Abstract
The dietary constituent inorganic nitrate, found in large concentrations in green leafy vegetables, has beneficial effects on cardiometabolic health. Contemporary studies employing nitrate have demonstrated that the anion has anti-obesity and anti-diabetic properties; however the nitrate-mediated mechanisms for improving metabolic health remain unclear. Recently, we employed a combined histological, metabolomics, and transcriptional and protein analysis approach to establish that nitrate promoted the “browning” of white adipose tissue via the xanthine oxidoreductase catalyzed reductive nitrate-nitrite-nitric oxide pathway. Interestingly, it was observed that nitrate-stimulated brown adipose-associated gene expression in white adipose tissue was augmented in hypoxia. These findings not only suggest that protection from metabolic disease offered by vegetable consumption may, in part, be mediated through the effects of nitrate on white adipose tissue, but also, since hypoxia is a serious co-morbidity affecting adipose tissue in obese individuals, that nitrate may be effective in promoting the browning of adipose tissue to improve metabolic fitness.
Abbreviations
| cGMP | = | cyclic guanosine monophosphate |
| GTP | = | guanosine triphosphate |
| NO | = | nitric oxide |
| PKG | = | Protein Kinase G |
| sGC | = | soluble guanylyl cyclase |
| XOR | = | xanthine oxidoreductase |
Inorganic nitrate is a known oxidation end-product of nitric oxide (NO) and is a constituent of the human diet, with vegetables accounting for approximately 80% of daily dietary nitrate intake.Citation1 From the perspective of metabolic health and disease the small molecule has had a turbulent and mixed history. Originally considered to be biologically inactive,Citation2 nitrate (and indeed nitrite) were later thought to be detrimental to human health. It was suggested that dietary nitrate may contribute to the risk of cancer development through the formation of the carcinogenic N-nitrosamines in vivo.Citation3 However, mounting epidemiological and mechanistic studies have been unable to link increased dietary nitrate with an elevated risk of cancer.Citation1,4-6 A World Health Organization Committee on Food Additives has since determined there was no evidence of nitrate-induced carcinogenicity.
Epidemiological studies have suggested that consumption of vegetables reduces the risk of cancerCitation7,8 and dietary nitrate at a concentration comparable to a high vegetable diet also offers beneficial effects on cardiovascular health.Citation9-14 In recent years, a picture of inorganic nitrate as a potential anti-diabetic or anti-obesity mediator has also emerged. Anti-diabetic effects of inorganic nitrate in both endothelial nitric oxide synthase-deficient mice, a strain prone to a metabolic syndrome-like phenotypeCitation15 and in Sprague-Dawley ratsCitation16 have been described. Interestingly, low nitrate diets have also been shown to reduce the concentration of cyclic guanosine monophosphate (cGMP) in certain tissuesCitation17 while dietary inorganic nitrate increases the concentration of cGMP in circulation in humans.Citation14 cGMP signaling is known to have a key role in systemic energy balance.Citation18-20
Of particular interest is the observation that cGMP induces “browning” of white adipose tissue.Citation21 During the browning process white adipose tissue develops a brown adipocyte-like phenotype characterized by the activation of thermogenesis, the utilization of cellular energy stores to produce heat.Citation22,23 Initiation of the browning program in white adipocytes results in increased fatty acid oxidation, mitochondrial biogenesis, and oxygen consumption.Citation24 Thermogenesis occurs via the activity and increased expression of numerous brown adipocyte-associated genes, including the mitochondrial membrane protein uncoupling protein 1, which uncouples the mitochondrial proton gradient from oxidative phosphorylation.Citation25 Physiological metabolite signals are known to promote the browning of white adipose tissue.Citation26 Cells expressing brown-adipocyte associated genes can be found within white adipose tissue depots of both rodents and humans and have been termed “beige” or “brite” cells.Citation27,28 Beige adipocytes demonstrate cardiometabolic protective effects in rodent models of metabolic disease.Citation29-32
