Citation2022). ADHD is also associated with a significant risk of educational failure, interpersonal problems, mental illness, and delinquency, with a significant burden on health, social care, and criminal justice systems (Biederman et al., Citation2006; Fredriksen et al., Citation2014).
When EEG (electroencephalography) is conducted so that the recorded neural activity is limited to correspond to a specific, repeated stimulus, this is called Event Related Potentials (ERP). Auditory Brainstem Response (ABR) refers to the kind of ERP where the stimulus is sound. ABR constitutes a direct test of both hearing status and integrity of brainstem pathways. ABR traditionally uses click sounds at given intervals and electrodes placed on the skull and calibrated to measure the processing that occurs in the basic auditory pathways situated in the brainstem to avoid recording later responses from cerebral clusters of higher processing complexity left out. Using complex stimuli, as in forward and backward masking, the sounds in ABR are similar to sounds in everyday hearing (Skoe & Kraus, Citation2010). Complex stimuli may reveal aberrations that would be missed by standard audiological ABR procedures (E. Baghdassarian et al., Citation2014; Källstrand et al., Citation2010, Citation2012; Sköld et al., Citation2014).
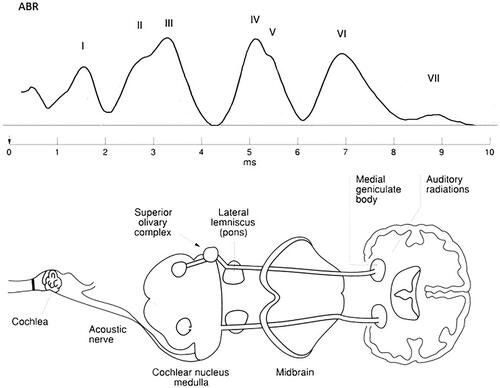
The ABR consists of seven positive peaks (Wave I–VII) recorded by surface electrodes on the mastoid processes of each ear and the forehead (Jewett & Williston, Citation1971). The auditory nerve produces Waves I–II. In contrast, the subsequent peaks are due to the combined electrical activity of nuclei at gradually higher levels of the ascending auditory pathway in the brainstem. Wave III is generated in the cochlear nucleus. Wave IV and V represents the mid-brain. Waves VI and VII are thought to represent Thalamus more specific the medial geniculate body (MGB), but the findings are still inconclusive (Habib & Habib, Citation2021; Klin, Citation1993; Parkkonen et al., Citation2009). The ABR waves are illustrated by Claesdotter-Hybbinette et al. (Citation2016; ).
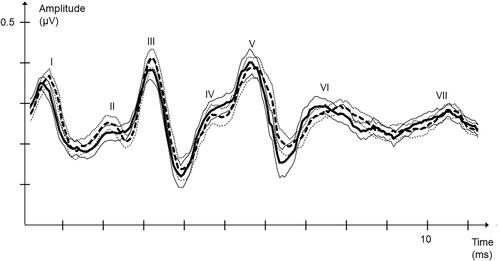
Figure 2. Right FM ABR amplitudes for the seven ABR peaks. Average ABR curves for ADHD; dotted black, thin dotted black standard error curves and HC; black, thin black standard error curves.
ABR has been used since the 1960s by pediatricians to diagnose possible defects in hearing (World Health Organization, Citation2010) and has been suggested as a complementary diagnostic tool to diagnose schizophrenia (Källstrand et al., Citation2012; Nielzén et al., Citation2008) and bipolar disorders (Sköld et al., Citation2014), autism spectrum disorder (ASD) (Claesdotter-Knutsson et al., Citation2019; Dabbous, Citation2012). ABR has also been studied in ADHD (E. J. Baghdassarian et al., Citation2018; Claesdotter-Hybbinette et al., Citation2016; Johnston et al., Citation2014).
The paradigm is built on the hypothesis that specific ABR peaks correlate to certain psychiatric symptoms.
