?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The adipose cell-size distribution is a quantitative characterization of adipose tissue morphology. At a population level, the adipose cell-size distribution is insulin-sensitivity dependent, and the observed correlation between obesity and insulin resistance is believed to play a key role in the metabolic syndrome. Changes in fat mass can be induced by altered energy intake or even diet composition. These macroscopic changes must manifest themselves as dynamic adipose cell-size distribution alterations at the microscopic level. The dynamic relationship between these 2 independent measurements of body fat is unknown. In this study, we investigate adipose tissue dynamics in response to various isocaloric diet compositions, comparing gender- and insulin sensitivity-dependent differences. A body composition model is used to predict fat mass changes in response to changes in diet composition for 28 individuals, separated into 4 subgroups according to gender and insulin sensitivity/resistance. Adipose cell-size distribution changes in each individual are simulated with a dynamic model and parameters corresponding to lipid turnover and cell growth rates are determined for each subgroup to match the relative change of fat mass for each diet composition, respectively. We find that adipose cell-size dynamics are associated with different modulations dependent on gender and insulin resistance. Larger turnover and growth/shrinkage rates in insulin resistant individuals suggest they may be more sensitive to changes in energy intake and diet composition than insulin sensitive subjects. The different cell-size distribution changes of adipose cells of various sizes in different subject groups further suggest distinct modulations of adipose cell dynamics.
Keywords:
Introduction
Obesity and its correlated dysfunctions have become a major health issue all over the world.Citation1 Both body weight and composition (fat mass and fat-free mass) can be influenced by various factors.Citation2,3 It is well known that the net energy intake level can lead to increase or loss of fat mass and body weight.Citation4 Even diet composition (percent of carbohydrate, protein and fat) can induce changes in body composition.Citation5,6 Adipose tissue is composed of a large number of adipose cells with cell sizes ranging from less than 20 microns to over 200 microns, and thus a thousand fold difference in lipid storage capacity.Citation7,8 Therefore, fat mass is determined by both adipose cell numbers and sizes. It has been reported that male or female subjects have different adipose cell-size distribution characteristics.Citation9 Moreover, obese individuals have distinct cell-size distribution patterns compared with normal weight individuals in both human and animal studies.Citation8,10 Therefore, the adipose cell-size distribution may be an important factor associated with the etiology of obesity and type 2 diabetes.Citation8,11
Adipose cell number and size are not static. Various processes (e.g. new cell recruitment, cell growth/shrinkage, lipid turnover, and cell death) can lead to changes in adipose cell-size distributions over the life of an individual.Citation10,12,13 Correspondingly, a change in fat mass is associated with a change in the adipose cell-size distribution, which includes information on cell sizes and numbers. Quantitative elucidation of the relationship of whole body fat mass changes, the macroscopic level, and adipose cell-size and number changes, the microscopic level, in response to physiological stimuli and energy intake is essential to understand the development of obesity, as adipose tissue state is history-dependent.Citation9,10,12,13 However, little is known about the relationship between metabolic state, changes in macroscopic fat mass, and associated changes in microscopic adipose tissue morphology.
Although whole body composition and fat mass can be measured by techniques such as computerized tomography,Citation14,15 it is not feasible to use these to gather data on dynamic changes of body composition in humans. The adipose cell-size distribution is even more difficult to determine, but can be obtained from biopsies of subcutaneous fat.Citation7 However, it is not possible to repeat such biopsies multiple times on humans or small mammals. Therefore, dynamic changes in adipose tissue in response to physiological stimuli are not well understood.
In recent years, physiology-based computational models have been widely applied in metabolic studies. Specifically, a mathematical body composition model (BCM) has been successfully validated as a predictor of diet-dependent changes in body composition.Citation16,17 This model can predict dynamic changes in body weight and fat mass by incorporating factors such as sex, age, weight, physical activity and diet. Therefore, it can predict the time courses of macroscopic body composition changes for specific individual characteristics under different dietary regimens. Moreover, a mathematical model of adipose cell growth dynamics has been developed to simulate changes in cell-size distributionsCitation10,12,13 in various conditions. This model can provide the detailed microscopic dynamics of the adipose cell-size distribution over time. The total lipid content can then be obtained by summing over all adipose cell-sizes. Although these 2 models correspond to macroscopic and microscopic views of adipose tissue, the fat mass changes predicted by the 2 models must agree.