In light of these previous observations, we hypothesized that the anti-diabetic and anti-obesity effects of dietary inorganic nitrate may be partially mediated through effects on white adipose tissue, and particularly the induction of browning.Citation33 Using a combination of gene and protein expression analysis, histology, enzyme activity assays and liquid and gas chromatography-mass spectrometry metabolomics techniques we were able to demonstrate that treatment with dietary inorganic nitrate induced a brown adipose-like phenotype in subcutaneous white adipose tissue of Wistar rats.Citation33 By treating stromal vascular fraction derived mouse primary adipocytes with nitrate as they differentiated in vitro and employing RT qPCR, respirometric and mass spectrometry-based stable isotope metabolic analysis it was determined that nitrate directly induced a brown adipocyte-like transcriptional and functional phenotype.Citation33
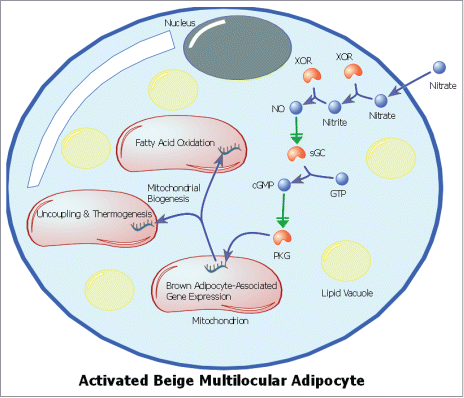
From these experiments it became clear that the nitrate-mediated activation of adipocyte browning required transcriptional reprogramming during the differentiation of the adipocytes. Treating the adipocytes with nitrate as they differentiated over 6-days resulted in an increased population of brown adipocyte-like cells, whereas a short (24 hr) exposure of mature adipocytes to nitrate had little effect. Subsequently we sought to identify the signaling mechanisms acting downstream of nitrate in the adipocyte. With the knowledge that NO can be produced in vivo from nitrate via xanthine oxidoreductase (XOR) catalyzed reduction first to nitrite and then to NO,Citation34 we hypothesized that nitrate might be functioning via NO to produce the browning response in white adipose tissue. Using both pharmacological agents and siRNA transcriptional knockdown we demonstrated that the nitrate-induced expression of brown adipocyte-associated genes in primary adipocytes was indeed mediated through the NO synthase independent, XOR catalyzed reduction of nitrate to NO. Having identified NO as a key intermediary in nitrate-induced browning, we sought to characterize the proximate signaling/effector mechanisms, the primary candidate being cGMP signaling through NO stimulation of soluble guanylyl cyclase.Citation35 Liquid chromatography-mass spectrometry identified an increase in the concentration of cGMP in both the adipose tissue of rats and primary adipocytes treated with nitrate. Employing pharmacological inhibitors it was confirmed that soluble guanylyl cyclase activation increased cGMP concentrations in primary adipocyte cultures, activating the cGMP-dependent protein kinase G, and leading to increased expression of brown adipocyte-associated genes in the primary adipocytes ().
Figure 1. Schematic of an activated beige adipocyte highlighting the signaling mechanism for the nitrate-induced browning response. Nitrate is reduced to nitrite, then to nitric oxide (NO) via xanthine oxidoreductase (XOR) activity. NO activates soluble guanylyl cyclase (sGC), which catalyzes the formation of cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP), activating Protein Kinase G (PKG) and leading to increased expression of mitochondrial browning genes. Increased expression of brown adipocyte-associated genes results in mitochondrial biogenesis and a brown adipocyte-like functional phenotype.
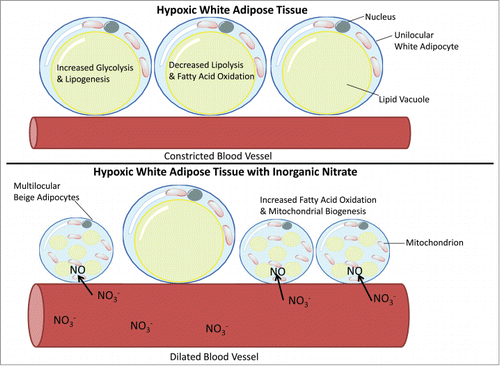
One of the most surprising and significant findings from this study was the effect of hypoxia on the nitrate-facilitated browning of white adipose tissue. It has previously been observed that the production of NO through the nitrate-nitrite-NO pathway is augmented as oxygen concentrations decrease.Citation36 We found that the exposure of both rats and primary adipocytes to low oxygen conditions augmented the nitrate-stimulated expression of brown adipocyte-associated genes within adipocytes. This observation allows us to speculate as to the likely physiological role of this adaptation. The white adipose tissue of obese humans and genetic and dietary models of obesity in rats and mice are characterized by low oxygen concentrations, which lead to a metabolic reconditioning of the adipocytes involving increased glycolysis and de novo lipogenesis, and decreased β-oxidation of lipid.Citation37-40 This reprogramming of metabolism in the adipocyte is thought to contribute to the pathological nature of obesity. The augmentation of the nitrate-stimulated browning response during hypoxia, mediated through enhanced reduction of nitrate to NO by XOR, may represent a physiological adaptation to low oxygen conditions that can be exploited to partly overturn the obesity-related pathological metabolic state of adipose tissue and maintain some oxidative capacity to metabolize fatty acids.