An ADHD diagnosis should be based on a neurodevelopmental and clinical history (DSM-5; American Psychiatric Association, Citation2013;). Neuropsychological testing and rating scales are used to provide indicative evidence in the diagnostic process. Many recent studies focus on trying to find objective neuropsychological testing methods for the diagnostics of ADHD (Abdolmaleki & Abadeh, Citation2020; Amado-Caballero et al., Citation2020; Gau & Shang, Citation2010; Häger et al., Citation2021). The diagnostic procedure of ADHD has been accused of being inadequate and unobjective, resulting in different prevalence rates but also over- and under-diagnose (Bell et al., Citation2022; Gascon et al., Citation2022; Hirvikoski et al., Citation2022). The has been a strong call for more objective methods in diagnosing ADHD.
In our study, we wanted to look at possible differences in the ABR of healthy controls (HC) and drug naïve patients with ADHD.
Materials and methods
Subjects
This study included 32 drug-naïve patients with ADHD (19 males and 13 females) and 35 HC (15 males and 20 females). Patients and HC were in the age range of 7–17. Patients with ADHD had a median age of 11, SD 2.61, and HC had a median age of 12, SD 2.58. All patients were recruited from the child and adolescent outpatient clinic in Lund, a city in the south of Sweden. All patients were diagnosed according to the DSM-5. A senior consultant in child and adolescent psychiatry confirmed all diagnoses.
Patients with concurrent psychiatry diagnoses were excluded to avoid comorbidity. All patients were drug naïve. Control subjects and ADHD patients with hearing impairment, verified by interviews with parents, were excluded from the study. The HC were recruited from schools in Lund, and recruited subjects had no previous record of any psychiatric disorder. Both groups were in the standard intellectual range.
Written informed consent was obtained from all the subjects and their parents/guardians. The study was approved by the regional ethics committee at Lund University (Dnr: 2021-00351).
Apparatus and stimuli
ABR was recorded using SensoDetect BERA (Brainstem Evoked Response Audiometry) A1000. Binaural sound stimuli were presented in phases via TDH-50 P headphones with Model 51 cushions (Telephonics, Farmingdale, NY, United States). The click pulses were repeated until 1024 accepted evoked potentials were collected for each sound stimulus. Transistor-transistor logic (TTL) triggers pulses coordinated recording sweeps with the auditory stimuli. Sound levels were calibrated with a Bruel & Kjaer 2203 sound level meter and type 4152 artificial ear (Bruel & Kjaer S & VMeasurement, Naerum, Denmark). The acoustic output power from the headphones corresponded to SPL: 80 dB HL or 109 peSPL (peak equivalent). The collected potentials for each sound stimulus from each ear of each individual were imported into Microsoft Excel (Microsoft Corp, Redmond, WA, United States) for statistical analysis.
The participants were presented with a forward masked sound (FM) during a 20 min. A square-shaped click pulse was used as a probe for both the standard ABR condition and the auditory forward masking stimulus. The duration time of the probe was 0.136 ms with a rise and fall time of 0.023 ms. The clicks of the stimulus train presented during recordings had an interstimulus interval (ISI) from onset to onset of 0.192 s. In the forward masking paradigm the square-shaped click pulse is preceded by a masker. As masker, a 1,500 Hz low-pass filtered noise (Butterworth filter) was used. The duration of the masking noise was 0.015 s including a 0.004 s rise and fall time. The gap between masker and target stimulus was 0.012 s. The interval between onset to onset of clicks in the forward masking stimulus was 0.192 s. All stimuli were constructed using MATLAB Signal Processing Toolbox (The MathWorks, Inc., Natick, MA, United States) and stored in flash memory in the SensoDetect® BERA system.