This raises a puzzle: The BCM does not have any parameter associated with insulin sensitivity in an individual. Insulin is the primary regulator of lipolysis through its action on hormone-sensitive lipase and other lipases,Citation18 and therefore insulin sensitivity is a major factor in microscopic adipose tissue state.Citation11 It follows then that the parameters governing the dynamic model of adipose tissue development must be implicitly dependent on insulin sensitivity in such a way that fat mass changes predicted by the 2 completely independent models coincide. This is not at all obvious.
In this study, we set out to resolve this puzzle. We match the 2 mathematical models of adipose tissue, using published clinical data involving 4 groups of subjects distinguished by insulin sensitivity (insulin sensitive or insulin resistant) and sex (male or female). We find the parameters governing the dynamics of the adipose cell-size distribution in each group for various diet compositions. The BCM is used to provide the dynamics of fat mass according to individual characteristics with varying diet compositions (% carbohydrate) as the input. We consider isocaloric, weight maintenance diets and the short term responses of fat mass (∼14 days) and the corresponding changes in adipose cell-size distributions of different subject groups.
Materials and Methods
Experimental data
Adipose cell-size distributions were obtained from McLaughlin et al.'s study.Citation7 In brief, data from 28 age and weight matched obese subjects was available, either insulin sensitive IS (4 male ISM and 11 female ISF) or insulin resistant IR (4 male IRM and 9 female IRF), determined by insulin suppression test.Citation7 The detailed individual characteristics are shown in Table S1. Biopsy samples were obtained from subcutaneous adipose tissue inferior to the umbilicus for each subject, and the adipose cell-size probability distributions were determined using a Beckman-Coulter counter.
Change of body composition with isocaloric diets
Both body weight and composition are influenced by diet composition, even with isocaloric diets.Citation5,6 Changes in body composition in response to specific diets were simulated using a validated mathematical model.Citation16
The body composition model (BCM) and anthropometric data for each subject (body weight, height) along with age and gender were used to predict the changes in body and fat mass of the subjects. All subjects were assumed to be at steady state (no significant weight changes in previous months) and they were assumed to have a physical activity level of 1.5 (an inactive work style, sitting or more or less sedentary, and very light exercise activities outside of work such as light walking). Using this information, the model was used to estimate the change of body weight and fat mass over specific short time periods (∼2 week days) under isocaloric diets with varying carbohydrate contribution in total dietary energy (carbohydrate intake index, CI. The fraction of dietary protein is assumed to be a constant 14%. The CI values were chosen covering the possible range 10–70%, avoiding extreme CI values close to 0 or 86%.
The BCM simulated fat mass changes in response to specific diets were then used as data to compare with the changes of adipose tissue mass generated from a dynamic model of adipose tissue (detailed below). To avoid the influence of body weight transients during the transition in diet, the first week predictions of the BCM after diet change were not utilized.
Changes of adipocyte distribution
The distributions of adipose cells n(s,t) are modeled quantitatively by a computational model detailed in Jo et al.'s studyCitation10,12 with minor modifications. Here, the size s is the cell diameter. This model can describe the dynamics of size–dependent cell growth/shrinkage and fluctuations due to lipid turnover. In the present study, because we consider a short-term response on an isocaloric diet with minor weight changes, both recruitment of new adipose cells from differentiation of precursor cells and death of existing adipose cells were neglected. A brief model description is given in the supplemental materials (section 3).
Simulation strategy
Parameters
All parameters in the body composition model are fixed. Model simulations are determined by the subject characteristics (gender, weight, height, age) and diet composition. Most of the parameters in the adipocyte distribution model are fixed as well. The unknown parameters are the cell growth/shrinkage rate coefficient (Vm) and cell size turnover rate coefficient (D), which reflects lipid turnover randomly occurring in adipose cells. The two parameters are optimized for each subject group at each diet composition level, respectively.