Although not examined in the study described, the vasculature of adipose tissue may represent an additional target for the nitrate-dependent adaptive response to hypoxia. During obesity adipose tissue has reduced angiogenesis and elevated vasoconstriction, which may be related to decreased oxygen concentrations.Citation41 Compounding these effects, obese individuals also have a decreased capacity for NO production.Citation42 NO and nitrate have well established vasodilatory properties. Therefore the increased activity of the XOR catalyzed nitrate-nitrite-NO pathway may function as an adaptive response to hypoxia in adipose tissue, especially in the setting of obesity, where it may have the tandem effect of dilating the blood vessels to improve tissue oxygenation and activating the browning process in adipocytes to reverse hypoxia-mediated pathological metabolic reprogramming (). Incidentally, the mechanism for natriuretic peptide induced browning of white adipose tissue was recently revealed to signal through the cGMP cascade.Citation18 Since natriuretic peptide also has a powerful vasodilatory effect this may underscore the importance of the NO-cGMP signaling pathway for the metabolic regulation of white adipose tissue both through physiological activation of thermogenesis and effects on the vasculature.
Figure 2. Diagram showing how the increased activity of the xanthine oxidoreductase catalyzed nitrate-nitrite-NO pathway may function as an adaptive response to hypoxia in adipose tissue. In addition to activating the browning process in adipocytes it is speculated that increased concentrations of nitrate will dilate the blood vessels to improve tissue oxygenation and partly reverse hypoxia-mediated pathological metabolic reprogramming of adipose tissue.
Our study not only identifies nitrate as a novel activator of the browning response in white adipose tissue which may have a role in the protection from metabolic disease afforded by vegetable consumption, but also highlights this small anion as a potentially important element of the adipose adaptive response to hypoxia. Therefore nitrate may provide an important therapy for the treatment of obesity and diabetes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Dr. Lee Roberts is supported by the MRC-Human Nutrition Research Elsie Widdowson Fellowship and supported by the Lipid Profiling and signaling program (MC_UP_A90_1006).
References
- Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 2009; 90:1-10; PMID:19439460; http://dx.doi.org/10.3945/ajcn.2008.27131
- Moncada S. The L-arginine: nitric oxide pathway, cellular transduction and immunological roles. Adv Second Messenger Phosphoprotein Res 1993; 28:97-9; PMID:8398422
- Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol 1976; 14:545-8; PMID:1017769; http://dx.doi.org/10.1016/S0015-6264(76)80005-3
- van Loon AJ, Botterweck AA, Goldbohm RA, Brants HA, van Klaveren JD, van den Brandt PA. Intake of nitrate and nitrite and the risk of gastric cancer: a prospective cohort study. Br J Cancer 1998; 78:129-35; PMID:9662263; http://dx.doi.org/10.1038/bjc.1998.454
- Tang Y, Jiang H, Bryan NS. Nitrite and nitrate: cardiovascular risk-benefit and metabolic effect. Curr Opin Lipidol 2011; 22:11-5; PMID:21102328; http://dx.doi.org/10.1097/MOL.0b013e328341942c
- Pannala AS, Mani AR, Spencer JP, Skinner V, Bruckdorfer KR, Moore KP, Rice-Evans CA. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med 2003; 34:576-84; PMID:12614846; http://dx.doi.org/10.1016/S0891-5849(02)01353-9
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer 1992; 18:1-29; PMID:1408943; http://dx.doi.org/10.1080/01635589209514201
- Terry P, Terry JB, Wolk A. Fruit and vegetable consumption in the prevention of cancer: an update. J Intern Med 2001; 250:280-90; PMID:11576316; http://dx.doi.org/10.1046/j.1365-2796.2001.00886.x
- Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A 2007; 104:19144-9; PMID:18025468; http://dx.doi.org/10.1073/pnas.0706579104
- Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide-Biol Ch 2008; 19:333-7; PMID:18793740; http://dx.doi.org/10.1016/j.niox.2008.08.003
- Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008; 51:784-90; PMID:18250365; http://dx.doi.org/10.1161/HYPERTENSIONAHA.107.103523
- Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol 2008; 4:411-7; PMID:18516050; http://dx.doi.org/10.1038/nchembio.92
- Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med 2006; 355:2792-3; PMID:17192551; http://dx.doi.org/10.1056/NEJMc062800
- Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 2010; 56:274-81; PMID:20585108; http://dx.doi.org/10.1161/HYPERTENSIONAHA.110.153536
- Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci U S A 2010; 107:17716-20; PMID:20876122; http://dx.doi.org/10.1073/pnas.1008872107
- Essawy S, Khaled AS, Amani E. Comparing the effects of inorganic nitrate and allopurinol in renovascular complications of metabolic syndrome in rats: role of nitric oxide and uric acid. Acta Endocrinologica-Bucharest 2012; 8:387-401.
- Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 2005; 1:290-7; PMID:16408059; http://dx.doi.org/10.1038/nchembio734
- Bordicchia M, Liu DX, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang CY, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes (vol 122, pg 1022, 2012). J Clin Invest 2012; 122:1584; PMID:22307324; http://dx.doi.org/10.1172/JCI63775
- Haas B, Mayer P, Jennissen K, Scholz D, Berriel Diaz M, Bloch W, Herzig S, Fassler R, Pfeifer A. Protein kinase G controls brown fat cell differentiation and mitochondrial biogenesis. Sci Signal 2009; 2:ra78; PMID:19952371
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 2003; 299:896-9; PMID:12574632; http://dx.doi.org/10.1126/science.1079368
- Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P, Haas B, Sassmann A, Pfeifer A, Kilic A. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J 2013; 27:1621-30; PMID:23303211; http://dx.doi.org/10.1096/fj.12-221580
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277-359; PMID:14715917; http://dx.doi.org/10.1152/physrev.00015.2003
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 2000; 404:652-60; PMID:10766252
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999; 98:115-24; PMID:10412986; http://dx.doi.org/10.1016/S0092-8674(00)80611-X
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997; 387:90-4; PMID:9139827; http://dx.doi.org/10.1038/387090a0
- Roberts LD, Bostrom P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, et al. beta-aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and Is inversely correlated with cardiometabolic risk factors. Cell Metab 2014; 19:96-108; PMID:24411942; http://dx.doi.org/10.1016/j.cmet.2013.12.003
- Ishibashi J, Seale P. Beige Can Be Slimming. Science 2010; 328:1113-4; PMID:20448151; http://dx.doi.org/10.1126/science.1190816
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPAR gamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285:7153-64; PMID:20028987; http://dx.doi.org/10.1074/jbc.M109.053942
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose-tissue - molecular and morphological characterization. J Cell Sci 1992; 103:931-42; PMID:1362571
- Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res 1997; 38:2125-33; PMID:9374134
- Melnyk A, Harper ME, HimmsHagen J. Raising at thermoneutrality prevents obesity and hyperphagia in BAT-ablated transgenic mice. Am J Physiol 1997; 272:R1088-R93; PMID:9140006
- Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 1995; 96:2914-23; PMID:8675663; http://dx.doi.org/10.1172/JCI118363
- Roberts LD, Ashmore T, Kotwica AO, Murfitt SA, Fernandez BO, Feelisch M, Murray AJ, Griffin JL. Inorganic nitrate promotes the browning of white adipose tissue through the nitrate-nitrite-nitric oxide pathway. Diabetes 2014; [Epub ahead of print]; PMID:25249574
- Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol 2009; 5:865-9; PMID:19915529; http://dx.doi.org/10.1038/nchembio.260
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993; 329:2002-12; PMID:7504210; http://dx.doi.org/10.1056/NEJM199312303292706
- Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab 2006; 3:277-87; PMID:16581005; http://dx.doi.org/10.1016/j.cmet.2006.02.011
- Ye JP, Gao ZG, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol-Endocrinol Meta 2007; 293:E1118-E28; PMID:17666485; http://dx.doi.org/10.1152/ajpendo.00435.2007
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56:901-11; PMID:17395738; http://dx.doi.org/10.2337/db06-0911
- Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, et al. Dietary obesity-associated Hif1 alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD(+) system. Genes Dev 2012; 26:259-70; PMID:22302938; http://dx.doi.org/10.1101/gad.180406.111
- Wellen KE, Thompson CB. Cellular Metabolic Stress: Considering How Cells Respond to Nutrient Excess. Mol Cell 2010; 40:323-32; PMID:20965425; http://dx.doi.org/10.1016/j.molcel.2010.10.004
- Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009; 33:54-66; PMID:19050672; http://dx.doi.org/10.1038/ijo.2008.229
- Siervo M, Jackson SJ, Bluck LJC. In-vivo nitric oxide synthesis is reduced in obese patients with metabolic syndrome: application of a novel stable isotopic method. J Hypertens 2011; 29:1515-27; PMID:21720276; http://dx.doi.org/10.1097/HJH.0b013e3283487806