Procedure
All tests were performed in a quiet and darkened room. Participants were comfortably seated in an armchair in a resting position. A Farraday’s cage was put around the patient to avoid electrical interference (Paliarin et al., Citation2018). Surface electrodes were attached to the skin over the mastoid bones behind the left and right ear, with a ground electrode and a reference electrode placed on the vertex and forehead, respectively. Before the test session, the procedure was fully explained to the test subject, and the click sounds were presented beforehand to make him/her acquainted with the stimuli. Absolute impedances and interelectrode impedance were measured before and after the experiments to verify that electrode contact was maintained (below 5,000 Ω). The subjects were instructed to relax with their eyes closed and were permitted to fall asleep. The test required no active participation other than being subjected to sound stimulation. The subjects were tested one at a time, and the duration of the testing procedure was 15 min.
Data analysis
The ABR curve quality check was measured through whole curve correlation (Spearman’s rho, 0–10 ms) between the specific patient and the norm ABR curve. The norm ABR curve consisted of the median representation from a group of normal hearing HC. This method is a standard operating procedure to grant ABR quality. Collected evoked potentials for each sound stimulus from each individual were imported to Microsoft Excel (Microsoft Corp, Redmond, WA, United States) and analyzed using SensoDetect® BAS. We studied the peak-to-trough amplitudes. For each subject, amplitudes for left and right were identified for the square-shaped click as the target stimulus preceded by no masker (ABR) or a masker of 70 dB (FM). The amplitudes were calculated by subtracting the minimum value from the preceding max value in the peak-to-trough area for each peak, as defined by the HC group average curves. Four ABR waveforms were obtained from each test subject (two left and two right) and 20 values. The nonparametric test Mann–Whitney U was used to measure statistical differences between groups. Bonferroni correction was applied on all p values as a total of 10 comparisons were made (the non-masked conditions were not the scope of investigation in this study).
Results
We studied the peak-to-trough amplitudes of the characterized peaks in the brainstem audiograms. This approach gave us five values: P1, P2, P3, P5, and P6, as P4 was excluded due to missing values. Although the non-masked conditions were not the scope of investigation in this study, these values are presented for descriptive purposes (). No significant differences were found here. In the masked condition, activity in P6 was significantly lower on both the left and right sides for ADHD patients ( and ).
Table 1. Peak-to-trough amplitudes for the five ABR peaks for standard ABR square-shaped click pulses (non-masked condition).
Table 2. Peak-to-trough amplitudes for the five ABR peaks (masked condition).
For the left side ABRs, P6 had an average amplitude of 0.32 µV for the group of HC whereas the ADHD group had an average amplitude of 0.27 µV. This difference was statistically significant (Mann–Whitney U test, p = .0050, Z = −3.51, U = 280) with Cohen’s d = 0.000825 ().
For the right side ABRs, P6 had an average amplitude of 0,29µV for the HC and the ADHD group had an average amplitude of 0,23µV. The difference between the studied groups were statistically significant (Mann–Whitney U test, p = .045, Z = −2.84, U = 333) with Cohen’s d = 0.001648 ().
Discussion
The aim of this study was to explore possible alterations in the ABR of 32 patients with ADHD compared to 35 HC. We found underactivity in a part of the ABR that is believed to correspond to the Thalamic area.
Previous studies of aberrant ABR in neurodevelopmental disorders are mostly done on ASD, results being inconsistent and even contradictory (Hitoglou et al., Citation2010; Kwon et al., Citation2007; Maziade et al., Citation2000; Taylor et al., Citation1982). The most frequent finding being an increase of interpeak latencies of Wave III, V, I-III and I-V (Talge et al., Citation2018). In a review Talge et al (Citation2021) found a stronger association in studies on children than on adults (Talge et al., Citation2021). ADHD being such a common neurodevelopmental disorder with an estimated worldwide prevalence of around 5% in children (Sayal et al., Citation2018) there are surprisingly few studies focusing on ABR and ADHD in children. As with ABR and ASD, findings are mixed, some reporting no associations and others reporting slower and even faster ABR latencies in ADHD (Azzam & Hassan, Citation2010; Ismail & Amin Citation1999; Schochat et al., Citation2002; Vaney et al., Citation2011). Talge et al. (Citation2021) goes as far as to state that ABR findings in ADHD might not be linked to ADHD itself and urge for more research in the field.