Cost function
For each diet composition and each subject group Subϵ(ISM, ISF, IRM, IRF, and all IS and IR), parameters are determined to minimize the relative differences between the simulated whole body fat mass change and the relative change of adipose cell-size probability distributions. The cost function (F) is the sum of these squared relative differences in the 2nd week for the whole subject group.(1)
(1) in which t0 is the time applied as the basal state. Here t0 = 8 days because the transient response during the first week is neglected. tm is the total simulation period. nSub is the number of subjects in each subject group. FM is the fat mass simulated from the body composition model. ATM is the adipose tissue mass at specific times, calculated as
, in which s is the cell size bins, and ρ is the known density of adipose cells.Citation10,12
Simulation conditions
The predicted fat mass values obtained from the BCM are for whole body fat changes, but subcutaneous fat and visceral fat have distinct functions.Citation19 In particular, male and female humans have different ratiosCitation15 of the 2 kinds of adipose tissue. It is essential to distinguish between the 2.
MOD 1: We assume that the relative change of whole body fat is the same as the relative change of subcutaneous fat. Therefore the cost function (EquationEq. 1(1)
(1) ) is used to determine parameters for each subject group and each diet.
MOD 2: According to an allometric law,Citation20 the relative change of subcutaneous fat is proportional to the relative change of visceral fat. This constraint helps to determine the relative change of subcutaneous fat mass itself from the relative change of whole body fat. Therefore, in MOD 2, the cost function (EquationEq. 1(1)
(1) ) is modified as
(3)
(3) in which SM is the subcutaneous fat mass. The derivation associated with fat balance is shown in supplemental materials Section 4. Thus, we used the validated allometric law for human subcutaneous and visceral body fat distribution to model the differences between these 2 fat depots.
Calculations
Time courses of adipose cell-size distribution of each subject with specific diet can be simulated with the parameters obtained above, respectively. The change in the adipose cell-size distribution of a specific subject from the basal values is calculated as [n(s,ti)-n(s,t0)]k. in which n(s,t0) is the initial cell-size distribution; n(s,ti) is the cell-size distribution at a specific time, and k is the subject in each group (ISM, ISF, IRM, IRF). Various cell-size distribution variables (peak diameter and value, nadir diameter and value, percentage of small cells, ratio of small and large cells) were calculated as previously published.Citation9 In brief, the nadir is the cell diameter at which the adipose cell-size distribution reaches its minimum between the mode at small sizes and the mode at larger sizes. The adipocytes whose sizes are smaller (larger) than this nadir referred to as small (large) cells. The total number of small or large cells is calculated as the sum of frequencies of each population, respectively, and these numbers are then used to compute the percentage in each population and the ratio of small to large cells. The peak diameter is the mean diameter at which the frequency of the large cell population reaches a maximum. These variables are compared for different subject groups under different diet compositions. Moreover, the average values of the change of cell-size distribution for subject groups (ISM, ISF, IRM, IRF, and for all male or all female, or all IS or all IR) were calculated and compared.
Simulation environment
The partial differential equation system of adipose cell distribution model was solved by a discrete step method with adipose cell size interval 0.73 μm.Citation10,12 To investigate the dependence of parameters on the time step and total simulation time, parameters were estimated by choosing 10 different simulation times (11, 12, …, 20 days) and 2 time intervals (1 hour and 0.5 hour), respectively.
Results
In this study, the parameters associated with adipose cell-size distribution dynamics were estimated for different subject groups and different diets by matching macroscopic fat mass changes induced by the change of diet composition according to a macroscopic body composition model to the mass change calculated as the sum of microscopic changes in adipose cells of all sizes estimated by an adipose tissue dynamics model.
Cell-size distribution characteristics
The adipose cell-size distribution characteristics of each subject group in the basal state are shown in . There were no significant differences for the percent of small cells (%small) and the ratio of small and large cells (S/L) for all subject groups. Both the peak and nadir diameters of ISM were significantly smaller than the corresponding values for ISF and IRF. The peak values of various subject groups were similar. The nadir value of ISF was smaller than ISM.
Table 1. Comparison of cell-size distribution variable of each subject group at basal conditions
The relative changes in cell-size distribution characteristics at CI = 20% and 70% are shown in . There were no significant differences for the relative changes of peak and nadir diameters for all diet compositions (data not shown). At both CI = 20% and 70%, the fraction of small cells and the ratio of small and larger cell numbers have significant differences between ISM and ISF group, and between ISF and IRF groups.