The Thalamus is thought to play a significant role in ADHD symptomatology. Thalamus is looked upon as a kind of switchboard of information, acting as a relay between various subcortical areas and the cerebral cortex. Specifically, it is the MGB that represent the Thalamic relay influencing the direction and maintenance of attention. However, the Thalamus is not just a station connecting the basal ganglia, the cerebellum, and the cortex. It also plays a role in modulating excitatory and inhibitory functions of both the ascending and descending pathways to actively influence and modify behavioral outcomes leading to symptoms similar to the characteristics of ADHD. Crucial neural circuitry connecting cortical and subcortical structures passes through the Thalamus (Guillery & Sherman, Citation2002; Sherman & Guillery, Citation2002). It has been shown that the activity in the Thalamus results in brainwaves that EEG can measure and ABR (Howe & Sterman, Citation1972; J. Sterman et al., Citation2019; M. B. Sterman, Citation1996; M. B. Sterman et al., Citation1970). Without the thalamic-cortical connections, awareness, volition, or consciousness are not possible (Steriade & Deschenes, Citation1984). Ivanov et al. (Citation2010) found no significance in thalamus volume but regional differences, especially in the pulvinar nuclei of the Thalamus bilaterally, when comparing ADHD subjects to HC. The pulvinar nuclei are particularly important in arousal, selective attention, learning and memory, and orientation to visual and auditory stimuli. It is the pulvinar-cortical and pulvinar-limbic connections that contribute to this. The nuclei are also thought to contribute to decisions about the salience of visual objects and the filtering-out of no salient environmental distractors (Bailey & Joyce, Citation2015; Ivanov et al., Citation2010). Thalamus is key in arousal and alertness, a pervasive problem for children with ADHD (Stephens et al., Citation2013).
Our findings of lower activity in an area of the brain that is believed to correspond to the Thalamus align well with the above results.
Limitations
The results in this study are based on group differences. Other limitations are the small number of patients and HC participants and the unbalanced gender representation. It is well known that ADHD presents itself differently in boys than in girls. The imbalance in overweight male ADHD subjects and female HC might have influenced the result. Future research areas of interest would be to investigate whether medication would decrease the ABR differences between the groups of ADHD and HC and, in that case, which of the medications on the market would be best for which trait.
Another limitation is the ADHD diagnosis itself. De Wit et al. (Citation2018) showed in their review that there is a great overlap between Auditory Processing Disorder (APD) and developmental diagnoses such as ADHD. This raises the concern often debated in child and adolescent psychiatry regarding diagnoses and their validity. One cannot rule out that the patients in our study also meet the criteria for APD which might affect the outcome of the study. More and preferably longitudinal studies are needed to substantiate our findings further.
Data availability statement
The dataset is available upon request addressed to Dr. Lindvall.
Additional information
Funding
References
- Abdolmaleki, S., & Abadeh, M. S. (2020). Brain MR image classification for ADHD diagnosis using deep neural networks. In 2020 international conference on machine vision and image processing (MVIP) (pp. 1–5). IEEE. https://doi.org/10.1109/MVIP49855.2020.9116877
- Amado-Caballero, P., Casaseca-de-la-Higuera, P., Alberola-Lopez, S., Andres-de-Llano, J. M., Villalobos, J. A. L., Garmendia-Leiza, J. R., & Alberola-Lopez, C. (2020). Objective ADHD diagnosis using convolutional neural networks over daily-life activity records. IEEE Journal of Biomedical and Health Informatics, 24(9), 2690–2700. https://doi.org/10.1109/JBHI.2020.2964072
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed).
- Azzam, H., & Hassan, D. M. (2010). Speech-evoked auditory potentials in attention deficit hyperactivity disorder. Audiological Medicine, 8(3), 129–136. https://doi.org/10.3109/1651386X.2010.499738
- Baghdassarian, E., Källstrand, J., Nielzén, S., & Lewander, T. (2014). P. 1. c. 011 Brainstem evoked response audiometry biomarkers in patients with schizophrenia and adult ADHD. European Neuropsychopharmacology, 24(24), S187–S188. https://doi.org/10.1016/S0924-977X(14)70288-3
- Baghdassarian, E. J., Markhed, M. N., Lindström, E., Nilsson, B. M., & Lewander, T. (2018). Auditory brainstem response (ABR) profiling tests as diagnostic support for schizophrenia and adult attention-deficit hyperactivity disorder (ADHD). Acta Neuropsychiatrica, 30(3), 137–147.
- Bailey, T., & Joyce, A. (2015). The role of the thalamus in ADHD symptomatology and treatment. Applied Neuropsychology. Child, 4(2), 89–96. https://doi.org/10.1080/21622965.2015.1005475
- Bell, Z. E., Fristad, M. A., Youngstrom, E. A., Arnold, L. E., Beauchaine, T. P., Findling, R. L., Birmaher, B., & Horwitz, S. M. (2022). Attention-deficit/hyperactivity disorder symptoms and externalizing progression in the LAMS study: A test of trait impulsivity theory. Journal of the American Academy of Child and Adolescent Psychiatry, 61(2), 298–307. https://doi.org/10.1016/j.jaac.2021.05.018
- Biederman, J., Monuteaux, M. C., Mick, E., Spencer, T., Wilens, T. E., Silva, J. M., Snyder, L. E., & Faraone, S. V. (2006). Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow-up study. Psychological Medicine, 36(2), 167–179. https://doi.org/10.1017/S0033291705006410
- Bruchmüller, K., Margraf, J., & Schneider, S. (2012). Is ADHD diagnosed in accord with diagnostic criteria? Overdiagnosis and influence of client gender on diagnosis. Journal of Consulting and Clinical Psychology, 80(1), 128–138. https://doi.org/10.1037/a0026582
- Chauhan, A., Sahu, J. K., Singh, M., Jaiswal, N., Agarwal, A., Bhanudeep, S., Pradhan, P., & Singh, M. (2022). Burden of attention deficit hyperactivity disorder (ADHD) in Indian children: A systematic review and meta-analysis. Indian Journal of Pediatrics, 89, 1–9.
- Claesdotter-Hybbinette, E., Cervin, M., Åkerlund, S., Råstam, M., & Lindvall, M. (2016). Gender specific differences in auditory brain stem response in young patients with ADHD. Neuropsychiatry, 06(01), 28–35. https://doi.org/10.4172/Neuropsychiatry.1000114
- Claesdotter-Knutsson, E., Åkerlund, S., Cervin, M., Råstam, M., & Lindvall, M. (2019). Abnormal auditory brainstem response in the pons region in youth with autism. Neurology, Psychiatry and Brain Research, 32, 122–125.