Table 2. Relative difference of cell-size distribution characteristics†
Parameters of stability and subject groups
Parameter values of various subject groups with specific diet composition conditions generated by the 2 models were in agreement. The stability of these parameters under changes in simulation time and integration time step were compared (Fig. S1-S2). With time step 0.5 hour, the parameter values from different simulation times were consistent compared with the values obtained with time interval 1 hour. Thus, parameter values are not sensitive to the simulation time if we chose short enough time steps. Therefore, in the following simulation, the simulation time was set as 2 weeks with 0.5 hour.
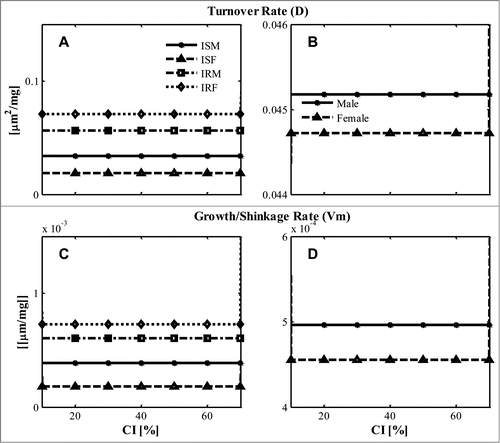
Parameter values were essentially independent of diet composition, which is due to the near constant fat mass changes predicted by BCM for the typical diet composition range (CI = 10–70%) (Fig. S3). The insulin resistant groups (IRM, IRF) tended to have larger turnover rate (D) () and growth/shrinkage rate (Vm) than insulin sensitive groups (ISM, ISF) (). The gender differences in the 2 parameters were negligible ()
Figure 1. Estimated parameter at specific diet composition (CI, %) (A, B) Turnover rate coefficient “D” distinguished by (A) ISM, ISF, IRM, IRF; (B) by the average values of all male or female; (C, D) Growth/shrinkage coefficient “Vm” distinguished (C) by ISM, ISF, IRM, IRF; (D) by average values of all male or female, which is calculated as the mean value of all male or all female subject based on the estimated parameter for ISM- IRM, ISF- IRF.
Dynamic changes in adipose cell-size distributions
With the estimated parameters, the time course of adipose cell-size distribution dynamics can be simulated for each subject in response to various diet compositions. The differences between cell-size distributions at specific times and the initial cell-size distribution can be calculated as n(s,tj) –n(s,t0). A typical graph (in this case, from one of the ISF subjects) is shown in , which explicitly demonstrates that adipose cells of different sizes have distinct changes in response to changes in diet composition.
Figure 2. Representative changes of adipose cell-size distribution time courses with specific diet composition (A) CI = 10%; (B) CI = 40%; (C) CI = 60%; (D) CI = 70%. The changes are calculated as the difference between the cell-size distribution at specific time n(s,tj) and the initial cell-size distribution n(s,t0). The results of different simulation time are compared: dashed line, 4 days; dotted line, 9 days; dash-dot line, 14 days. (One of the ISF subjects is shown here.)
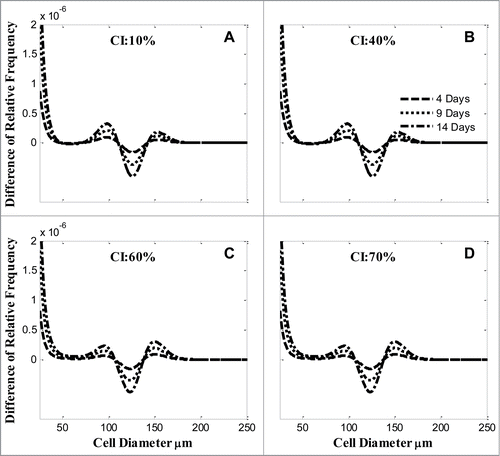
From CI = 10–70%, the changes of cell-size distributions are small (). The fractions of cells (∼100 and ∼150 μm) had small increases but the fraction of cells (∼125 μm) decreased instead.
Average changes of cell-size distribution dynamics
Based on the changes in adipose cell-size distributions in each subject, the average changes in the cell-size distribution of each subject group were calculated.