- Dabbous, A. O. (2012). Characteristics of auditory brainstem response latencies in children with autism spectrum disorders. Audiological Medicine, 10(3), 122–131. https://doi.org/10.3109/1651386X.2012.708986
- de Wit, E., van Dijk, P., Hanekamp, S., Visser-Bochane, M. I., Steenbergen, B., van der Schans, C. P., & Luinge, M. R. (2018). Same or different: The overlap between children with auditory processing disorders and children with other developmental disorders: A systematic review. Ear and Hearing, 39(1), 1–19. https://doi.org/10.1097/AUD.0000000000000479
- Faraone, S. V., & Biederman, J. (2005). What is the prevalence of adult ADHD? Results of a population screen of 966 adults. Journal of Attention Disorders, 9(2), 384–391. https://doi.org/10.1177/1087054705281478
- Fayyad, J., Sampson, N. A., Hwang, I., Adamowski, T., Aguilar-Gaxiola, S., Al-Hamzawi, A., Andrade, L. H. S. G., Borges, G., de Girolamo, G., Florescu, S., Gureje, O., Haro, J. M., Hu, C., Karam, E. G., Lee, S., Navarro-Mateu, F., O'Neill, S., Pennell, B.-E., Piazza, M., … Kessler, R. C. (2017). The descriptive epidemiology of DSM-IV adult ADHD in the world health organization world mental health surveys. ADHD: Attention Deficit and Hyperactivity Disorders, 9(1), 47–65. https://doi.org/10.1007/s12402-016-0208-3
- Fredriksen, M., Dahl, A. A., Martinsen, E. W., Klungsoyr, O., Faraone, S. V., & Peleikis, D. E. (2014). Childhood and persistent ADHD symptoms associated with educational failure and long-term occupational disability in adult ADHD. ADHD: Attention Deficit and Hyperactivity Disorders, 6(2), 87–99. https://doi.org/10.1007/s12402-014-0126-1
- Gascon, A., Gamache, D., St‐Laurent, D., & Stipanicic, A. (2022). Do we over‐diagnose ADHD in North America? A critical review and clinical recommendations. Journal of Clinical Psychology, 78(12), 2363–2380. https://doi.org/10.1002/jclp.23348
- Gau, S. S. F., & Shang, C. Y. (2010). Executive functions as endophenotypes in ADHD: Evidence from the Cambridge Neuropsychological Test Battery (CANTAB). Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51(7), 838–849.
- Guillery, R. W., & Sherman, S. M. (2002). Thalamic relay functions and their role in corticocortical communication: Generalizations from the visual system. Neuron, 33(2), 163–175. https://doi.org/10.1016/s0896-6273(01)00582-7
- Habib, S. H., & Habib, S. S. (2021). Auditory brainstem response: An overview of neurophysiological implications and clinical applications. JPMA: The Journal of the Pakistan Medical Association, 71(9), 2230–2236. https://doi.org/10.47391/JPMA.03-432
- Häger, L. A., Åsberg Johnels, J., Kropotov, J. D., Weidle, B., Hollup, S., Zehentbauer, P. G., Gillberg, C., Billstedt, E., & Ogrim, G. (2021). Biomarker support for ADHD diagnosis based on event related potentials and scores from an attention test. Psychiatry Research, 300, 113879.
- Hirvikoski, T., Billstedt, E., Lundström, S., & Brar, A. (2022). Screening and investigation – risk of both over- and under-diagnosis. Läkartidningen. 119, 21097.
- Hitoglou, M., Ververi, A., Antoniadis, A., & Zafeiriou, D. I. (2010). Childhood autism and auditory system abnormalities. Pediatric Neurology, 42(5), 309–314. https://doi.org/10.1016/j.pediatrneurol.2009.10.009
- Howe, R. C., & Sterman, M. B. (1972). Cortical-subcortical EEG correlates of suppressed motor behavior during sleep and waking in the cat. Electroencephalography and Clinical Neurophysiology, 32(6), 681–695. https://doi.org/10.1016/0013-4694(72)90104-6
- Ismail, N., & Amin, A. (1999). Auditory brainstem response in attention deficit hyperactivity disorders in children. Current Psychiatry, 6(1), 63–70.
- Ivanov, I., Bansal, R., Hao, X., Zhu, H., Kellendonk, C., Miller, L., Sanchez-Pena, J., Miller, A. M., Chakravarty, M. M., Klahr, K., Durkin, K., Greenhill, L. L., & Peterson, B. S. (2010). Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. The American Journal of Psychiatry, 167(4), 397–408. https://doi.org/10.1176/appi.ajp.2009.09030398
- Jewett, D. L., & Williston, J. S. (1971). Auditory-evoked far fields averaged from the scalp of humans. Brain: A Journal of Neurology, 94(4), 681–696. https://doi.org/10.1093/brain/94.4.681
- Johnston, B. A., Mwangi, B., Matthews, K., Coghill, D., Konrad, K., & Steele, J. D. (2014). Brainstem abnormalities in attention deficit hyperactivity disorder support high accuracy individual diagnostic classification. Human Brain Mapping, 35(10), 5179–5189. https://doi.org/10.1002/hbm.22542
- Källstrand, J., Nehlstedt, S. F., Sköld, M. L., & Nielzén, S. (2012). Lateral asymmetry and reduced forward masking effect in early brainstem auditory evoked responses in schizophrenia. Psychiatry Research, 196(2–3), 188–193.
- Källstrand, J., Olsson, O., Nehlstedt, S. F., Sköld, M. L., & Nielzén, S. (2010). Abnormal auditory forward masking pattern in the brainstem response of individuals with Asperger syndrome. Neuropsychiatric Disease and Treatment, 6, 289–296. https://doi.org/10.2147/ndt.s10593
- Klin, A. (1993). Auditory brainstem responses in autism: Brainstem dysfunction or peripheral hearing loss? Journal of Autism and Developmental Disorders, 23(1), 15–35. https://doi.org/10.1007/BF01066416
- Kwon, S., Kim, J., Choe, B. H., Ko, C., & Park, S. (2007). Electrophysiologic assessment of central auditory processing by auditory brainstem responses in children with autism spectrum disorders. Journal of Korean Medical Science, 22(4), 656–659.
- Maziade, M., Merette, C., Cayer, M., Roy, M. A., Szatmari, P., Côté, R., & Thivierge, J. (2000). Prolongation of brainstem auditory-evoked responses in autistic probands and their unaffected relatives. Archives of General Psychiatry, 57(11), 1077–1083. https://doi.org/10.1001/archpsyc.57.11.1077
- Nielzén, S., Olsson, O., Källstrand, J., & Nehlstedt, S. (2008). P0184-aberrant brain stem function in schizophrenia. European Psychiatry, 23(S2), S135–S135. https://doi.org/10.1016/j.eurpsy.2008.01.851
- Paliarin, F., Incrocci, R. M., & Nobre, M. J. (2018). Behavioral and auditory electrophysiological rebound as a compensatory response to the reinforcing effects of morphine. Neuroscience, 392, 66–76. https://doi.org/10.1016/j.neuroscience.2018.09.025
- Parkkonen, L., Fujiki, N., & Mäkelä, J. P. (2009). Sources of auditory brainstem responses revisited: Contribution by magnetoencephalography. Human Brain Mapping, 30(6), 1772–1782. https://doi.org/10.1002/hbm.20788
- Polanczyk, G., & Rohde, L. A. (2007). Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Current Opinion in Psychiatry, 20(4), 386–392. https://doi.org/10.1097/YCO.0b013e3281568d7a
- Sayal, K., Prasad, V., Daley, D., Ford, T., & Coghill, D. (2018). ADHD in children and young people: Prevalence, care pathways, and service provision. The Lancet Psychiatry, 5(2), 175–186. https://doi.org/10.1016/S2215-0366(17)30167-0
- Schochat, E., Scheuer, C. I., & Andrade, Ê R D. (2002). ABR and auditory P300 findings in children with ADHD. Arquivos de Neuro-Psiquiatria, 60(3B), 742–747. https://doi.org/10.1590/S0004-282X2002000500012