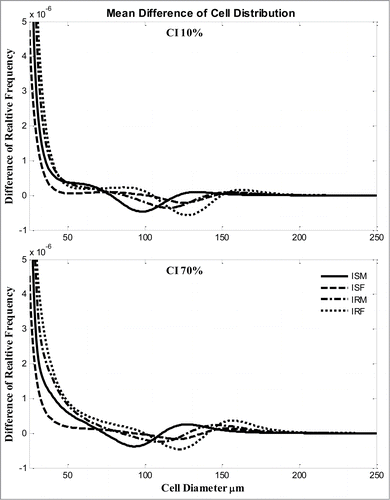
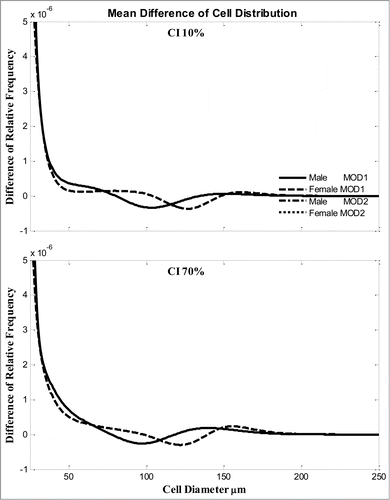
The average changes are clearly gender dependent () for both CI = 10 and 70%. Neglecting the very small cells, the male and female subjects had the maximal decrease for cells ∼100 μm and 125 μm, respectively; and the maximal increase for cells at ∼130 and ∼160 μm, respectively. There are no differences between the 2 models, MOD1 and MOD2.
Figure 3. Average changes of adipose cell-size distribution (relative frequency) after 2 weeks for all male or all female subjects with diet composition (A) CI = 10% or (B) CI = 70%. Two models simulations are compared. Solid line, male from MOD 1; dashed line, female from MOD 1; dash-dot line, male from MOD 2; dotted line, female from MOD 2.
The average changes of 4 groups were compared in . Compared with the average differences of male subjects, ISM dominated the changes. The changes of IRM were close to the 2 female groups.
Discussion
In this study, the consistency of macroscopic and microscopic quantitative models of adipose tissue is used to find gender and insulin sensitivity dependent dynamic differences in the size-dependent response of adipose cells to different isocaloric diet compositions. Simulated fat mass changes at various diet compositions provide the constraints for dynamics of the adipose cell-size distribution.
Short terms changes of fat mass in responses to diet compositions
While it is well known that energy intake is the key factor influencing fat massCitation1, recent studies showed that diet composition (e.g.,, percent of carbohydrate, fat and protein) also affects body weight and fat mass.Citation5,6 Hall's body composition model (BCM),Citation16,17,21 in particular, takes the overall energy content and the carbohydrate intake index (CI, percent of carbohydrate energy contribution) as the only model inputs in addition to specific subject characteristics (Fig. S3).
Dietary energy generally is generated from carbohydrate, lipid and protein. For short-terms responses, if we assume the constant fraction of dietary protein (typically ∼14%) with various CI conditions, the effects of protein on body composition can be assumed unchanged.Citation17 Correspondingly, only the altered fractions of carbohydrate and lipid influence short-term body composition changes. For typical diets (CI = 10–70%), the predicted fat mass changes are almost constant (Fig. S3) within the limited time interval considered (2wk).
Key parameters associated with adipose cell dynamics
In this study, only the short term (2 weeks) responses of adipose cell dynamics to isocaloric diet changes are considered, which helps to reduce model complexity by rendering the effect of new cell recruitment and cell death negligible. Other complex effects, e.g. inflammation on adipose tissue also can be neglected.Citation22 Therefore, dynamic changes in the adipocyte cell-size distribution are determined by the lipid turnover rate (D) and the growth/shrinkage rate (Vm). These two estimated parameters for the various subject groups have almost no diet dependent changes () Especially, insulin resistant groups (IRM, IRF) have larger turnover rates and growth/shrinkage rates compared with insulin sensitive groups (ISF, ISM) ().
The development of insulin resistance is closely associated with obesity (accumulation of fat). As predicted by Hall's BCM,Citation16 the net energy balance directly influences body weight and fat mass, which should be reflected by the adipocyte distribution changes at the microscopic level. The dynamics of adipose cells are dependent on cell-size fluctuations due to lipid turnover and growth/shrinkage in this simplified condition, represented by 2 essential parameters (D, Vm). The larger values of (D, Vm) for insulin resistant groups suggest that these individuals may be more sensitive to changes in energy intake, i.e., quicker to gain weight and reach larger adipocyte cell-sizes. Both of these characteristics are closely associated with the anabolic effects of higher insulin levels.Citation23 Measured adipose cell-size distributions from biopsy samples also show different cell-size distributions between normal and obese individuals.Citation8,9,10 The present study provides evidence that insulin resistance can influence fundamental microscopic adipose cell dynamics even in an isocaloric diet composition change. The molecular mechanisms underlying these altered dynamics remain to be elucidated.