- Sciberras, E., Patel, P., Stokes, M. A., Coghill, D., Middeldorp, C. M., Bellgrove, M. A., Becker, S. P., Efron, D., Stringaris, A., Faraone, S. V., Bellows, S. T., Quach, J., Banaschewski, T., McGillivray, J., Hutchinson, D., Silk, T. J., Melvin, G., Wood, A. G., Jackson, A., … Westrupp, E. (2022). Physical health, media use, and mental health in children and adolescents with ADHD during the COVID-19 pandemic in Australia. Journal of Attention Disorders, 26(4), 549–562. https://doi.org/10.1177/1087054720978549
- Sherman, S. M., & Guillery, R. W. (2002). The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 357(1428), 1695–1708. https://doi.org/10.1098/rstb.2002.1161
- Skoe, E., & Kraus, N. (2010). Auditory brainstem response to complex sounds: A tutorial. Ear and Hearing, 31(3), 302–324. https://doi.org/10.1097/AUD.0b013e3181cdb272
- Sköld, M., Källstrand, J., Nehlstedt, S., Nordin, A., Nielzén, S., Holmberg, J., & Adolfsson, R. (2014). Thalamocortical abnormalities in auditory brainstem response patterns distinguish DSM-IV bipolar disorder type I from schizophrenia. Journal of Affective Disorders, 169, 105–111. https://doi.org/10.1016/j.jad.2014.08.002
- Stephens, R. J., Chung, S. A., Jovanovic, D., Guerra, R., Stephens, B., Sandor, P., & Shapiro, C. M. (2013). Relationship between polysomnographic sleep architecture and behavior in medication-free children with TS, ADHD, TS and ADHD, and controls. Journal of Developmental and Behavioral Pediatrics: JDBP, 34(9), 688–696. https://doi.org/10.1097/DBP.0000000000000012
- Steriade, M., & Deschenes, M. (1984). The thalamus as a neuronal oscillator. Brain Research Reviews, 8(1), 1–63. https://doi.org/10.1016/0165-0173(84)90017-1
- Sterman, J., Cunqueiro, A., Dym, R. J., Spektor, M., Lipton, M. L., Revzin, M. V., & Scheinfeld, M. H. (2019). Implantable electronic stimulation devices from head to sacrum: Imaging features and functions. Radiographics: A Review Publication of the Radiological Society of North America, Inc, 39(4), 1056–1074. https://doi.org/10.1148/rg.2019180088
- Sterman, M. B. (1996). Physiological origins and functional correlates of EEG rhythmic activities: Implications for self-regulation. Biofeedback and Self-Regulation, 21(1), 3–33. https://doi.org/10.1007/BF02214147
- Sterman, M. B., Howe, R. C., & Macdonald, L. R. (1970). Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science, 167(3921), 1146–1148. https://doi.org/10.1126/science.167.3921.1146
- Talge, N. M., Adkins, M., Kileny, P. R., & Frownfelter, I. (2021). Click-evoked auditory brainstem responses and autism spectrum disorder: A meta-analytic investigation of disorder specificity. Pediatric Research, 11(6), 1–7.
- Talge, N. M., Tudor, B. M., & Kileny, P. R. (2018). Click‐evoked auditory brainstem responses and autism spectrum disorder: A meta‐analytic review. Autism Research: Official Journal of the International Society for Autism Research, 11(6), 916–927. https://doi.org/10.1002/aur.1946
- Taylor, M. J., Rosenblatt, B., & Linschoten, L. (1982). Auditory brainstem response abnormalities in autistic children. Canadian Journal of Neurological Sciences, 9(4), 429–433.
- Thomas, R., Sanders, S., Doust, J., Beller, E., & Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics, 135(4), e994–e1001.
- Vaney, N., Anjana, Y., & Khaliq, F. (2011). No auditory conduction abnormality in children with attention deficit hyperactivity disorder. Functional Neurology, 26(3), 159–163.
- Willcutt, E. G. (2012). The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics, 9(3), 490–499. https://doi.org/10.1007/s13311-012-0135-8
- World Health Organization. (2010). Newborn and infant hearing screening: Current issues and guiding principles for action. WHO Press, World Health Organization.