Changes of adipocyte distribution due to altered diet composition
The total fat mass changes due to altering diet composition are accompanied by changes in adipose cell-sizes and numbers. Adipose tissue is broadly categorized as subcutaneous fat and visceral fat. They play different roles in the development of insulin resistance,Citation19 and have slightly different cell distribution profiles in obese insulin resistant women.Citation24 Typically biopsy samples are obtained from subcutaneous fat, which may not reflect the actual cell-size distributions in visceral fat. In the present study, we compared the corresponding cell-size distributions without (MOD 1) or with (MOD 2) distinguishing between the 2 kinds of fat tissue, using an allometric relation between the 2. As the models differentiating between visceral and subcutaneous fat cannot be distinguished, it may be that the role of visceral fat is evident only for larger diet changes or over longer time scales.
As previously mentioned, adipose cell-sizes have a large range.Citation7 Large cells provide the dominant contribution to total fat mass,Citation10,12 but the effect of small cells cannot be arbitrarily neglected due to the changes in the cell-size distribution under specific conditions. Model simulations show that the fractional changes of different size of adipose cells are not uniform (), especially for the major cell-size range 80–180 μm.
The adipocyte cell-size distribution changes can be further compared between various subjects. Clearly, male and female individuals have dramatic differences (). Similarly, if both gender and insulin resistance are controlled, the fractional changes of the ISM group are different compared with the other 3 groups in 2 conditions (). In fact, the profile of ISM is very close to the averaged profile of all male subjects (), suggesting that the average over all males is dominated by ISM in this cohort of subjects. Especially prominent is the fact that insulin resistance is correlated with a shift in cell-size distributions of IRM closer to female groups.
In the basal condition, there are significant differences between ISM-ISF, and between ISM-IRF subjects for peak diameter and nadir diameter (, Fig. S4). Clearly, gender difference is the primary factor for the differences between the adipose cell-size distributions. In response to diet changes, the values of peak and nadir diameter and corresponding magnitudes have no significant changes (data not shown).
However, the ratio of small and large cells is different between ISM-ISF and even between the 2 female groups (). These results provide additional evidence of gender and insulin resistance-dependent regulation of adipose cell-size distributions.
Limitations: Predictions of fat mass changes were generated from the BCM, so experimental measurements are needed for validation. To reduce model complexity, we only consider the short-term changes of fat mass to isocaloric diets, which are difficult to measure in typical physiological conditions. The absolute changes of fat mass under these isocaloric diets are relatively small. Therefore, data on the long-term fat mass changes induced by diets with different energy content are needed as well.
In conclusion, 2 quantitative perspectives on adipose tissue are combined together here to evaluate the adipose cell-size distribution characteristics of various subjects in response to isocaloric diet changes. We showed that insulin resistant subjects have larger values of parameters affecting lipid turnover (D) and cell-size increase (Vm). Thus insulin resistance is associated with adipose cells reaching larger sizes and with a wider range of cell sizes. Especially in weight loss, unraveling the complex mechanisms involved is an intriguing avenue for future research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental_Materials.docx
Download MS Word (144.5 KB)Acknowledgments
We thank Arthur Sherman and Kevin Hall for invaluable help and comments.
Funding
This work is supported by the Intramural Research Program of the National Institutes of Health (NIH), NIDDK.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Reference
- Friedman JM. Obesity: Causes and control of excess body fat. Nature 2009; 459:340-2; PMID:19458707; http://dx.doi.org/10.1038/459340a
- Blundell JE, Cooling J. Routes to obesity: phenotypes, food choices and activity. Br J Nutr 2000; 83 Suppl 1:S33-8; PMID:10889790
- Brozek J. Body Composition: The relative amounts of fat, tissue, and water vary with age, sex, exercise, and nutritional state. Science 1961; 134:920-30; PMID:17812916; http://dx.doi.org/10.1126/science.134.3483.920
- Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation 2012; 126:126-32; PMID:22753534; http://dx.doi.org/10.1161/CIRCULATIONAHA.111.087213
- Noakes M, Foster PR, Keogh JB, James AP, Mamo JC, Clifton PM. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr Metab (Lond) 2006; 3:7; PMID:16403234; http://dx.doi.org/10.1186/1743-7075-3-7
- Tay J, Brinkworth GD, Noakes M, Keogh J, Clifton PM. Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J Am Coll Cardiol 2008; 51:59-67; PMID:18174038; http://dx.doi.org/10.1016/j.jacc.2007.08.050
- McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, Reaven GM, Cushman SW. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia 2007; 50:1707-15; PMID:17549449; http://dx.doi.org/10.1007/s00125-007-0708-y
- Yang J, Eliasson B, Smith U, Cushman SW, Sherman AS. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity (Silver Spring) 2012; 20:932-8; PMID:22240722; http://dx.doi.org/10.1038/oby.2011.371
- McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, Cushman SW. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity (Silver Spring) 2014; 22:673-80; PMID:23666871; http://dx.doi.org/10.1002/oby.20209
- Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol 2009; 5:e1000324; PMID:19325873; http://dx.doi.org/10.1371/journal.pcbi.1000324
- Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Blüher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 2010; 299:E506-15; PMID:20570822; http://dx.doi.org/10.1152/ajpendo.00586.2009
- Jo J, Guo J, Liu T, Mullen S, Hall KD, Cushman SW, Periwal V. Hypertrophy-driven adipocyte death overwhelms recruitment under prolonged weight gain. Biophys J 2010; 99:3535-44; PMID:21112277; http://dx.doi.org/10.1016/j.bpj.2010.10.009
- Jo J, Shreif Z, Periwal V. Quantitative dynamics of adipose cells. Adipocyte 2012; 1:80-8; PMID:23700516; http://dx.doi.org/10.4161/adip.19705
- Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012; 55:2622-30; PMID:22898763; http://dx.doi.org/10.1007/s00125-012-2639-5
- Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. Computed tomography assessment of fat distribution in male and female patients with Cushing's syndrome. Eur J Endocrinol 2003; 149:561-7; PMID:14640998; http://dx.doi.org/10.1530/eje.0.1490561
- Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab 2010; 298:E449-66; PMID:19934407; http://dx.doi.org/10.1152/ajpendo.00559.2009
- Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011; 378:826-37; PMID:21872751; http://dx.doi.org/10.1016/S0140-6736(11)60812-X
- Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr 2007; 27:79-101; PMID:17313320; http://dx.doi.org/10.1146/annurev.nutr.27.061406.093734
- Mundi MS, Karpyak MV, Koutsari C, Votruba SB, O'Brien PC, Jensen MD. Body fat distribution, adipocyte size, and metabolic characteristics of nondiabetic adults. J Clin Endocrinol Metab 2010; 95:67-73; PMID:19890025; http://dx.doi.org/10.1210/jc.2009-1353
- Hallgreen CE, Hall KD. Allometric relationship between changes of visceral fat and total fat mass. Int J Obes (Lond) 2008; 32:845-52; PMID:18087265; http://dx.doi.org/10.1038/sj.ijo.0803783
- Hall KD. Modeling metabolic adaptations and energy regulation in humans. Annu Rev Nutr 2012; 32:35-54; PMID:22540251; http://dx.doi.org/10.1146/annurev-nutr-071811-150705
- Cummins TD, Holden CR, Sansbury BE, Gibb AA, Shah J, Zafar N, Tang Y, Hellmann J, Rai SN, Spite M, et al. Metabolic remodeling of white adipose tissue in obesity. Am J Physiol Endocrinol Metab 2014; 307:E262-77; PMID:24918202; http://dx.doi.org/10.1152/ajpendo.00271.2013
- Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013; 93:359-404; PMID:23303913; http://dx.doi.org/10.1152/physrev.00033.2011
- Liu A, McLaughlin T, Liu T, Sherman A, Yee G, Abbasi F, Lamendola C, Morton J, Cushman SW, Reaven GM, et al. Differential intra-abdominal adipose tissue profiling in obese, insulin-resistant women. Obes Surg 2009; 19:1564-73; PMID:19711137; http://dx.doi.org/10.1007/s11695-009-9949-9